Abstract
Background
Only one-third of patients with Major Depressive Disorder (MDD) achieve remission with initial treatment. Consequently, current clinical practice relies on a “trial-and-error” approach to identifying an effective treatment for each patient. The purpose of this report was to determine whether we could identify a set of clinical and biological parameters with potential clinical utility for prescription of exercise for treatment of MDD in a secondary analysis of the Treatment with Exercise Augmentation in Depression (TREAD) trial.
Methods
Participants with non-remitted MDD were randomized to one of two exercise doses for 12 weeks. Participants were categorized as “remitters” (≤ 12 on the IDS-C), non-responders (< 30% drop in IDS-C), or neither. The least absolute shrinkage and selection operator (LASSO) and random forests were used to evaluate thirty variables as predictors of both remission and non-response. Predictors were used to model treatment outcomes using logistic regression.
Results
Of the 122 participants, 36 were categorized as remitters (29.5%), 56 as non-responders (45.9%), and 30 as neither (24.6%). Predictors of remission were higher levels of brain derived neurotrophic factor (BDNF) and IL-1B, greater depressive symptom severity, and higher post-exercise positive affect. Predictors of treatment non-response were low cardiorespiratory fitness, lower levels of IL-6 and BDNF, and lower post-exercise positive affect. Models including these predictors resulted in predictive values greater than 70% (true predicted remitters/all predicted remitters) with specificities greater than 25% (true predicted remitters/all remitters).
Conclusions
Results indicate feasibility in identifying patients who will either remit or not respond to exercise as a treatment for MDD utilizing a clinical decision model that incorporates multiple patient characteristics.
Keywords: exercise, depression, treatment outcomes, decision support techniques
Introduction
Major Depressive Disorder (MDD) results in significant disease burden including impaired psychosocial functioning (Judd et al., 2008; Wells et al., 1989), greater risk of future MDD episodes (Burcusa and Iacono, 2007), and poorer general health outcomes (Barth et al., 2004; Cuijpers and Smit, 2002; Koponen et al., 2010; Pinquart and Duberstein, 2010). These detrimental effects of MDD result in an annual economic burden of $83 billion in the United States (Greenberg et al., 2003).
One factor that greatly contributes to significant disease burden of MDD is the heterogeneous treatment response patients experience with current treatment options. Initial response rates to treatment with SSRIs are approximately 50% (Depression Guideline Panel, 1993), with remission rates for SSRI treatment ranging from 30%–35% (Thase et al., 2001; Entsuah et al, 2001) (Thase et al., 2005). Similarly, exercise is an efficacious alternative treatment for MDD, with remission rates to exercise treatment in randomized controlled trials similar to those observed with SSRIs (Rethorst et al., 2009). Augmentation or treatment switches are therefore often necessary for a significant portion of patients with MDD.
This “trial and error” process results in prolonged disease presence that could be averted if patients received a personalized prescription for the treatment most likely to be effective and/ or avoid treatments that are likely to fail. Many individual patient characteristics are associated with desired treatment outcomes of SSRIs (Papakostas and Fava, 2008) and exercise (Schuch et al., 2016), but these individual factors are not strong enough predictors to be clinically meaningful. Given the heterogeneity of depressive disorders and the limited predictive capability of individual factors, composite measures of several patient characteristics are likely necessary to be of clinical utility. More recent work has attempted to use multiple patient characteristics to improve the accuracy of these predictions with SSRI and psychotherapy (Saveanu et al., 2015; Wallace et al., 2013).
The purpose of this report was to determine whether we could identify a set of pre-defined clinical and biological parameters that might have clinical utility for prescription of exercise for treatment of depression. This secondary analysis utilized data from the Treatment with Exercise Augmentation for Depression (TREAD) trial (Trivedi et al., 2011; Trivedi et al., 2006a). We assessed the utility of predictors to identify two distinct patient subgroups: 1) patients for whom exercise would likely be beneficial, and 2) patients who would be unlikely to benefit from exercise. We reduced the heterogeneity of the sample by eliminating those patients for whom the efficacy of exercise was uncertain (i.e., those with only marginal improvement in depressive symptomatology).
Materials and Methods
A comprehensive description of the TREAD trial methodology has been published (Trivedi et al., 2011; Trivedi et al., 2006a). Details relevant to the current analysis are provided below.
