Abstract
Objective
To determine the association of smoking and HIV status with tissue specific inflammation measured by 18flurodeoxyglucose positron emission tomography (FDG-PET).
Design
Cross-sectional
Methods
We prospectively enrolled 55 HIV+ subjects on stable antiretroviral therapy and 19 age-matched HIV-uninfected controls without known CVD. We measured aortic target-to-background ratio (TBR) and spleen standardized uptake values (SUV) three hours post-FDG, and used regression models to examine the independent association of HIV and smoking status with PET variables.
Results
Overall, median (IQR) age was 50 (42–55) years; 81% were male and 54% were current smokers [median 0.5 packs per day, 25 pack-years]. Median CD4+ of HIV+ subjects was 690 cells/ml and 88% had HIV-1 RNA <20c/ml; 43% were on a protease inhibitor. In fully-adjusted models, HIV was associated with 0.16 (95%CI 0.04–0.27; p=0.009) higher aortic TBR while current smoking was marginally associated with a lower TBR [−0.11 (95%CI −0.23 to 0.01); p=0.07]. Spleen SUVmean was not associated with HIV or smoking, and there was no evidence for an HIV*smoking interaction for aortic or spleen models (all p>0.1). Spleen SUVmean was positively associated with biomarkers of inflammation and coronary artery calcium, but adjustment for traditional CVD risk factors attenuated these relationships.
Conclusion
This FDG-PET study of HIV+ subjects suggests that HIV is associated with increased aortic inflammation independent of traditional risk factors, but smoking is not. Future studies should continue to explore the mechanistic roles of smoking and inflammation at various stages of clinical and subclinical atherosclerotic vascular disease in HIV.
Introduction
18Flurodeoxyglucose positron emission tomography (FDG-PET) imaging is used to identify metabolically active tissues such as malignancies, but uptake in other tissues may also reflect high levels of inflammatory cell infiltration. Uptake in the arterial wall, for example, reflects vascular inflammation[1], while increased metabolic activity in the spleen may reflect systemic immune activation[2]. Both measures have been associated with risk of cardiovascular disease (CVD) events in the general population[2, 3].
Increased arterial inflammation may be an early manifestation of vascular disease in HIV. HIV+ subjects appear to have higher aortic FDG uptake in two studies from the same research group[4, 5], but another group failed to detect a difference between HIV+ and controls[6]. Additionally, lymphatic tissue activity strongly correlates with measures of HIV disease, but not with aortic uptake[4]. These two PET measures may reflect different pathways of inflammation and immune activation[4].
We, therefore, sought to confirm the HIV associations and further explore the potential confounding effect of smoking status on aortic and spleen FDG uptake. Our secondary objectives were (a) to determine whether the smoking effect was modified by HIV status and (b) to explore relationships between PET measures, biomarkers of inflammation and immune activation, and coronary artery disease.
Methods
We prospectively enrolled HIV+ subjects and age-matched HIV-negative controls in a 3:1 fashion to undergo a dedicated FDG-PET/CT protocol designed to optimize large vessel vascular imaging. All subjects were >18 years old and free of known CVD. HIV+ subjects were on stable antiretroviral therapy for 12 weeks with at least 6 months of HIV-1 RNA ≤400 c/mL. Serum glucose was confirmed by glucometer and if >200mg/dL, the study was rescheduled. The protocol was approved by the University Hospitals IRB and all subjects signed written informed consent.
After a minimum fast of 4 hours, 18F-FDG was injected and subjects then rested quietly in a chair without talking to reduce muscle and jaw uptake. Approximately 3 hours +/− 10 minutes post-FDG, images were acquired from the orbits through the lower abdomen on a Philips Gemini TF Big Bore PET/CT scanner (Philips Healthcare; Andover, MA, USA). Images were analyzed offline on a dedicated workstation using MIM v. 6.6.6 by two readers blinded to clinical variables.
