SUMMARY
Tumors undergo nutrient stress and need to reprogram their metabolism to survive. The stroma may play a critical role in this process by providing nutrients to support the epithelial compartment of the tumor. Here we show that p62 deficiency in stromal fibroblasts promotes resistance to glutamine deprivation by the direct control of ATF4 stability through its p62-mediated polyubiquitination. ATF4 upregulation by p62 deficiency in the stroma activates glucose carbons flux through a pyruvate carboxylase-asparagine synthase cascade that results in asparagine generation as a source of nitrogen for stroma and tumor epithelial proliferation. Thus, p62 directly targets nuclear transcription factors to control metabolic reprogramming in the microenvironment and repress tumorigenesis, and identifies ATF4 as a synthetic vulnerability in p62-deficient tumor stroma.
eTOC
Tumors suffer nutrient stress and need to reprogram their metabolism to survive. Linares et al. elucidate a key role for p62 in rewiring the metabolism of tumor stromal fibroblasts to sustain growth of both stromal and epithelial tumor cells, thus making p62 a novel cancer vulnerability point.

INTRODUCTION
The extreme genetic complexity of tumors makes the targeting of individual signaling molecules an inefficient therapeutic strategy in most cases. However, collateral dependencies or synthetic vulnerabilities in the form of responses to the different stresses, including metabolic, caused by the mutated tumor genome or its microenvironment, can provide a rich source of therapeutic targets in cancer, irrespective of the mutational landscape of the tumor. A critical aspect of tumor growth is the requirement for cancer cells to adapt their metabolism to a highly proliferative phenotype, often under nutrient-scarce conditions (Boroughs and DeBerardinis, 2015; Kenific and Debnath, 2015). Our understanding of how tumors respond to these nutritionally challenging situations is becoming progressively apparent. Although this phenomenon has been investigated with certain detail in epithelial cancer cells, how the stroma, specifically cancer associated fibroblasts (CAFs), rewire their metabolism to cope with nutrient stress, promote their own survival, and drive tumor cell proliferation, is a highly significant but clearly understudied biological question with implications for cancer therapy.
It is well accepted now that the inappropriate activation of tumor stromal fibroblasts can embolden epithelial cancer cells to progress to more malignant stages (Barron and Rowley, 2012). Also, the mechanisms by which normal fibroblasts (NAFs) acquire the characteristics of CAFs are being progressively unveiled (Gascard and Tlsty, 2016). However, how the metabolic reprograming of CAFs influence the metabolism of the tumor epithelium still remains largely unknown. Germane to these fundamental questions is the ability of cells to survive situations of glutamine (Gln) deprivation, since Gln is the most commonly depleted amino acid in solid tumors, and serves as the obligate nitrogen donor for numerous biosynthetic pathways (Ahn and Metallo, 2015).
We have recently demonstrated that the autophagy substrate and signaling adaptor p62 (encoded by the SQSTM1 gene), is downregulated in prostate and liver cancer stroma, which decisively contributes to the NAF-to-CAF transition, creating a tumor microenvironment conducive to aggressive tumor progression (Duran et al., 2016; Valencia et al., 2014). However, whether p62 controls the ability of CAFs to reprogram their metabolism to survive nutrient challenging conditions, and to support the survival and growth of epithelial cancer cells, especially upon Gln deprivation, is a fundamental question in cancer biology that has not been addressed yet. This is very important because the identification of metabolic and signaling pathways unleashed by p62 deficiency in the stroma, under conditions of nutrient deprivation, may lead to the discovery of synthetic vulnerabilities created in CAFs that can be instrumental for better cancer therapies. Since it is accepted that the stroma is more genetically stable than the cancer epithelial cells, targeting these collateral addictions in CAFs might offer therapies less prone to resistance. Here we have addressed the role that p62 plays in the rewiring of the CAF metabolic pathways to confer resistance to Gln deprivation, and how that reprogramming promotes the growth of the tumor epithelium.
RESULTS
p62 deficiency induces resistance to Gln deprivation
To test whether p62 might play a role in allowing stromal cells to endure nutrient stress and more specifically Gln deprivation, we generated human prostate stromal fibroblasts (WPMY-1) in which p62 was inactivated either by CRISPR-mediated gene editing (sgp62), or by shRNA-mediated knockdown (shp62). When these p62-deficient cells, and their corresponding p62-proficient controls (sgC and shNT), were incubated in the absence of Gln, p62-deficient cells proliferated while p62-proficient controls did not (Figures 1A and 1B). Similar results were obtained in experiments using prostate stromal fibroblasts from p62 KO mice as compared with their wildtype (WT) controls (Figure 1C). This resistance of p62-deficient stromal fibroblasts to nutrient stress is selective for Gln and not due to a hypothetical general reduction in apoptosis, since p62-proficient and deficient cells underwent cell death equally when incubated in medium that is free of glucose (Figures 1D), or when treated with TNF plus cycloheximide (a bona fide inducer of cell death) (Figure 1E). However, Gln starvation-induced cell death in control stromal cells was rescued by p62 deficiency (Figures S1A and S1B). Interestingly, the resistance to Gln deprivation conferred by p62 loss was specific to stromal cells since p62-deficient PC3 and DU145 epithelial PCa cells, like their p62-proficient controls, did not survive Gln deprivation (Figures 1F and 1G).
Figure 1. Stromal p62-deficiency confers selective resistance to Gln deprivation.
(A–C) Growth curves of CRISPR/Cas9-mediated WPMY-1 p62-Knockout (sgp62) and control (sgC) cells (A), WPMY-1 shNT and shp62 cells (B) and mouse stroma WT and p62KO cells (C) under Gln-depleted conditions (n = 3, biological replicates). (D and E) Four-day growth of WPMY-1 sgC and sgp62 cells in absence of glucose (D) or in the presence of CHX and TNFα (E) (n = 3, biological replicates). (F and G) Growth curves of shNT and shp62 cells PC3 (F) or DU145 (G) in absence of Gln (n = 3, biological replicates). Error bars, S.E.M., two-way ANOVA test (A, B, C, F, G), two-tailed t-test (D, E). **p < 0.01, ***p < 0.001, ****p < 0.0001. See also Figure S1.
We next investigated the metabolic mechanisms whereby p62 deficiency conferred resistance to Gln-deprivation in stromal fibroblasts. Since our previously published data demonstrated that p62 deficiency results in reduced c-Myc expression in stromal cells as consequence of mTORC1 inhibition (Valencia et al., 2014), we next determined whether c-Myc or mTORC1 inactivation would mimic the ability of p62-deficient cells to survive in Gln-free medium. mTORC1 inhibition by two well characterized mTOR inhibitors (INK128 and Torin) or knockdown of c-Myc did not promote the survival of p62-proficent cells in the absence of Gln (Figures S1C–S1E). These results suggest that p62 utilizes unexplored c-Myc-mTORC1-independent pathways to promote Gln-free survival of stromal cells.