Participants
Individuals meeting the following inclusion/exclusion criteria were eligible for participation. Inclusion criteria: 1) age 18–70; 2) diagnosis of nonpsychotic MDD, 3) 2–6 months of treatment with an SSRI with at least 6 weeks of adequate dose, 4) residual depressive symptomatology (defined as a score of ≥ 14 on the Hamilton Depression Rating Scale), 5) not engaged in regular exercise, 6) capable of exercise, and 7) able and willing to provide informed consent. Exclusion criteria included: 1) significant medical condition contraindicative of exercise, 2) depression due to a comorbid psychiatric disorder, 3) current pharmacological treatment other than SSRI, 4) failure of 2 or more pharmacologic treatments of adequate dose and duration during current depressive episode, and 5) pregnant or planned pregnancy.
Potential Baseline Predictors
An initial pool of 30 baseline characteristic variables was selected for analysis as potential predictors of remission and non-response (Table 1). Full description of these measures have been published previously (Greer et al., 2016; Rethorst et al., 2013a; Rethorst et al., 2013b; Suterwala et al., 2016; Toups et al., 2011; Trivedi et al., 2011; Trivedi et al., 2006a). These variables were selected based on the results of these previously published findings and other factors that have been associated with treatment outcomes in depression (Sotsky et al., 1991; Trivedi et al., 2006b; Warden et al., 2007).
Table 1.
Pool of potential predictor variables.
| Age | Length of Current Episode (weeks) | Baseline IL-1B |
| Antidepressant Treatment History | MEI Mental subscale | Baseline IL6 |
| Atypical Depression (y/n) | MEI Physical subscale | Baseline TNF-a |
| Baseline Body Mass Index | MEI Social subscale | Recurrent MDD (y/n) |
| Employment Status | Number of Axis I Disorders | Baseline SF12 Physical subscale |
| Family History of Mental Illness (y/n) | Number of Episodes of MDD | Baseline SF12 Mental subscale |
| Gender | Post-exercise positive affect (PANAS) | Baseline SHAPS |
| IDS Hypersomnia (y/n) | Post-exercise negative affect (PANAS) | Time on Adequate Dose of Antidepressant |
| Baseline IDS-SR | Baseline Patient Perception of Benefits | IDS Insomnia |
| Exercise dose (high/low) | Baseline BDNF | Cardiorespiratory Fitness (VO2max) |
IDS - Inventory of Depressive Symptomatology
IDS-SR - Inventory of Depressive Symptomatology - Self-Rated
MEI - Motivation and Energy Inventory
PANAS - Positive and Negative Affect Scale
MDD - Major Depressive Disorder
SHAPS - Snaith-Hamilton Pleasure Scale
Intervention
Participants were randomized to one of two exercise dose groups for 12 weeks: 4 kcal/kg/week or 16 kcal/kg/week. Participants completed the entire dose in supervised sessions during Week 1, completed two supervised sessions during Week 2, and completed 1 supervised session during Weeks 3–12. The remaining exercise dose was completed in unsupervised exercise sessions. Exercise intensity was self-selected and monitored with a heart rate monitor (Polar 610i) in supervised and unsupervised sessions.
Statistical Analysis
To minimize heterogeneity in the sample, we focused on two groups within the sample; those defined as treatment successes (those who achieved remission defined as a score of ≤ 12 on the IDS-C) or treatment failures (those who experienced less than a 30% drop in IDS-C total score by the end of their last week in the program (‘non-responders’). Of the 122 patients, 36 were remitters (29.5%), 56 were non-responders (45.9%), and 30 were neither (24.6%).
To utilize the maximum potential of the data, we followed the recommendations of Schomaker and Heumann (2014) and carried out both multiple imputation and the bootstrap. Starting with the initial pool of 30 variables, a filtering process was used to identify a subset of variables (after standardizing them to have a mean of 0 and standard deviation of 1) that seemed to contain predictive power. The process was carried out on each bootstrapped, multiply imputed sample and utilized both the least absolute shrinkage and selection operator, or LASSO (Tibshirani, 1996) and random forests (Breiman, 2001); these two methods were chosen so that both parametric and non-parametric modelling techniques were represented. We deemed predictive power to be added by variables with large effect sizes in both modeling paradigms. The LASSO was implemented via the glmnet package in R (Friedman et al., 2009; Friedman et al., 2010) with the penalty parameter chosen via 10-fold cross validation. Random forests were implemented using the random Forest function (Liaw and Wiener, 2002). The ranks of the relative strengths of the variables in each method were summed and the variables for inclusion in the final models were selected by identifying a natural “elbow” in the rank sums. Note that this process was repeated twice – once using remission status as the dependent variable, and once using non-response status as the dependent variable.