Standard uptake dose values (SUV) were defined per standard convention as the decay-corrected tissue concentration of 18F-FDG (in kBq/g) divided by the injected dose per body weight (in kBq/g). “Mean-mean” aortic target-to-background ratio was defined as the average aortic SUVmean measured on consecutive axial slices through the ascending aorta from the sinus of Valsalva to the aortic arch divided by the average of superior vena cava blood pool SUVmean measured from the same axial slices [Mean aortic SUVmean ÷ mean superior vena cava SUVmean = mean-mean aortic TBR]. Aortic SUVmax was used in place of aortic SUVmean to generate the “mean-max” TBR [Mean aortic SUVmax ÷ mean superior vena cava SUVmean = mean-max aortic TBR]. Both mean-mean[7] and mean-max[5] measures are widely used in prior literature; although, most HIV studies have used mean-max[4–6, 8, 9]. We chose mean-mean as our primary dependent variable of interest because it was normally distributed and did not require log-transformation, making interpretation of the effect sizes more intuitive. As a sensitivity analysis, we repeated our models using log-transformed mean-max TBR.
For spleen, we traced the borders of the entire spleen using a three-dimension region of interest tool to determine SUVmean. Inter-reader variability was determined using ten randomly selected cases and was excellent for both mean-mean aortic TBR [intraclass correlation coefficient (95% CI) = 0.979 (0.921–0.995)], mean-max aortic TBR [0.994 (0.997–0.998)], and spleen SUVmean [0.995 (0.981–0.999)].
In addition to the PET/CT scan, all subjects had cardiac CT for coronary calcium (CAC) scoring as previously described[10]. Demographics and medical history were obtained by self-report and medical record review. Smoking status was categorized as current, past, and never. Biomarkers of inflammation and immune activation measured from frozen plasma stored at −80° C. Interleukin-6, soluble tumor necrosis factor-α receptor II (TNF-RII), soluble vascular cell adhesion molecule-1, oxidized low density lipoprotein cholesterol, and two soluble markers of monocyte activation (sCD14 and sCD163) were measured by ELISA (R&D Systems; Minneapolis, MN, USA). High sensitivity C-reactive protein (hsCRP) was measured by particle enhanced immunonephelometric assay on a BNII nephelometer (Siemens; Munich, Germany).
We first compared baseline characteristics using t-tests, Wilcoxon rank-sum tests, or Fisher’s exact tests as appropriate. All non-normally distributed variables were log-transformed prior to analyses. We then used hierarchical linear regression to examine the association of HIV and smoking status with PET variables. The two primary dependent variables of interest were (a) mean-mean aortic TBR and (b) spleen SUV. Initial models included HIV and smoking status only. We subsequently adjusted these models for age, gender, and race. A fully adjusted model added hypertension, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, body-mass index, homeostatic model of insulin resistance[11], and statin use. Smoking effect modification by HIV status was assessed by adding an HIV*smoking interaction term to the models. Models were repeated using log-transformed mean-max aortic TBR. Exploratory analyses of the association between biomarkers of inflammation, CAC, and PET variables were conducted using a similar modeling approach. For CAC analyses, CAC>0 was the dependent variable and spleen SUVmean and mean-mean aortic TBR were the primary explanatory variables in logistic regression models, with multivariable adjustment as above. All analyses were performed using STATA 14.2 (StataCorp; College Station, TX, USA).
Results
The characteristics of the 55 HIV+ subjects and 19 HIV-negative controls are shown in Table 1. Overall, median (IQR) age was 50 (42–55) years; 81% were male and 54% were current smokers. The groups were generally well-matched (all p>0.2) except HIV+ were more likely to be African American (p=0.05) and to have hypertension (p=0.003).
Table 1. Baseline characteristics of study subjects by HIV status.
Results are displayed as median (IQR) or percentage. HOMA-IR, homeostatic model of insulin resistance; GFR, glomerular filtration rate; LDL, low-density lipoprotein cholesterol concentration; HDL, high-density lipoprotein cholesterol concentration.