Activation of macropinocytosis and/or autophagy has been proposed as mechanisms engaged by cells to endure nutrient stress (Kenific and Debnath, 2015). To address these possibilities, WPMY-1 cells were treated with a widely accepted inhibitor of macropinocytosis (EIPA) or with bafilomycin A1 (BafA1), which inhibits the last step in the autophagy cascade. Of note, although both treatments impaired stromal cell proliferation, there were not specific differences between both genotypes (Figures S1F and S1G). To further test the potential role of autophagy in this process, the autophagic flux was determined by different experimental approaches. Autophagosomes were visualized by the appearance of green fluorescent protein (GFP)-labelled autophagosomes in WPMY-1 cells transfected with GFP-tagged LC3 upon treatment with chloroquine. No differences in number of GFP-LC3-positive puncta (Figures S1H and S1I) or in the levels of the lipidated form of LC3, LC3-II in basal conditions or in the presence of Bafilomycin A1, an inhibitor of autophagosomal and lysosomal fusion existed between both genotypes in Gln-deprived conditions (Figure S1J). Similar results were obtained when the autophagic flux was measured using the reporter GFP-mCherry-LC3 (Linares et al., 2015), which allows the identification of autolysosomes (mCherry-positive/GFP-negative: red dots) and autophagosomes (mCherry-positive/GFP-positive; yellow dots). Consistently, no changes were observed in the total number of autophagosomes and autolysosomes in p62-deficient cells as compared to normal controls in Gln-deprived conditions (Figures S1K and S1L). Likewise, macropinocytosis was equally activated in both types of WPMY-1 cells (Figures S1M and S1N) Therefore, neither autophagy nor macropinocytosis account for the ability of p62-deficient stromal fibroblasts to withstand Gln deprivation.
p62 loss reprograms stromal cell metabolism to endure nutrient stress
We next investigated the metabolic changes that might support Gln independence upon stromal p62 deficiency. First, we noted that glucose consumption by sgp62 was higher than by sgC WPMY-1 cells when both cell genotypes were cultured under Gln-deprived conditions (Figure 2A). Furthermore, the analysis of metabolite abundance in sgp62 and sgC WPMY-1 cells cultured in Gln-free medium revealed that, although p62-deficient cells exhibited a slight decrease in the levels of intracellular lactate, they displayed dramatic changes in several TCA-associated metabolites (Figure 2B). Specifically, citrate, fumarate, and malate were all elevated, whereas succinate and αKG, which is derived predominantly from Gln carbon under normal culture conditions (Vacanti et al., 2014), were similar in abundance (Figure 2B). Significant increases in several non-essential amino acids were also noted in p62-deficient prostate stromal cells, with both glutamate and aspartate/asparagine (Asp/Asn) increased significantly (Figure 2B). Alanine, on the other hand, was not significantly elevated in sgp62 cells (Figure 2B). Interestingly, the increment of glucose consumption (Figure 2A) and the significant increases in TCA-associated metabolites (Figure 2B) conferred by p62 loss were specific to stromal cells since no differences were observed upon p62 knock-down in PCa epithelial cells in Gln-deprived conditions (Figures S2A and S2B). Since most TCA intermediates and associated amino acids were elevated in p62-deficient stromal fibroblasts, these results suggest that loss of p62 influenced the glucose flux into TCA metabolism. To gain more detailed insights into the metabolic reprogramming caused by p62 deficiency, we cultured cells with [U-13C6]glucose and quantified isotope incorporation in mitochondrial-derived metabolites over time (Figure 2C). Consistent with the targeted metabolomics data above, we observed significant increases in flux from glucose into TCA intermediates (citrate, fumarate, malate) and downstream amino acids (Glu, Asp/Asn) in sgp62 cells under Gln-deprived conditions (Figure 2C). These data indicate that p62-deficient stromal cells reprogram their metabolism to maintain flux through the TCA cycle and production of non-essential amino acids critical for proliferation in the absence of Gln.
Figure 2. Stromal metabolic reprogramming by p62 deficiency in Gln-deprived conditions.
(A) Glucose consumption was determined by spent medium analysis (YSI2950 analyzer) from WPMY-1 sgp62 and sgC cells (n = 2, biological replicates) under Gln-deprived conditions. Results are normalized to total protein content. (B) Intracellular metabolite abundances in WPMY-1 sgC and sgp62 cells after 48 hr under Gln-deprived conditions normalized to cell number (n = 3, biological replicates). (C) Dynamics of [13C] labeled citrate, fumarate, malate, glutamate, and aspartate/asparagine in WPMY-1 sgC and sgp62 cells under Gln-deprived conditions. Metabolites at each time were normalized to control cell line at 1 hr post tracer addition. Graphs represent per cell abundance of 13C-labeled metabolites in cells cultured with [U-13C6]glucose (n = 3, biological replicates). (D) Metabolic map depicting pyruvate anaplerosis, TCA metabolism, and non-essential amino acid synthesis downstream of a [U-13C6]glucose tracer. Pyr, pyruvate; OAA, oxaloacetate; Cit, citrate; αKG, α-ketoglutarate; SucCoA, succinyl-CoA; Suc, succinate; Fum, fumarate; Mal, malate; Asp, aspartate; Asn, asparagine; Glu, glutamate; Gln, glutamine; PC, pyruvate carboxylase; PDH, pyruvate dehydrogenase. ASNS, asparagine synthetase. (E–G) Knockout of p62 in WPMY-1 cells increases abundances of M5 citrate (E), M3 malate (F), and M3 Asp/Asn (G) compared to control conditions. Cells were exposed to [U-13C6]glucose for 48 hr under Gln-deprived conditions (n = 3, biological replicates). Results depict per cell abundances of 13C-labeled metabolites. (H) M1 citrate (Cit), malate (Mal), and glutamate (Glu) abundances from [3-13C]glucose increase in shp62 compared to shNT cells after 48 hr under Gln-deprived conditions (n = 3, biological replicates). Error bars, S.E.M., two-tailed t-test (A, B, E, F, G, H), two-way ANOVA test (A, C). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. See also Figure S2.
Non-essential amino acid synthesis removes intermediates from the TCA cycle, and these metabolites must be replenished via anaplerosis. While Gln serves as the primary anaplerotic substrate in most cultured cell systems (Vacanti et al., 2014), pyruvate carboxylase (PC) is another important anaplerotic pathway, particularly in the context of limited Gln availability (Cheng et al., 2011). Because p62 deficiency induced glucose metabolism to generate TCA intermediates and Asp/Asn under Gln-deprived conditions, we hypothesized that enhanced flux through PC and pyruvate dehydrogenase (PDH) could serve to promote the synthesis of oxaloacetate and acetyl-CoA (Figure 2D). Both combined will generate citrate, and subsequently α-ketoglutarate which could compensate for the lack of Gln-derived α-ketoglutarate available under these conditions (Figure 2D). PC activity could promote Asn production via asparagine synthetase (ASNS) by increasing flux to oxaloacetate and Asp (Sellers et al., 2015) (Figure 2D). This possibility offers a rationale to link the reprogramming of glucose anaplerosis induced by p62-deficiency in Gln-free medium to the production of Asn. To confirm whether PC flux was changed in the context of p62 loss, we quantified the abundance of specific isotopologues generated via PC and TCA metabolism in prostate fibroblasts cultured with [U-13C6]glucose. As depicted in Figure 2D, [U-13C6]glucose yields M5 citrate, M3 malate, and M3 aspartate (i.e., each labeled with five or three 13C atoms, respectively) through the combined activity of PC and PDH. Consistent with this, the abundance of these isotopologues was significantly increased in p62-deficient cells compared to control cells when both were cultured without Gln (Figures 2E–2G). To more directly quantify relative flux through PC, we did tracing experiments with [3-13C]glucose in Gln-deprived conditions, which labels TCA intermediates via PC activity but not through PDH. Relative labeling in citrate, malate, and glutamate pools was significantly increased in p62-deficient cells compared to control conditions (Figure 2H).