After selecting a set of variables for each classification problem, multiple imputation in combination with separate logistic regression models was used to estimate the probabilities of both remitting and non-responding for the subjects in the study. Because the patients for whom clinicians can take action to prescribe a treatment are those who can be predicted with substantial certainty to either remit or to have no meaningful benefit (i.e., no substantial response) to a particular treatment, we utilized the ratio of the estimated probability of remission to the estimated probability of no substantial response, subject to a minimum probability threshold:
| (1) |
where a is the largest ratio value for which we would not call someone a substantial non-responder, b is the smallest ratio value for which we would call someone a remitter, and c is the minimum probability threshold we require to call someone a remitter or non-responder, given that the appropriate ratio threshold is met. Assume that a ≤ b.
Four adjusted metrics were defined to evaluate the efficacy of a specific decision rule (phrased as “adjusted” because they are not defined as in the traditional sense. Remitters and non-responders – while mutually exclusive – are not complementary events, resulting in the creation of a third group of people who are not cured but do have some degree of response to treatment. ).
Positive Predictive Value (PPV):
Sensitivity:
Negative Predictive Value (NPV):
Specificity:
Results
For each bootstrap/imputation combination (we bootstrapped the data 200 times and imputed each bootstrapped data set 10 times), a lasso model was fit and the estimated coefficients were stored. The results from each of the 10 imputed data sets per bootstrap replicate were averaged, and the average of these averages are reported in Tables 2a and 2b. We also report the 95% bootstrap confidence interval based on the 200 bootstrapped averages. Note that only the ten strongest variables are reported, and that the sign of the bootstrap means indicates whether a one standard deviation increase in the variable increases or decreases the odds of remission/non-responding. With respect to random forests, we followed the same protocol but instead tracked the mean decrease in Gini index1 for each variable in each imputation of each bootstrap sample and present the results in Tables 3a and 3b below.
Table 2.
| a – Bootstrap Estimated Parameters – Lasso (Dependent = Remitter)
| |||
|---|---|---|---|
| Variable | Bootstrap Mean |
Lower 95% Bootstrap CI |
Upper 95% Bootstrap CI |
|
| |||
| BDNF | 0.835 | 0.111 | 1.754 |
| PANAS (positive) | 0.514 | 0 | 1.365 |
| IDS-SR | −0.461 | −1.441 | 0.002 |
| IL-1B | 0.331 | −0.083 | 1.047 |
| SF-12 mental subscale | 0.325 | −0.006 | 1.347 |
| Hypersomnia | 0.306 | −0.032 | 1.033 |
| SF-12 physical subscale | −0.298 | −0.948 | 0 |
| PANAS (negative) | −0.243 | −1.129 | 0.034 |
| MEI Physical subscale | −0.227 | −1.324 | 0.041 |
| Recurrent MDD | −0.219 | −0.933 | 0.071 |
| b – Bootstrap Estimated Parameters – Lasso (Dependent = Non-Responder)
| |||
|---|---|---|---|
| Variable | Bootstrap Mean |
Lower 95% Bootstrap CI |
Upper 95% Bootstrap CI |
|
| |||
| VO2max | 0.303 | 0 | 0.9 |
| PANAS (positive) | −0.247 | −0.803 | 0 |
| BDNF | −0.221 | −0.721 | 0.003 |
| Hypersomnia | −0.206 | −0.627 | 0 |
| IL-6 | −0.18 | −0.802 | 0.03 |
| IDS-SR | 0.153 | −0.015 | 0.86 |
| Gender | −0.136 | −0.571 | 0.01 |
| Exercise dose | 0.131 | −0.017 | 0.554 |
| Age | 0.124 | 0 | 0.625 |
| TNF-a | −0.112 | −0.825 | 0.246 |
Table 3.