| HIV+ (n=55) | HIV-negative (n=19) | |
|---|---|---|
| Age (years) | 49 (43–55) | 50 (34–56) |
| Male gender | 84% | 74% |
| African American | 73% | 47% |
| Current smoker | 55% | 53% |
| Packs per day | 0.5 (0.2–1) | 1 (0.5–1) |
| Body mass index (kg/m2) | 26 (23–31) | 27 (26–30) |
| Diabetes | 1.8% | 0% |
| HOMA-IR | 3.1 (1.6–4.8) | 2.1 (1.8–3.8) |
| Hypertension | 56% | 16% |
| Estimated GFR (mL/min/1.73m2) | 88 (76–102) | 84 (73–107) |
| LDL cholesterol (mg/dL) | 87 (70–114) | 88 (76–116) |
| HDL cholesterol (mg/dL) | 47 (37–60) | 45 (35–58) |
| Statin use | 11% | 5% |
| Coronary artery calcium >0 | 43% | 39% |
| CD4+ T-cell count (cells/μl) | 690 (443–818) | N/A |
| Nadir CD4+ T-cells (cells/μl) | 189 (46–303) | N/A |
| HIV-1 RNA <20 copies/ml | 88% | N/A |
| Current protease inhibitor | 43% | N/A |
| Current abacavir | 7% | N/A |
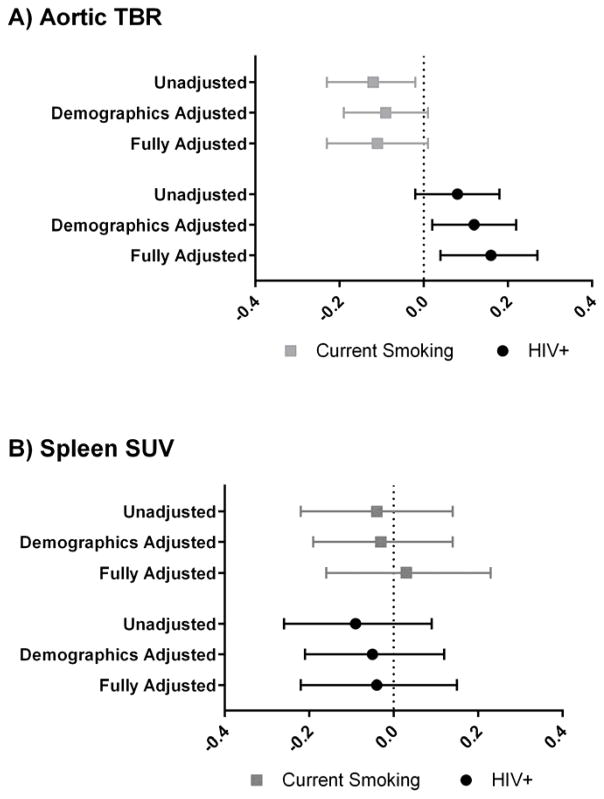
Figure 1a compares the effects of HIV and smoking status on mean-mean aortic TBR in unadjusted, demographic adjusted, and fully adjusted models. Overall median (IQR) mean-mean aortic TBR was 1.50 (1.39–1.61) and normally distributed. After adjustment for age, sex, race, and traditional cardiovascular risk factors, HIV was associated with 0.16 (95%CI 0.04–0.27; p=0.009) higher aortic TBR while current smoking was marginally associated with a lower TBR [−0.11 (95%CI −0.23 to 0.01); p=0.07]. Overall mean-max aortic TBR was 2.72 (2.45–3.23), but was skewed toward the high end and therefore log-transformed for subsequent analyses. For HIV, results of adjusted and unadjusted models were similar as for mean-mean aortic TBR (fully-adjusted β=0.12, p=0.043); but current smoking was not associated with mean-max aortic TBR in unadjusted or adjusted models (all p>0.30). Neither HIV status nor current smoking status was associated with spleen SUVmean in any of the models (all p>0.34; Figure 1b). There was no evidence of an HIV*smoking interaction in any of the models (all p for interaction > 0.14).
Figure 1. Association of HIV and smoking status with (A) aortic TBR and (B) spleen SUV.
Symbols represent parameter estimates and error bars represent 95% confidence intervals. Unadjusted models include smoking and HIV status only. Demographics adjusted models include age, gender, and race. Fully adjusted models include demographics, hypertension, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, body-mass index, and homeostatic model assessment of insulin resistance. TBR, target-to-background ratio; SUV, standardized uptake value.
Log-transformed TNF-RII was positively but only borderline statistically significantly associated with spleen SUVmean in fully adjusted models (p=0.078); however, it was not with aortic TBR (p=0.27). Log-transformed hsCRP was also positively associated with spleen SUVmean in unadjusted models (p=0.006), but adjustment for demographics (p=0.21) and further adjustment for traditional risk factors (p=0.33) significantly attenuated the relationship. None of the other biomarkers were associated with aortic TBR or spleen SUV in unadjusted or adjusted models (all p>0.10).
Spleen SUVmean was associated with higher odds of detectable CAC in a univariate logistic regression model [OR per 1 SD of spleen SUV 1.70 (95%CI 1.0–2.9); p=0.047]. In fully adjusted models, the adjusted OR was similar, but the association was no longer statistically significant [OR 1.80 (95%CI 0.8–3.9); p=0.14]. Aortic TBR was not associated with CAC>0 in univariate or multivariate models (all p>0.4).