PC and ASNS control growth of p62-deficient stromal cells in Gln-deprived conditions
The results described above suggest that flux through PC in Gln-deficient media is elevated because of p62 loss. Notably, although the levels of PDHA1 transcripts were unchanged in p62-deficient cells (not shown), PC mRNA and protein levels were higher in p62-deficient cells (Figure 3A). Furthermore, knockdown of PC impaired the ability of p62-deficient stromal cells to survive in Gln-deprived medium (Figure 3B). Taken together, these results demonstrate p62’s control of specific steps in pyruvate metabolism to support TCA anaplerosis, and potentially non-essential amino acid synthesis, is critical for stromal cell growth in Gln-deprived conditions. Interestingly, in addition to increased TCA anaplerosis via PC, we observed increased levels of, and glucose flux to, Asp/Asn in p62-deficient prostate stromal cells cultured in the absence of Gln (Figures 2B and 2C). Also, our recent analysis of stromal gene expression demonstrated that p62 levels were downregulated in human tumor stroma in several types of cancers, including prostate (Valencia et al., 2014). While searching for the expression of correlation neighbors of p62 in the same human prostate stroma datasets, we found that p62 levels negatively correlated with those of ASNS (Figure 3C). This is consistent with a model whereby p62 deficiency promotes the upregulation of ASNS. To test this idea we determined the mRNA and protein expression levels of ASNS in p62-deficient prostate stromal cells, and discovered that p62 loss, indeed, resulted in increased expression of ASNS under conditions of Gln deprivation, both at the mRNA and protein levels (Figure 3D). To determine the functional relevance of this observation, we knocked down the expression of ASNS by using siRNA and examined cell numbers over time in the absence of Gln. Notably, knockdown of ASNS eliminated the proliferation advantage afforded by p62-deficiency in Gln-deprived stromal fibroblasts (Figure 3E). Incubation of p62/ASNS doubly deficient human stromal cells with exogenous Asn completely rescued proliferation under Gln-deprived conditions (Figure 3F). These results demonstrate that increased generation of Asn by p62-deficient stromal cells is essential for their growth in Gln-deprived conditions.
Figure 3. p62 loss regulates ASNS and PC in Gln-deprived stroma.
(A) qPCR of PC levels in WPMY-1 sgC and sgp62 cells under Gln-depleted conditions for 48 hr (n = 3, biological replicates) and western blot (n = 2, independent experiments). (B) Growth curves of WPMY-1 sgC and sgp62 cells, transduced with the indicated siRNAs under Gln-depleted conditions for 48 hr (n = 3, biological replicates). (C) Negative correlation between ASNS and p62 levels in the stroma. (D) qPCR of ASNS levels in WPMY-1 sgC and sgp62 cells under Gln-depleted conditions for 48 hr (n = 3, biological replicates) and western blot (n = 2, independent experiments). (E) Growth curves of WPMY-1 sgC and sgp62 cells transduced with the indicated siRNAs under Gln-depleted conditions (n = 3, biological replicates). (F) Four-day growth of WPMY-1 sgC and sgp62 cells, transduced with the indicated siRNAs under Gln-depleted conditions, in presence or not of 0.5 mM asparagine (n = 3, biological replicates). Error bars, S.E.M., two-tailed test (A, D, F); two-way ANOVA test (B, E). **p < 0.01, ***p < 0.001.
Upregulation of stromal ATF4 by p62 deficiency in Gln-deprived conditions
Gene set enrichment analysis (GSEA) of a transcriptomic profiling of sgp62 and sgC cells cultured in the absence of Gln revealed that p62-deficient WPMY-1 cells displayed enrichment of a gene expression signature that corresponds to the “UNFOLDED PROTEIN RESPONSE” category (Figures 4A and S3A). The transcription factor ATF4, and its downstream target ASNS, appear to be significantly increased in this gene category (Figure S3A). ATF4 has been shown to regulate the metabolic adaptation of cells to situations in which the availability of amino acids is reduced (DeNicola et al., 2015; Qing et al., 2012; Ye et al., 2015). We speculated that p62 deficiency would directly upregulate ATF4 to promote the generation of TCA-associated metabolites, which will drive the synthesis of Asp (facilitated by the upregulation of a PC-generated OAA) that provides the substrate for Asn production mediated by ASNS, a bona fide downstream target of ATF4 (Barbosa-Tessmann et al., 1999). Therefore, we next tested the possibility that p62 deficiency might regulate stromal cell proliferation under Gln-deprived conditions by upregulating the levels of ATF4. Consistent with our hypothesis, shp62 stromal fibroblasts showed a higher induction of ATF4 than shNT controls in response to Gln deprivation (Figure 4B). Re-expression of p62 into shp62 cells restored ATF4 to the levels of shNT cells (Figure S3B). Similar results were obtained in two different clones of human sgp62 stromal cells (Figure 4C), which could also be rescued by the re-expression of p62 (Figure S3C). These effects of p62 were stroma-specific since no differences in ATF4 levels were observed between p62-deficient and proficient PCa epithelial cells, PC3 and DU145, when exposed to Gln-deprived conditions (Figure S3D). Importantly, the knockdown of ATF4 completely abolished the growth of p62-deficient stromal cells in Gln-free medium (Figure 4D), demonstrating the functional relevance of ATF4 upregulation in p62-deficient stromal fibroblasts for Gln-free proliferation. Furthermore, the addition of extracellular Asn completely rescued the growth defect of p62/ATF4 double KD cells in the absence of Gln (Figure 4E).
Figure 4. Stromal p62 deficiency upregulates ATF4 in Gln-deprived conditions.
(A) GSEA plot of enrichment in “unfolded protein response” signatures in WPMY-1 sgp62 cells (n = 3, biological replicates) under Gln-depleted conditions using H MSigDB database. (B and C) Western blot (n = 2, independent experiments) in WPMY shNT and shp62 cells (B) and WPMY sgC and sgp62 (C) cells under Gln-depleted conditions (48 hr). (D) Growth curves of WPMY-1 sgC, and sgp62 cells transduced with the indicated siRNAs under Gln-depleted conditions (n = 3, biological replicates). (E) Four-day growth of WPMY-1 shNT, shp62 and shp62/shATF4 cells, transduced with the indicated siRNAs under Gln-depleted conditions, in presence or not of 0.5 mM asparagine (n = 3 biological replicates). (F) Western blot (n = 2 independent experiments) in WPMY-1 sgC and sgp62 cells transduced with the indicated siRNAs under Gln-depleted conditions (48 hr). (G) Schematic representation of the epigenetic status of the PC locus in human mesenchymal cells as mapped with the ENCODE project. CHIP-seq data for ATF4 reveal a potential binding site in PC promoter that was identified through H3K27ac signals. (H) ChiP-qPCR analysis (n = 3, biological replicates) of PC promoter occupancy of ATF4 in WPMY-1 sgC and sgp62 cells under Gln-depleted conditions (12 hr). (I) Schematic representation of the binding site of ATF4 in ASNS promoter as mapped with the ENCODE project. (J) ChiP-qPCR analysis (n = 3, biological replicates) of ASNS promoter occupancy of ATF4 in WPMY-1 sgC and sgp62 cells under Gln-depleted conditions (12 hr). Error bars, S.E.M., two-tailed test (E, H, J), two-way ANOVA test (D). *p < 0.05, **p < 0.01. See also Figure S3.