| a – Bootstrap Estimated Mean Decrease in Gini – Random Forests (Dependent = Remitter)
| |||
|---|---|---|---|
| Variable | Bootstrap Mean | Lower 95% Bootstrap CI | Upper 95% Bootstrap CI |
|
| |||
| BDNF | 4.392 | 2.169 | 7.258 |
| PANAS (positive) | 3.64 | 1.801 | 6.552 |
| IDS-SR | 3.416 | 1.828 | 5.485 |
| IL-1B | 3.061 | 1.723 | 5.896 |
| TNF-a | 2.67 | 1.575 | 4.781 |
| Time on adequate SSRI dose | 2.517 | 1.666 | 3.964 |
| IL-6 | 2.404 | 1.428 | 4.139 |
| Perceived benefit | 2.203 | 1.266 | 3.493 |
| BMI | 2.193 | 1.499 | 3.149 |
| VO2max | 2.022 | 1.412 | 2.954 |
| b – Bootstrap Estimated Mean Decrease in Gini – Random Forests (Dependent = Non-Responder)
| |||
|---|---|---|---|
| Variable | Bootstrap Mean | Lower 95% Bootstrap CI | Upper 95% Bootstrap CI |
|
| |||
| PANAS (positive) | 4.358 | 2.537 | 7.282 |
| IL-6 | 3.918 | 2.581 | 6.313 |
| BDNF | 3.674 | 2.295 | 6.002 |
| VO2max | 3.444 | 2.15 | 5.527 |
| TNF-a | 3.431 | 2.114 | 6.187 |
| BMI | 2.948 | 2.211 | 4.021 |
| Time on adequate SSRI dose | 2.879 | 2.153 | 4.105 |
| IL-1B | 2.811 | 2.049 | 4.054 |
| SF-12 physical subscale | 2.71 | 1.716 | 4.097 |
| Age | 2.683 | 1.907 | 3.871 |
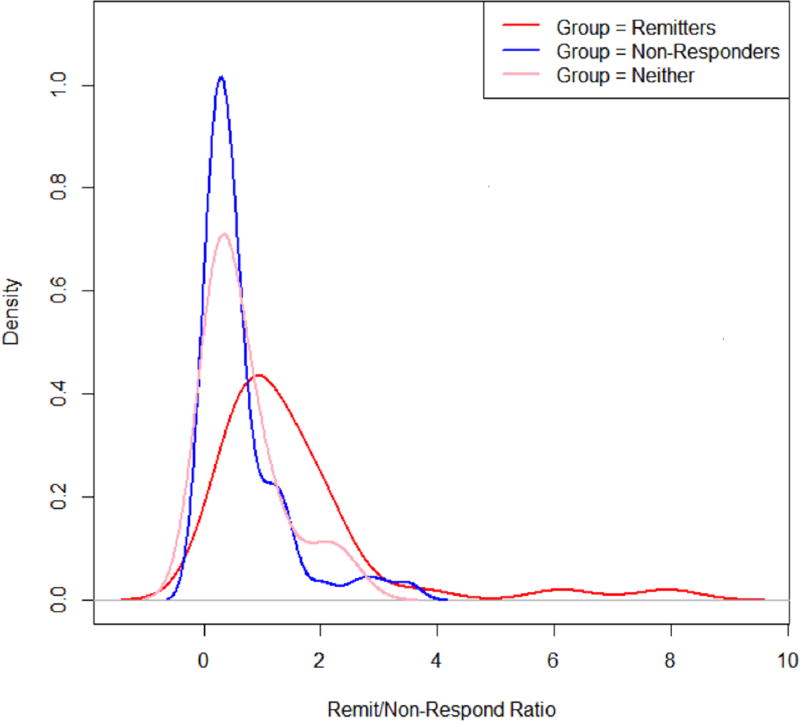
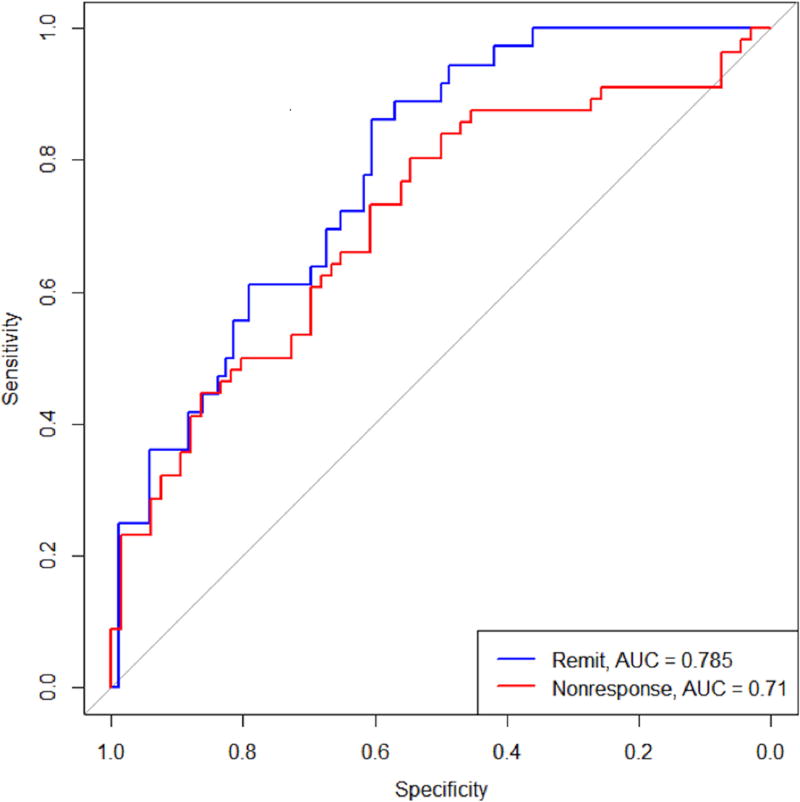
There was a strong amount of agreement between the two variable filtering approaches. The following variables increased the probability of remission: higher baseline BDNF levels, higher baseline IL-1B levels, lower baseline IDS-SR total score, and higher positive affect following initial exercise (PANAS). A similar process yielded the following variables that predicted high probability of non-response: higher cardiorespiratory fitness (VO2max), lower baseline IL-6, lower baseline BDNF levels, and lower positive affect following exercise (PANAS). An ROC analysis (Figure 1) was carried out on the average fitted probabilities, with an AUC of 0.785 for the remission model and 0.710 for the non-response model; this indicates a moderate fit to the data. Figure 2 shows the distribution of the ratios for the three groups of people. The distributions show a narrow range of ratios for the non-responders, suggesting they may be easier to identify using this metric. The remitters have the widest range of ratios but are skewed more towards the larger ratios, and the ‘neither’ group was somewhere in between.
Figure 1.
Estimated density plots for remission and non-response ratios
Figure 2.
ROC curves for remission and non-response
Actionable decisions can be made when PPV or NPV are large for a substantial portion of true remitters or substantial non-responders. Specifically, we considered this to be at least 70% PPV and NPV at sensitivities and specificities of at least 20%. Table 4 summarizes the optimal combination of cut-points as specified in equation (1). Using these cutpoints results in 70.6% of predicted remitters actually achieving remission and 77.8% of predicted non-responders failing to respond to treatment.