Discussion
In one of the largest prospective vascular PET studies of treated HIV+ to date, we confirm that HIV is associated with increased aortic 18FDG uptake. Furthermore, smoking was not clearly associated with 18FDG uptake in aorta or spleen. Biomarkers of inflammation were weakly associated with spleen uptake, which was in turn associated with coronary artery calcification.
Our findings contribute to a growing literature on subclinical aortic inflammation measured by 18FDG PET. The field is complicated in part by different populations (on ART vs. treatment naïve vs. elite controllers) and different imaging protocols. For example, Subramanian et al. used different methods for HIV+ (prospective protocol performed two hours post-FDG) and HIV-negative subjects (post hoc analysis of clinical scans performed one hour post-FDG)[5]. The HIV effect was substantially smaller in a subsequent prospective study that imaged all subjects two hours post FDG[4]. Similar to Knudsen et al[6], we chose a longer three hour protocol to maximize venous blood pool washout. In contrast to Knudsen et al, however, we did find an association between HIV status and aortic FDG update. Additionally, prior studies in HIV have used mean-max aortic TBR[4–6, 8, 9], but some of the earlier protocols reported in the general population used mean-mean aortic TBR[7]. We report both measures and show that results may vary somewhat depending on the method used.
The bulk of these data currently suggest that HIV is associated with higher aortic FDG uptake, presumably representing inflammatory cell infiltration. This vascular inflammation has been associated with features of high-risk coronary plaque[8], but was not associated with CAC in our study. Statin therapy substantially improved high-risk plaque features in a clinical trial; however, there was no effect on aortic 18FDG uptake[12]. Similarly, ART initiation reduced 18FDG uptake in lymphoid tissue but not in the aorta[9]. Early pilot evidence suggests that interventions targeting specific inflammatory pathways—such as IL-1β blockade[13]—may reduce aortic inflammation in subjects with HIV, but it is not clear if the vascular benefit will outweigh the risks of treatment.
Smoking is strongly associated with CVD events[14], mortality[15], and subclinical carotid disease[16] in treated HIV infection—an association that appears to be stronger in HIV than in the HIV-uninfected population[15, 16]. However, smoking was not associated with aortic TBR in a prior study[5]. Similarly, we found no evidence of an independent association of smoking with aortic TBR. Together, these studies suggest that vascular inflammation does not explain the potent association of smoking with CVD risk in HIV. Alternatively, smoking may promote vascular inflammation very early in the disease process which is not captured in studies of longer-term smokers.
Studies have differed on whether systemic inflammation, as measured by various circulating soluble and cellular markers, relates to aortic FDG uptake. Recent systematic reviews suggest that these biomarkers are strongly related to CVD events[17], but are less consistently related to markers of subclinical vascular disease including aortic TBR[18]. For example, soluble CD163, a marker of monocyte activation, positively correlated with aortic TBR in one study[5], but was not correlated in other studies including ours[4, 6]. Inflammation within secondary lymphoid tissue may provide additional information on CVD risk in HIV. In our study, spleen SUVmean was modestly associated with some markers of inflammation and CAC. Future studies should explore these intriguing associations between lymphoid tissue inflammation and CVD.
Our study is one of the largest vascular PET/CT studies to date, which allowed us to explore associations with risk factors while accounting for important confounders; however, we cannot rule out that our findings may be explained by residual or unknown confounders. Furthermore, although ours is the first to include HIV+ and control women, the numbers were small and results may not be generalizable to this group.
In conclusion, our study suggests that HIV is associated with increased aortic inflammation independent of traditional risk factors, but smoking is not. Future studies should continue to explore the mechanistic roles of smoking and inflammation at various stages of clinical and subclinical atherosclerotic vascular disease in HIV.
Acknowledgments
Funding: This work was supported by the National Institutes of Health (R56 HL126526 and R56 HL126539 to GAM, K23 HL123341 to CTL and K23 HL116209 to COH). Additional support was provided by the Center for AIDS Research, Case Western Reserve University (P30 AI36219) and the Clinical and Translational Science Collaborative of Cleveland (UL1TR000439) from the National Center for Advancing Translational Sciences component of the NIH and NIH roadmap for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest:
CTL has received research grants from the Medtronic Global Health Foundation and the Wolf Family Foundation; and has served as a scientific advisor and speaker for Gilead Sciences. GAM has served as a scientific advisor for Gilead Sciences and Merck; has received research grants from Bristol-Myers Squibb, GlaxoSmithKline, and Gilead Sciences. COH has served as a scientific advisor for Gilead Sciences. CES, JM, DAZ, RG, and JO have no disclosures.