Collectively, these results establish a model whereby the loss of p62 upregulates ATF4, which promotes Asn synthesis profiting from an enhanced flux of glucose to the TCA and, specifically, through PC to OAA. However, how PC is transcriptionally regulated in this context was unknown. Since p62 deficiency led to the upregulation of ATF4, an attractive unifying hypothesis would be that both ASNS and PC transcription are directly regulated by ATF4. Consistent with this notion, PC upregulation by p62 deficiency in human stromal cells under Gln-deprived conditions was inhibited by ATF4 knockdown (Figure 4F and S3E). ENCODE interrogation of the regulatory region of the PC gene revealed the potential for recruitment of ATF4 (Figure 4G). Analysis of ATF4 Chip-seq data (Cohen et al., 2015) revealed three putative binding sites in close proximity of PC transcription start site (TSS). Further bioinformatic analysis revealed that one of these sites overlapped with a strong regulatory region, defined by high H3K27Ac signal, and contained a canonical ATF4 binding site confirmed by JASPAR analysis (Figure 4G). Therefore, it is possible that the high levels of ATF4 in p62-deficient stromal cells might result in enhanced interaction with the regulatory regions of the PC gene promoter, which would account for the increase in ATF4-dependent expression of PC in p62-deficient stromal fibroblasts. To test this possibility, we performed chromatin immunoprecipitation (ChIP) analysis in sgp62 and sgC cells under Gln-deprived conditions and found that the recruitment of ATF4 to the PC gene regulatory region was significantly increased in p62-deficient cells as compared to p62-proficient controls (Figure 4H). Likewise, ChIP analysis also detected an increase in the recruitment of ATF4 to the ASNS gene regulatory region (Cohen et al., 2015) in p62-deficient cells under Gln-deprived conditions (Figures 4I and 4J). These results demonstrate that ATF4 upregulation accounts for the increased growth of p62-deficient stromal fibroblasts in the absence of Gln, and established the importance of PC and ASNS in this process.
p62 controls ATF4 stability through ubiquitin-mediated proteasomal degradation
Amino acid deprivation activates GCN2, the kinase that phosphorylates eIF2α, which results in the general inhibition of translation initiation, with the exception of a select group of mRNAs, including ATF4 (Ye et al., 2010). We showed that the loss of p62 does not affect eIF2α phosphorylation in stromal fibroblasts incubated in Gln-free medium (Figure S4A), suggesting that mechanisms other than eIF2α kinases are the critical regulators of ATF4 under situations of p62-deficiency. To dissect these pathways, we next treated sgC and sgp62 human stromal fibroblasts with cycloheximide to block protein synthesis, and then determined the levels of ATF4 at different times thereafter to determine its stability. We found that ATF4 is more stable in sgp62 than in sgC cells (Figures 5A and 5B). Similar results were obtained when shp62 cells were compared with shNT controls (Figure S4B). To further explore this observation, we determined whether p62 could interact with ATF4. Co-transfection experiments demonstrated that p62 and ATF4 indeed interacted (Figure 5C). This interaction was direct, as shown in pulldown experiments using recombinant purified MBP-p62 and His-ATF4 protein (Figure 5D). Furthermore, we found that endogenous p62 interacted with endogenous ATF4 in human fibroblasts when incubated under Gln-deficient conditions (Figure 5E). Consistent with ATF4 being a nuclear protein, Gln deprivation promoted the nuclear translocation of p62 (Figure 5F), which allowed their direct interaction. This is of great relevance because it establishes an insufficiently explored mechanism of action of p62 by regulating the activity of transcription factors in the nucleus.
Figure 5. p62 controls ATF4 stability through ubiquitin-mediated proteasomal degradation.
(A) WPMY-1 sgC and sgp62 cells were incubated with cycloheximide (CHX) and protein stability was determined by immunoblotting at indicated time points. (B) ATF4 protein levels were normalized to β-actin at basal level (n = 2, independent experiments). (C) Co-immunoprecipitation (Co-IP) of ATF4 and p62 (n = 2, independent experiments) in HEK293T cells. (D) In vitro Co-IP of ATF4 and p62 (n = 2, independent experiments). (E) Co-IP of ATF4 and p62 (n = 2, independent experiments) in nuclear fraction of WPMY-1 cells under Gln-depleted conditions for 48 hr. (F) Immunofluorescence of p62 in WPMY-1 cells under Gln-depleted conditions at the indicated times (n = 2, biological replicates). (G) ATF4 ubiquitination assay in WPMY-1 sgC and sgp62 cells in presence or absence of 10 μM MG132 for 4 hr (n = 2, independent experiments). (H) Co-IP of β-TrCP and p62 (n = 2, independent experiments) in HEK293T cells. (I) Co-IP of β-TrCP and ATF4 (n = 2, independent experiments) in WPMY-1 sgC and sgp62 cells in presence 10 μM of MG132 for 4 hr. Error bars, S.E.M., two-way ANOVA test (B). ****p < 0.0001. See also Figure S4.
ATF4 stability has been shown to be regulated by proteasome-mediated degradation following its K48-polyubiquitination by the E3-ubiquitin ligase β-TrCP (Lassot et al., 2001). We found that p62 is an essential component in this process. That is, whereas treatment with the proteasome inhibitor MG132 promoted the accumulation of poly-ubiquitinated ATF4 in sgC cells, it did not in sgp62 cells (Figure 5G). Similar results were obtained in shp62 as compared with shNT cells (Figure S4C). This means that p62 deficiency impairs the polyubiquitination of ATF4, which explains its higher stability and accumulation, especially under Gln-deprived conditions. Importantly, p62 interacted with β-TrCP in transfection experiments (Figure 5H), and was required for β-TrCP to interact with ATF4 (Figure 5I). Collectively, these results demonstrate that p62 bridges the interaction between β-TrCP and ATF4 to promote its ubiquitination and proteasome-mediated degradation, which is important for the control of its stability.