Table 4.
Efficacy of final models to predict remission and non-response.
| c | δ1 < a | δ1 < b | PPV | Sensitivity | NPV | Specificity |
|---|---|---|---|---|---|---|
| 0.5 | 0.2 | 2.9 | 0.706 | 0.333 | 0.778 | 0.250 |
c = minimum probability threshold needed to make a decision
δ1 = ratio of P(remission) to P(substantial nonresponse)
a = largest ratio value for which we would not call someone a substantial nonresponder
b = smallest ratio value for which we would not call someone a remitter
Discussion
The aim of this study was to determine whether we could identify clinical and biological characteristics that could collectively be used to predict treatment response to exercise for patients with non-remitted MDD. Using only 6 variables identified using data driven procedures, we were able to use the proposed methodology to obtain greater than 70% positive predictive and negative predictive values for more than 25% of the population in the study. These results suggest that there is substantial potential clinical utility, as one quarter of patients who would not respond to exercise could instead be offered an alternative treatment should these results be replicable.
The goal of this approach would be to ultimately create a clinical decision tool that would assist clinicians in choosing treatment for patients based on the presence of these characteristics. While individual predictors of treatment response to exercise (Schuch et al., 2016) have been identified, these individual characteristics do not have the predictive power to be of clinical utility. Our results suggest that by combining multiple patient characteristics into a decision model can provide the necessary level of certainty for the likely outcome.
Our findings also provide some insight into the potential mechanisms underlying the antidepressant effects of exercise. Inflammatory markers and BDNF have both been suggested as potential mechanisms, as reviewed by Medina et al. (Medina et al., 2015). Our previous analyses of the TREAD data found each were associated with treatment response (Rethorst et al., 2013b; Toups et al., 2011). In addition, a recent paper form the Regassa study (Hallgren et al., 2015) also found elevated baseline inflammation (IL-6) to predict better treatment response to exercise (Lavebratt et al., 2017). The fact that these markers remained among the most significant predictors of treatment outcomes when also considering several other baseline characteristics as potential predictors reinforces the role these biological factors in the antidepressant effects of exercise.