References
- 1.Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48(9):1818–1824. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 2.Emami H, Singh P, MacNabb M, Vucic E, Lavender Z, Rudd JH, et al. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC Cardiovasc Imaging. 2015;8(2):121–130. doi: 10.1016/j.jcmg.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging. 2013;6(12):1250–1259. doi: 10.1016/j.jcmg.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Tawakol A, Ishai A, Li D, Takx RA, Hur S, Kaiser Y, et al. Association of Arterial and Lymph Node Inflammation With Distinct Inflammatory Pathways in Human Immunodeficiency Virus Infection. JAMA Cardiol. 2017;2(2):163–171. doi: 10.1001/jamacardio.2016.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. Jama. 2012;308(4):379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knudsen A, Hag AM, Loft A, von Benzon E, Keller SH, Moller HJ, et al. HIV infection and arterial inflammation assessed by (18)F-fluorodeoxyglucose (FDG) positron emission tomography (PET): a prospective cross-sectional study. J Nucl Cardiol. 2015;22(2):372–380. doi: 10.1007/s12350-014-0032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudd JH, Myers KS, Bansilal S, Machac J, Rafique A, Farkouh M, et al. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50(9):892–896. doi: 10.1016/j.jacc.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 8.Tawakol A, Lo J, Zanni MV, Marmarelis E, Ihenachor EJ, MacNabb M, et al. Increased arterial inflammation relates to high-risk coronary plaque morphology in HIV-infected patients. J Acquir Immune Defic Syndr. 2014;66(2):164–171. doi: 10.1097/QAI.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanni MV, Toribio M, Robbins GK, Burdo TH, Lu MT, Ishai AE, et al. Effects of Antiretroviral Therapy on Immune Function and Arterial Inflammation in Treatment-Naive Patients With Human Immunodeficiency Virus Infection. JAMA Cardiol. 2016;1(4):474–480. doi: 10.1001/jamacardio.2016.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longenecker CT, Sattar A, Gilkeson R, McComsey GA. Rosuvastatin slows progression of subclinical atherosclerosis in patients with treated HIV infection. Aids. 2016;30(14):2195–2203. doi: 10.1097/QAD.0000000000001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 12.Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. The Lancet HIV. 2015;2(2):e52–e63. doi: 10.1016/S2352-3018(14)00032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsue P, Deeks SG, Ishai AE, Hur S, Li D, Sterman F, et al. IL-1β Inhibition Significantly Reduces Atherosclerotic Inflammation in Treated HIV. Conference on Retroviruses and Opportunistic Infections; Seattle, WA, USA. 2017. [Google Scholar]
- 14.Rasmussen LD, Helleberg M, May MT, Afzal S, Kronborg G, Larsen CS, et al. Myocardial infarction among Danish HIV-infected individuals: population-attributable fractions associated with smoking. Clin Infect Dis. 2015;60(9):1415–1423. doi: 10.1093/cid/civ013. [DOI] [PubMed] [Google Scholar]
- 15.Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, et al. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis. 2013;56(5):727–734. doi: 10.1093/cid/cis933. [DOI] [PubMed] [Google Scholar]
- 16.Fitch KV, Looby SE, Rope A, Eneh P, Hemphill L, Lee H, et al. Effects of aging and smoking on carotid intima-media thickness in HIV-infection. Aids. 2013;27(1):49–57. doi: 10.1097/QAD.0b013e328358b29c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vos AG, Idris NS, Barth RE, Klipstein-Grobusch K, Grobbee DE. Pro-Inflammatory Markers in Relation to Cardiovascular Disease in HIV Infection. A Systematic Review. PLoS One. 2016;11(1):e0147484. doi: 10.1371/journal.pone.0147484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vos AG, Hulzebosch A, Grobbee DE, Barth RE, Klipstein-Grobusch K. Association between Immune Markers and Surrogate Markers of Cardiovascular Disease in HIV Positive Patients: A Systematic Review. PLoS One. 2017;12(1):e0169986. doi: 10.1371/journal.pone.0169986. [DOI] [PMC free article] [PubMed] [Google Scholar]