Asn produced by p62-deficient stroma supports PCa growth in Gln-free conditions
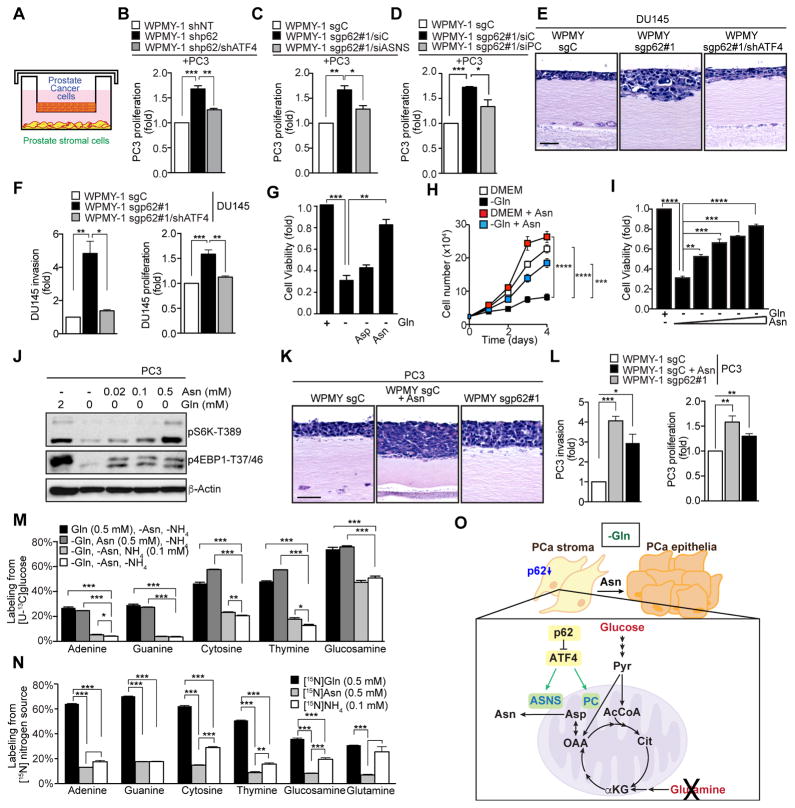
The results described above show that p62 deficiency drives a metabolic program that allows prostate stromal fibroblasts to grow under Gln-deprived conditions by producing Asp/Asn. One attractive possibility is that, in addition to promoting resistance to nutrient stress in stromal cells, metabolic reprogramming induced by p62 deficiency might also facilitate production of metabolites in the stroma that would serve to prevent nutrient stress in the epithelial fraction of the tumor. To address this question, we cultured stromal fibroblasts in the presence of PCa epithelial cells, as shown in Figure 6A. Specifically, we co-cultured mouse stromal fibroblasts (either WT or KO) with mouse Myc-CaP or TRAMP PCa cells (Figure S5A). We also co-cultured human prostate sgC and sgp62 stromal cells with human PCa PC3 cells (Figure S5A). Results from these experiments indicate that p62-deficiency in the stroma can sustain PCa proliferation in the absence of Gln in human and mouse co-cultures (Figure S5A), establishing for the first time that the lack of p62 in the stroma not only made stromal cells resistant to Gln deprivation, but also allowed epithelial PCa cells to become Gln resistant. Notably, the knockdown of ATF4 (Figure 6B) or ASNS (Figure 6C) in stromal cells severely inhibited the ability of p62-deficient stromal cells to promote the growth of PCa epithelial cells under Gln-free conditions. Consistent with the relevance of PC in the metabolic reprogramming of p62-deficient stromal fibroblasts to produce Asn, its knockdown severely inhibited the ability of sgp62 cells to promote PC3 proliferation under Gln-deprived conditions (Figure 6D). This demonstrates that the same mechanisms that regulate stromal cell growth upon Gln deprivation accounts for the ability of these cells to promote the proliferation of PCa epithelial cells in co-culture experiments. We next carried out a set of experiments using a previously described 3D organotypic model that recapitulates key aspects of epithelial-stromal crosstalk in the tumor microenvironment (Valencia et al., 2014). We used this organotypic system to co-culture sgp62 and sgC with PC3 cells and found that sgp62 fibroblasts enhanced the invasiveness and proliferation index of PC3 cells (Figures S5B and S5C). Importantly, the invasive and proliferative phenotype promoted by sgp62 stromal cells was reverted by knockdown of ATF4 (Figures 6E and 6F). Therefore, a reasonable prediction would be that the Asp/Asn produced by p62-deficient stromal cells could alleviate the nutrient stress promoted by Gln deficiency in PC3 cells. To test this hypothesis, we incubated PC3 cells without Gln in the absence or presence of exogenous Asp or Asn. Importantly, addition of Asn was sufficient to drive the growth of PC3 cells in the absence of Gln, whereas Asp had no effect (Figure 6G). Since Ala has been shown to support tumor metabolism through autophagic stromal secretion (Sousa et al., 2016), we determined the ability of Ala as compared to Asn in the same system. Of note, whereas Asn efficiently rescued PC3 growth in Gln-deprived conditions, Ala supplementation did not (Figure S5D). Asn rescued PCa growth in a time and dose-dependent manner in nutrient stress conditions (Figures 6H and 6I), and served to sustain PC3 mTORC1 activity in the absence of Gln (Figure 6J). In fact, addition of Asn to sgC stromal cells co-cultured with PC3 cells in low Gln conditions was able to mimic the response of sgp62 cells (Figures 6K and 6L). These are important observations because Asn has been shown to be critical for the survival and growth of cancer cells in several settings (Krall et al., 2016; Zhang et al., 2014). Moreover, they established that stromal cells can produce the Asn necessary to withstand Gln deprivation in the tumor microenvironment and sustain epithelial cancer cell growth under nutrient challenging conditions.
Figure 6. p62-deficient stroma supports PCa growth in Gln-free conditions through Asn.
(A) Schematic of co-cultures in double chamber. (B) Proliferation of PC3 cell in co-culture experiments with WPMY shNT, shp62 and shp62/shATF4 cells under Gln-depleted conditions for 4 days (n = 3, biological replicates). (C, D) Proliferation of PC3 cell in co-culture experiments with WPMY sgC and sgp62 cells transduced with the indicated siRNAs under Gln-depleted conditions for 4 days (n = 3, biological replicates). (E) H&E staining of organotypic gels combining DU145 cells with WPMY sgC, sgp62 and sgp62/shATF4 cells in low Gln conditions (0.2 mM), (n = 4, biological replicates). (F) Quantification of DU145 cells invasion and proliferation of experiments shown in e. (G) Four-day growth of PC3 cells in the presence or absence of Gln and Asn or Asp supplementation (n = 3, biological replicates). (H) Growth curves of PC3 cells in the presence or absence of Gln and Asn (n = 3, biological replicates). (I) Four-day growth of PC3 cells in the presence or absence of Gln and Asn (n = 3, biological replicates). (J) Western blot (n = 2 independent experiments) in PC3 cells in the presence or absence of Gln and Asn. (K) H&E staining of organotypic gels combining PC3 cells with WPMY sgC and sgp62 cells in low Gln conditions (0.2 mM), and sgC in low Gln conditions (0.2 mM) plus asparagine (Asn) (0.5 mM), (n = 3, biological replicates). (L) Quantification of PC3 cells invasion and proliferation of experiments shown in K. (M) Gln (black) and Asn (dark grey) increases labeling on nucleobases adenine, guanine, cytosine, thymine as well as glucosamine from [U-13C6]glucose in PC3 cells compared to conditions with NH4+ (light grey) or without a nitrogen source (white). Cells were cultured in [U-13C6] glucose labeling medium for four days supplemented with 0.5 mM Gln, 0.5 mM Asn, 0.1 mM NH4+ or without a nitrogen source (n = 3, biological replicates). (N) Labeling on adenine, guanine, cytosine, thymine, glucosamine and glutamine from various labeled nitrogen [15N] sources. PC3 cells were exposed for 4 days to 0.5 mM [γ-15N]Gln, 0.5 mM [γ-15N]Asn or 0.1 mM [15N]NH4+ (n = 3, biological replicates). (O) Model for the role of p62-deficient stroma in Gln-deprived conditions. Error bars, S.E.M., two-tailed t-test (B, C, D, F, G, I, L, M, N), two-way ANOVA test (H). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. See also Figure S5.