While previous research has similarly aimed to identify models to predict treatment outcomes to antidepressant medication and psychotherapy in MDD (Wallace et al., 2013), we are not aware of reports focusing on remission versus “total” lack of response. Given that other treatment options are be available in clinical settings (i.e., antidepressant medications, psychotherapy, etc), this approach would optimally be implemented in conjunction with tools that predict treatment response to these other treatments. The utility of a tool to aid in clinical decision-making for MDD is enhanced if the probability of treatment outcomes were available for multiple treatment options. For example, clinicians might tolerate a less optimal negative predictive value if a tool indicated the patient was likely to respond to another treatment.
This study has several limitations. This is a secondary analysis of the TREAD study, which was not designed to develop or evaluate a clinical decision model. Therefore, these results serve only to generate hypotheses that require replication. Ultimately, testing the clinical utility of such a clinical tool will require a priori testing. The patient sample in the TREAD study consisted of patients willing to participate in an exercise trial; as a result, our findings may not generalize to all patients with MDD. Similarly, all patients were enrolled in the trial following non-remission to adequate dose/duration of treatment with an SSRI. As a result, our results may not generalize to treatment-naive patients. From a statistical point of view, the small sample size and uniqueness of this clinical trial did not allow us to validate the methodology on an independent sample. Consequently, the results reported are likely overly optimistic. Further, the solution to the lasso is found via optimization and does not result in any tests of statistical significance. So, we cannot speak to the statistical significance of the predictors reported in this analysis – we can only say that they were selected by the lasso to have some predictive power.
Conclusion
Our results suggest it is possible to identify a subset of patients with MDD for which exercise is unlikely to be an effective treatment. Avoiding ineffective treatment options helps to properly target the treatment to patients who are more responsive, which should enhance outcomes and perhaps reduce costs compared to the current “trial and error” approach to treatment selection.
Acknowledgments
Funding source: This work was supported by the National Institute for Mental Health (1-R01-MH067692-01; PI: MH Trivedi) and in part by a National Alliance for Research on Schizophrenia and Depression (NARSAD) Independent Investigator Award (MHT), and Young Investigator Award (TLG). Chad D. Rethorst is supported by the National Institute Of Mental Health of the National Institutes of Health (K01MH097847). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures: Dr. Rush has received consulting fees from Brain Resource Ltd, Eli Lilly, Emmes Corp, Liva Nova, Medavante Inc., Mind Linc Inc., Montana State University, National Institute of Drug Abuse, Otsuka, Santium Inc., SingHealth/ Duke-NUS, Stanford University, Sunovion, Takeda-USA, Texas Tech, University of Colorado, University of Texas Southwestern Med Cntr.; royalties from Guilford Publications and the University of Texas Southwestern Medical Center.
Dr. Greer has received research funding from NARSAD and has received honoraria and consulting fees from H. Lundbeck A/S and Takeda Pharmaceuticals International, Inc.
Dr. Trivedi has received funding support from the Agency for Healthcare Research and Quality (AHRQ), Cyberonics Inc., National Alliance for Research in Schizophrenia and Depression, National Institute of Mental Health (NIMH), National Institute on Drug Abuse (NIDA), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Johnson & Johnson. He has received advisor/consultant fees from Abbott Laboratories Inc., Akzo (Organon Pharmaceuticals Inc.), Allergan Sales LLC, Alkermes, Arcadia Pharmaceuticals Inc., AstraZeneca, Axon Advisors, Brintellix, Bristol-Myers Squibb Company, Cephalon Inc., Cerecor, Eli Lilly & Company, Evotec, Fabre Kramer Pharmaceuticals Inc., Forest Pharmaceuticals, GlaxoSmithKline, Global Medical Education Inc., Health Research Associates, Johnson & Johnson, Lundbeck, MedAvante, Medscape, Medtronic, Merck, Mitsubishi Tanabe Pharma Development America Inc., MSI Methylation Sciences Inc., Nestle Health Science-PamLab Inc., Naurex, Neuronetics, One Carbon Therapeutics Ltd., Otsuka Pharmaceuticals, Pamlab, Parke-Davis Pharmaceuticals Inc., Pfizer Inc., PgxHealth, Phoenix Marketing Solutions, Rexahn Pharmaceuticals, Ridge Diagnostics, Roche Products Ltd., Sepracor, SHIRE Development, Sierra, SK Life and Science, Sunovion, Takeda, Tal Medical/Puretech Venture, Targacept, Transcept, VantagePoint, Vivus, and Wyeth-Ayerst Laboratories.