To better define how exogenous Asn promotes the growth of PC3 cells in Gln-deprived medium, we conducted stable isotope tracing experiments to quantify biosynthetic fluxes. Importantly, the amido-nitrogen on Gln is a critical substrate for the synthesis of Asn, as well as nucleotides and glycans. To quantify how Gln, Asn, or free ammonium (NH4+) influence biosynthesis in prostate cancer cells, we cultured PC3 cells with each nitrogen substrate along with [U-13C6]glucose, and quantified isotope enrichment in glucosamine and nucleobases (adenine, guanine, cytosine, thymine) from hydrolyzed biomass. Supplementation of either Gln or Asn similarly supported hexosamine and nucleotide biosynthesis, whereas 0.1 mM free ammonium (which can also promote autophagy) did not appreciably alter biosynthetic flux as compared to the negative control (Figure 6M). We next explored how exogenous Asn was used by prostate cancer cells metabolically. We cultured PC3 cells in the presence of isotopically labeled [γ-15N]Asn, [γ-15N]Gln, and [15N]NH4+ and quantified incorporation into free Gln or hydrolyzed biomass metabolites. As expected, [γ-15N]Gln effectively labeled all downstream metabolites (Figure 6N). In the absence of Gln, [γ-15N]Asn also provided nitrogen for biosynthesis, albeit at lower levels (Figure 6N). However, label incorporation was similar, or less than that from [15N]NH4+ (Figure 6N). Since overall biosynthesis (Figure 6M) was not increased by ammonium supplementation, these data suggest that Asn availability generally enhances tumor cell growth through signaling (Krall et al., 2016; Zhang et al., 2014), and also by limiting the need for Gln to be diverted to Asn production rather than directly providing nitrogen. Altogether, these data support a model whereby p62-deficient stroma reprograms its metabolism under Gln scarce conditions to provide Asn to support epithelial growth in an ATF4-dependent mechanism (Figure 6O).
In vivo effect of fibroblast-specific p62 deletion in the prostate epithelium
To determine the in vivo role of stromal p62 deficiency in prostate epithelium growth, we generated fibroblast-specific conditional p62 KO mice by crossing p62fl/fl mice with Fsp1-Cre mice (Bhowmick et al., 2004) to produce p62FSP1-KO mice. Analysis of the prostates of these mice at age 52 weeks showed that the loss of p62 in stromal fibroblasts was sufficient to promote prostate epithelial hyperplasia (Figure 7A) with a strong stromal reaction characterized by increased collagen deposition, as determined by Masson’s Trichrome staining (Figure 7B). Furthermore, immunofluorescence analysis of these samples demonstrated the highly dysplastic response (positive for αSMA) surrounding the hyperplastic prostate glands in the conditional p62 KO mice, but not in WT controls (Figure 7C). Double p62/αSMA immunofluorescence staining of these prostates demonstrated the selective loss of p62 in the stromal fraction (positive for αSMA) of the conditional p62 mice whereas staining for p62 remained intact in the epithelial compartment (Figure 7D).
Figure 7. Fibroblast-specific deletion of p62 promotes stromal activation through ATF4 in vivo.
(A) H&E staining of prostate sections from p62f/f (n = 14) and p62fspKO (n = 12) mice. (B) Masson’s Trichrome staining of prostate sections from p62f/f and p62fspKO mice (n = 5 mice per genotype). (C and D) Immunofluorescence of CK8 (C), p62 (D) and α-SMA levels in prostate tumors from p62f/f and p62fspKO mice (n = 5 mice per genotype). (E) GSEA plot of enrichment in “unfolded protein response” signatures in p62fspKO prostates (n = 3 mice per genotype) using H MSigDB database. (F) Heatmap representation of upregulated genes in p62fspKO relative to p62f/f prostates (n = 3 mice per genotype), including Log2FC and adjusted p value. (G) Top canonical pathways from Ingenuity Pathway Analysis (IPA) of upregulated genes in p62fspKO compared with p62f/f mice. (H and I) Immunofluorescence of ATF4 (H), ASNS (I) and α-SMA levels in prostate tumors from p62f/f and p62fspKO mice (n = 5 mice per genotype). See also Figure S6.
To determine the signaling cascades activated in these prostates in vivo, we carried out GSEA and IPA of RNA-Seq transcriptomics data from prostates of both genotypes. Interestingly, like in the WPMY-1 cells when subjected to Gln deprivation, the conditional p62FSP1-KO cells displayed enrichment in the “UNFOLDED PROTEIN RESPONSE” with enhanced expression of the ASNS and ATF4 genes (Figures 7E–7G). Notably, immunofluorescent analysis of these prostates revealed strong staining for ATF4 (Figure 7H) and ASNS (Figure 7I), which showed a clear co-localization with the αSMA staining of the stroma in the conditional p62FSP1-KO prostates. Likewise, increased staining of stromal ATF4 and ASNS was also observed in prostates from PTEN+/−/p62 KO mice as compared to PTEN+/− samples (Figures S6A and S6B). These results demonstrate that activation of the ATF4-ASNS axis identified in p62-deficient stromal fibroblasts is also found in the p62-deficient stromal compartment of mouse prostates, supporting the in vivo relevance of our findings.
DISCUSSION
The identification of non-oncogenic addictions and synthetic vulnerabilities is an innovative strategy to target metabolism in cancer epithelial cells but likely also in the stroma. Here we show that under conditions of Gln deprivation, p62 plays an essential role in rewiring the metabolism of tumor stromal fibroblasts through the upregulation of ATF4, which not only sustains stromal growth but also, indirectly, that of the epithelial tumor cells, making both cell lineages addicted to stromal ATF4-derived signals that culminate in the generation of Asn. This creates a potentially actionable synthetic vulnerability since downregulation of ATF4, or its downstream targets, prevents the growth of p62-deficient stromal cells, as well as their ability to sustain the proliferation of the cancer epithelial compartment under conditions of nutrient stress.
Recent data show how the activation of autophagy in the stroma of pancreatic tumors generates alanine (Ala), which serves to substitute the carbons provided by glucose and glutamine to fuel the TCA, and is instrumental for lipid and amino acid synthesis (Sousa et al., 2016). The data shown in our study demonstrates that the enhanced production of Asn by p62-deficient CAFs serves to provide the nitrogen required by cells under conditions of Gln deprivation. The generation of Asn through this pathway is likely synergistic to the activation of autophagy in the stroma. That is, whereas stromal autophagy generates Ala to substitute the carbons provided by glucose and glutamine (Sousa et al., 2016), the Asn generated by the p62 loss in the stroma serves to provide the nitrogen that is not available during Gln deficiency. Therefore, autophagy and p62 (acting independently of its autophagy adaptor function) are part of a symbiotic stromal-epithelial mechanism to maintain and promote tumorigenesis even under nutrient challenging conditions. Because the activation of autophagy results in p62 degradation (Moscat and Diaz-Meco, 2011; Moscat et al., 2016), we can speculate that in a nutrient-stressed tumor microenvironment, stromal cells activate autophagy to generate Ala, and simultaneously downregulate p62 to promote the metabolic reprogramming necessary to generate Asn by upregulating ATF4. The generation of Asn and their utilization by cancer cells have attracted recent interest in the cancer metabolism field (Krall et al., 2016; Ye et al., 2010; Zhang et al., 2014), and our data further support its relevance in cancer. Whereas our results indicate that Asn provides the nitrogen necessary for cells to grow under Gln-deficient conditions, other roles equally important can also be envisioned. This might include the ability of Asn to maintain mTORC1 activation through its actions as an exchange factor to promote the uptake of amino acids (Krall et al., 2016), or its ability to prevent Chop-driven cell death upon the accumulation of ATF4, its upstream regulator (Zhang et al., 2014).
We also show here that key downstream elements of this pathway are also activated in vivo in mice in which p62 has been specifically knocked out in the fibroblastic compartment. The fact that the global loss of p62 promotes tumorigenesis due to the metabolic reprogramming of the stroma, indicates that p62 should be considered a tumor suppressor, because the stromal signals unleashed by p62 deficiency supersede the requirement of p62 for the oncogenic transformation of the epithelium (Duran et al., 2016; Valencia et al., 2014). Therefore, the results reported here demonstrate that, in contrast to the prevailing view of p62 as a pro-tumorigenic molecule due to its actions in the epithelial compartment of the tumor, p62 suppresses cancer progression by inhibiting the NAF-to-CAF transition (Valencia et al., 2014); and by restraining the metabolic and signaling pathways of stromal fibroblasts that sustain their growth, as well as their ability to maintain the growth of epithelial cells through the generation of Asn.
Ample evidence suggests that the constant degradation of p62 in the epithelium via autophagy serves to keep p62 at levels that do not promote oncogenic transformation while still allowing p62 to perform its quality-control role as an autophagy adaptor (Moscat and Diaz-Meco, 2009; Moscat et al., 2016). Indeed, p62 has recently emerged as an important activator of mTORC1 and of ROS detoxifying pathways (NF-κB and NRF2), which are all instrumental in the oncogenic transformation of epithelial cells (Duran et al., 2011; Linares et al., 2015; Umemura et al., 2016). Notably, p62 deficiency in prostate stromal fibroblasts results in the reduction of mTORC1 activity without changes in NF-κB or NRF2, promoting an inflammatory response and the expression of TGFβ, which are both instrumental in the activation of the CAF differentiation program (Valencia et al., 2014). However, although this metabolic reprogramming through the p62-mTORC1 axis serves to control the NAF-to-CAF differentiation, it is not involved in the response to Gln deprivation reported here since the manipulation of neither mTORC1 nor Myc impacts the growth of stromal cells under Gln-deprived conditions. Furthermore, these actions of p62 in the stroma are not mediated by autophagy since inhibition of this catabolic process negatively impacts stromal cell survival independently of their p62 content. Therefore, whereas cancer epithelial cells upregulate p62 to activate mTORC1 and the NRF2/NF-κB detoxifying cassette, in the stroma p62 is inactivated, which results into two intertwined but independent consequences: (1) mTORC1 inhibition that (through IL-6 and TGFβ) promotes the NAF-to-CAF transition; (2) upregulation of ATF4 that under conditions Gln deprivation serves to trigger the synthesis of Asn. Our results expand beyond the better-known concept that tumor growth requires cancer cells to adapt their metabolism to a highly proliferative phenotype, often under nutrient-scarce conditions (Boroughs and DeBerardinis, 2015; Kenific and Debnath, 2015), to the involvement of the stroma in the metabolic control of epithelium growth through the stromal downregulation of p62. Importantly, the function of p62 in the metabolic rewiring of the stroma described here is not accounted for by its role as a cytosolic regulator of gene expression through NF-κB or NRF2, but rather as a direct regulator of the nuclear levels of ATF4. This is consistent with an emerging concept of p62 as a nuclear regulator of transcription, first established by the discovery of p62’s ability to modulate the activity of nuclear vitamin D receptor complexes in hepatic stellate cells during liver inflammation and cancer progression (Duran et al., 2016). Interestingly, these two nuclear activities of p62 are specific to stromal fibroblasts, which raise the possibility of selectively targeting these p62 actions in the stroma to prevent the pro-tumorigenic effects of p62 deficiency.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Maria T Diaz-Meco (mdmeco@sbpdiscovery.org)
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mouse models
p62fspKO mice were generated by breeding Sqstm1f/f mice (Duran et al., 2016) with FSP1-Cre mice (Jackson Laboratory, stock number 012641). Animals were maintained under conditions of controlled temperature (22.5 °C) and illumination (12 hr dark/light cycle). All mice were born and maintained under pathogen-free conditions. All animal handling and experimental procedures performed in this study were approved by the Institute Institutional Animal Care and Use Committee (IACUC) at the SBP Medical Discovery Institute. All genotyping was done by PCR. Experimental mice were all males, and aged 52 weeks at the time of experimentation.
Cell culture experiments
PCa cells (PC3 and DU145), stromal cells (WPMY-1), HEK293T and Phoenix-GP cells were cultured in DMEM (Cellgro, #15-017-CV) supplemented with 10% FBS, 2 mM of Glutamine and 100 U/ml penicillin and 100 μg/ml streptomycin, in an atmosphere of 95% air and 5% CO2. WT and p62 KO stromal prostate fibroblasts were isolated from mouse prostate, as described previously (Valencia et al., 2014), and were cultured DMEM supplemented with 5% (vol/vol) fetal bovine serum (FBS), 5% (vol/vol) NuSerum IV, 1% glutamine, 1% penicillin-streptomycin, 0.01 μM testosterone, and 25 μg/ml of insulin, in an atmosphere of 95% air and 5% CO2 at 37°C. For glutamine or glucose deprivation, cells were plated overnight in complete DMEM, briefly washed with phosphate-buffered saline (PBS) and then transferred into glutamine-free medium (Cellgro, #15-017-CV) or glucose-free medium (Cellgro, #17-207-CV) supplemented with 10% dialyzed FBS (Gibco, #26400) and 1 mM sodium pyruvate (Cellgro, #25-000-CI). Cell viability was determined by Trypan Blue exclusion at the indicated times. For mTOR pathway inhibition, cells were incubated with 25 nM of INK128 (Selleckchem) or 100 nM of Torin (Tocris) for 4 days. For cell death assays, cells were treated with 10 ng/ml of TNFα (Sigma) and 25 μg/ml of CHX (Sigma) for 4 days. For the protein stability assay, CHX (100 μg/ml) was added 24 hr after seeding, and protein was extracted at the indicated time points. For blocking autophagy or macropinocytosis, cells were incubated with 20 nM of Bafilomycin A1 (Sigma), 20 μM of Chloroquine (Sigma) or 50nM of EIPA (Sigma), respectively at the indicated time. To knock down p62 in WPMY, DU145 and PC3 cells, TRC lentiviral shRNA targeting human p62 (TRCN000007234) was obtained from Sigma-Aldrich. shRNA- encoding plasmids were co-transfected with psPAX2 (Addgene plasmid #12260; Trono Lab Packaging and Envelope Plasmids, unpublished) and pMD2.G (Addgene plasmid #12259; Trono Lab Packaging and Envelope Plasmids, unpublished) packaging plasmids into actively growing HEK293T cells by using XtremeGene HP transfection reagent (Roche). Virus-containing supernatants were collected 48 hr after transfection, filtered to eliminate cells, and then used to infect WPMY, DU145 and PC3 cells in the presence of 8 μg/ml polybrene (Millipore). Cells were analyzed after puromycin selection (1μg/ml) to confirm knockdown. To knock down ASNS, c-myc, PC and ATF4 in WPMY cells, siRNAs from Santa Cruz were used. siRNAs were co-transfected into actively growing cells by using Lipofectamine transfection reagent. Cells were analyzed on the second day after transfection. To knock out p62 in WPMY-1 cells, a 20-nucleotide single-guide RNA sequence targeting the first exon of the human p62 gene (5′ GAAGATCGCCTTGGAGTCCG) was designed using the CRISPR design tool at http://crispr.mit.edu/. The single guide RNA was cloned in the lentiCRISPR v2 vector (a gift from Feng Zhang; Addgene plasmid # 52961) and were transfected into WPMY-1 cells using Lipofectamine 2000 according to manufacturer’s instructions. 48 hr post transfection. Cells were selected with puromycin (1μg/ml), trypsinized, washed with PBS, and re-suspended in DMEM with 2% FBS and penicillin/streptomycin. Cells were single-sorted by FACS (SBP FACS core, FACS ARIA) into 96-well plates containing DMEM with 20% FBS and 50 μg/ml penicillin/streptomycin. Single clones were expanded and screened for p62 expression by protein immunoblotting.