Footnotes
The Gini index represents the average gain of purity by splits of a given variable; larger values indicate more importance.
References
- Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom. Med. 2004;66:802–813. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- Breiman L. Random forests. Machine Learning. 2001;45:5–32. [Google Scholar]
- Burcusa SL, Iacono WG. Risk for recurrence in depression. Clin. Psychol. Rev. 2007;27:959–985. doi: 10.1016/j.cpr.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, Smit F. Excess mortality in depression: a meta-analysis of community studies. J. Affect. Disord. 2002;72:227–236. doi: 10.1016/s0165-0327(01)00413-x. [DOI] [PubMed] [Google Scholar]
- Friedman J, Hastie T, Tibshirani R. glmnet: Lasso and elastic-net regularized generalized linear models. R package version. 2009:1. [Google Scholar]
- Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey-Lisle PK. The economic burden of depression in the United States: how did it change between 1990 and 2000? J. Clin. Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- Greer TL, Trombello JM, Rethorst CD, Carmody TJ, Jha MK, Liao A, Grannemann BD, Chambliss HO, Church TS, Trivedi MH. Improvements in Psychosocial Functioning and Health-Related Quality of Life Following Exercise Augmentation in Patients with Treatment Response but Nonremitted Major Depressive Disorder: Results from the Tread Study. Depress. Anxiety. 2016;33:870–881. doi: 10.1002/da.22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren M, Kraepelien M, Ojehagen A, Lindefors N, Zeebari Z, Kaldo V, Forsell Y. Physical exercise and internet-based cognitive-behavioural therapy in the treatment of depression: randomised controlled trial. Br. J. Psychiatry. 2015;207:227–234. doi: 10.1192/bjp.bp.114.160101. [DOI] [PubMed] [Google Scholar]
- Judd LL, Schettler PJ, Solomon DA, Maser JD, Coryell W, Endicott J, Akiskal HS. Psychosocial disability and work role function compared across the long-term course of bipolar I bipolar II and unipolar major depressive disorders. J. Affect. Disord. 2008;108:49–58. doi: 10.1016/j.jad.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Koponen H, Jokelainen J, Keinanen-Kiukaanniemi S, Vanhala M. Depressive symptoms and 10-year risk for cardiovascular morbidity and mortality. World J. Biol. Psychiatry. 2010;11:834–839. doi: 10.3109/15622975.2010.486842. [DOI] [PubMed] [Google Scholar]
- Lavebratt C, Herring MP, Liu JJ, Wei YB, Bossoli D, Hallgren M, Forsell Y. Interleukin-6 and depressive symptom severity in response to physical exercise. Psychiatry Res. 2017;252:270–276. doi: 10.1016/j.psychres.2017.03.012. [DOI] [PubMed] [Google Scholar]
- Liaw A, Wiener M. Classification and regression by random Forest. R news. 2002;2:18–22. [Google Scholar]
- Medina JL, Jacquart J, Smits JA. Optimizing the Exercise Prescription for Depression: The Search for Biomarkers of Response. Curr Opin Psychol. 2015;4:43–47. doi: 10.1016/j.copsyc.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakostas GI, Fava M. Predictors, moderators, and mediators (correlates) of treatment outcome in major depressive disorder. Dialogues Clin. Neurosci. 2008;10:439–451. doi: 10.31887/DCNS.2008.10.4/gipapakostas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol. Med. 2010;40:1797–1810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethorst CD, Sunderajan P, Greer TL, Grannemann BD, Nakonezny PA, Carmody TJ, Trivedi MH. Does exercise improve self-reported sleep quality in non-remitted major depressive disorder? Psychol. Med. 2013a;43:699–709. doi: 10.1017/S0033291712001675. [DOI] [PubMed] [Google Scholar]
- Rethorst CD, Toups MS, Greer TL, Nakonezny PA, Carmody TJ, Grannemann BD, Huebinger RM, Barber RC, Trivedi MH. Pro-inflammatory cytokines as predictors of antidepressant effects of exercise in major depressive disorder. Mol. Psychiatry. 2013b;18:1119–1124. doi: 10.1038/mp.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethorst CD, Wipfli BM, Landers DM. The antidepressive effects of exercise: a meta-analysis of randomized trials. Sports Med. 2009;39:491–511. doi: 10.2165/00007256-200939060-00004. [DOI] [PubMed] [Google Scholar]
- Saveanu R, Etkin A, Duchemin AM, Goldstein-Piekarski A, Gyurak A, Debattista C, Schatzberg AF, Sood S, Day CV, Palmer DM, Rekshan WR, Gordon E, Rush AJ, Williams LM. The international Study to Predict Optimized Treatment in Depression (iSPOT-D): outcomes from the acute phase of antidepressant treatment. J. Psychiatr. Res. 2015;61:1–12. doi: 10.1016/j.jpsychires.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Schuch FB, Dunn AL, Kanitz AC, Delevatti RS, Fleck MP. Moderators of response in exercise treatment for depression: A systematic review. J. Affect. Disord. 2016;195:40–49. doi: 10.1016/j.jad.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Sotsky SM, Glass DR, Shea MT, Pilkonis PA, Collins JF, Elkin I, Watkins JT, Imber SD, Leber WR, Moyer J, et al. Patient predictors of response to psychotherapy and pharmacotherapy: findings in the NIMH Treatment of Depression Collaborative Research Program. Am. J. Psychiatry. 1991;148:997–1008. doi: 10.1176/ajp.148.8.997. [DOI] [PubMed] [Google Scholar]
- Suterwala AM, Rethorst CD, Carmody TJ, Greer TL, Grannemann BD, Jha M, Trivedi MH. Affect Following First Exercise Session as a Predictor of Treatment Response in Depression. J. Clin. Psychiatry. 2016;77:1036–1042. doi: 10.4088/JCP.15m10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase ME, Haight BR, Richard N, Rockett CB, Mitton M, Modell JG, VanMeter S, Harriett AE, Wang Y. Remission rates following antidepressant therapy with bupropion or selective serotonin reuptake inhibitors: a meta-analysis of original data from 7 randomized controlled trials. J. Clin. Psychiatry. 2005;66:974–981. doi: 10.4088/jcp.v66n0803. [DOI] [PubMed] [Google Scholar]
- Tibshirani R. Regression shrinkage and selection via the Lasso. Journal of the Royal Statistical Society Series B-Methodological. 1996;58:267–288. [Google Scholar]
- Toups MS, Greer TL, Kurian BT, Grannemann BD, Carmody TJ, Huebinger R, Rethorst C, Trivedi MH. Effects of serum Brain Derived Neurotrophic Factor on exercise augmentation treatment of depression. J. Psychiatr. Res. 2011;45:1301–1306. doi: 10.1016/j.jpsychires.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Greer TL, Church TS, Carmody TJ, Grannemann BD, Galper DI, Dunn AL, Earnest CP, Sunderajan P, Henley SS, Blair SN. Exercise as an augmentation treatment for nonremitted major depressive disorder: a randomized, parallel dose comparison. J. Clin. Psychiatry. 2011;72:677–684. doi: 10.4088/JCP.10m06743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Greer TL, Grannemann BD, Church TS, Galper DI, Sunderajan P, Wisniewski SR, Chambliss HO, Jordan AN, Finley C, Carmody TI. TREAD: TReatment with Exercise Augmentation for Depression: study rationale and design. Clin. Trials. 2006a;3:291–305. doi: 10.1191/1740774506cn151oa. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am. J. Psychiatry. 2006b;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Wallace ML, Frank E, Kraemer HC. A novel approach for developing and interpreting treatment moderator profiles in randomized clinical trials. JAMA Psychiatry. 2013;70:1241–1247. doi: 10.1001/jamapsychiatry.2013.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden D, Trivedi MH, Wisniewski SR, Davis L, Nierenberg AA, Gaynes BN, Zisook S, Hollon SD, Balasubramani GK, Howland R, Fava M, Stewart JW, Rush AJ. Predictors of attrition during initial (citalopram) treatment for depression: a STAR*D report. Am. J. Psychiatry. 2007;164:1189–1197. doi: 10.1176/appi.ajp.2007.06071225. [DOI] [PubMed] [Google Scholar]
- Wells KB, Stewart A, Hays RD, Burnam MA, Rogers W, Daniels M, Berry S, Greenfield S, Ware J. The functioning and well-being of depressed patients. Results from the Medical Outcomes Study. JAMA. 1989;262:914–919. [PubMed] [Google Scholar]