METHODS DETAILS
Cell lysis, immunoprecipitation and western blotting
Cells for protein analysis were lysed in RIPA buffer (20 mM Tris-HCl, 37 mM NaCl, 2 mM EDTA, 1% Triton-X, 10% glycerol, 0.1% SDS, and 0.5% sodium deoxycholate, with phosphatase and protease inhibitors). For immunoprecipations, cells were pretreated with 10 μM of MG132 (Sigma) for 4 hr and then cells were harvested and lysed in 1% NP-40 lysis buffer (1% Nonidet P-40, 150 mM NaCl, 50 mM Tris-HCl [pH 7.5], 1 mM EDTA with phosphatase and protease inhibitors). 1 mg of cell lysates were pre-cleared with 30 μl of a 50% slurry of protein A sepharose (GE healthcare) for 30 min. Then 1 μg of primary antibody or control immnunoglobulin (Santa Cruz Biotech) was added to the lysates and incubated with rotation overnight at 4°C. The next day, 30 μl of a 50% slurry of protein A sepharose was added and the incubation was continued for 1 hr. After a 15 min centrifugation at 2,500 rpm at 4 °C, the immunoprecipitates were washed three times with lysis buffer, once with high salt (500 mM NaCl), and once more with lysis buffer. For nuclear protein extraction, cells were incubated with buffer A (20 mM Tris-HCl at pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, 1 mM PMSF) for 10 min on ice, centrifuged at 3500 rpm, resuspended in buffer C (20 mM Tris-HCl at pH 7.9, 1.5 mM MgCl2, 0.42 M NaCl, 0.5 mM DTT, 0.2 mM PMSF, 0.2 mM EDTA), sonicated, and centrifuged at 14,000 rpm. For immunoprecipitation, 2 mg of nuclear extract was incubated with the antibody bound to protein A/G beads overnight, then the beads were washed three times with BC300 buffer (50 mM Tris at pH 7.9, 300 mM KCl, 2 mM EDTA, 10% glycerol, protease inhibitors) plus 0.1% NP40, and eluted with 2× SDS loading buffer. Cell extracts and immunoprecipitated proteins were denatured, subjected to SDS-PAGE, transferred to PVDF membranes (GE Healthcare), and immunoblotted with the specific antibodies. For MBP pull-down assays, 50 μl of Amylose resin-bound MBP-p62 were mixed with 100 ng of his-ATF4 (Origene) in 500 μl of binding buffer (20 mM HEPES pH 8.0, 100 mM KCl, 0.2 mM EDTA and 20% glycerol) and incubated for 2 hr. Samples were washed three times with 1 ml of binding buffer and proteins were denatured by adding 10 μl of sample buffer followed by boiling for 5 min, and subjected to immunoblotting.
Chromatin immunoprecipitation analysis
p62 knockout and control WPMY-1 cells were growth without glutamine for 12 hr. Cells were fixed by adding directly to the culture medium formaldehyde (HCHO; from a 37% HCHO–10% methanol stock, Calbiochem) to a final concentration of 1%. After, 20 min of incubation with 125 mM glycine, cells were washed with ice-cold phosphate-buffered saline (PBS) and lysed with in 50 mM Tris pH 8.0, 10 mM EDTA, 1% SDS and protease inhibitors and incubated 30 min at 4 °C. Chromatin was sheared in a COVARIS S220 Focused-ultrasonicator to yield DNA fragment sizes of 200–1000 base pairs, and diluted 10 times in dilution buffer (20 mM Tris pH 8.0, 2 mM EDTA, 1% triton X-100, 150 mM NaCl and protease inhibitors). Immunoprecipitations were carried out overnight at 4°C using the following protein A-antibodies complexes: ATF4 (Cell signaling #11815). Immunocomplexes were washed three times with buffer TSEI (20 mM Tris pH 8.0, 2 mM EDTA, 1% triton X-100, 150 mM NaCl, 0.1% SDS and protease inhibitors), three washes with buffer TSEII (20 mM Tris pH 8.0, 2 mM EDTA, 1% triton X-100, 500 mM NaCl, 0.1% SDS and protease inhibitors), one wash with buffer TSEIII (10 mM Tris pH 8.0, 250 mM LiCl, 1 mM EDTA, 1% NP40, 1% Deoxycholate and protease inhibitors) and one wash with TE pH 8.0. Immunocomplexes were extracted in TE containing 1% SDS, and protein–DNA cross-links were reverted by heating at 65°C overnight. DNA was extracted by using a PCR purification kit (Qiagen). One-tenth of the immunoprecipitated DNA was used in each PCR, for which the promoter-specific primers were used.
Ubiquitination Assay
To detect endogenous in vivo ATF4 ubiquitination, WPMY-1 cells were lysed with cell lysis buffer (2% SDS, 150 mM NaCl, 10 mM Tris-HCl, pH 8.0, with 2mM sodium orthovanadate, 50 mM sodium fluoride, and protease inhibitors). Cell lysates were boiled for 10 min to dissociate protein-protein interactions. The samples were diluted with dilution buffer (10 mM Tris-HCl [pH 8.0], 150 mM NaCl, 2 mM EDTA, 1% Triton). The diluted samples were incubated at 4°C for 60 min with rotation and then centrifuged for 30 min. Cell lysate was incubated with ATF4 antibody (Cell signaling #11815) overnight, after which Protein A beads were added for an additional 1 hr. Immunoprecipitates were washed four times with washing buffer (10 mM Tris-HCl, pH 8.0, 1 M NaCl, 1 mM EDTA, 1% NP-40). Proteins were eluted in SDS-sample buffer, subjected to SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted with anti-ubiquitin.
Co-culture proliferation assays
Proliferation assays were performed as described previously (Valencia et al., 2014). Briefly, stromal cells were seeded in TC-treated 6-well plates and allowed to attach for 6 hr before PCa cells were seeded on top of Milicell Cell Culture Inserts (Millipore). Inserts were then placed in the pre-seeded 6-well plate. Proliferation was quantified by Trypan Blue exclusion after 4 days growing in glutamine-depleted conditions.
Organotypic cultures
Organotypic cultures were performed as described previously (Valencia et al., 2014). Briefly, gels were composed of one ml of a mixture of 1.75 volumes of Matrigel, 5.25 volumes of collagen type I, 1 volume of 1x DMEM, 1 volume of 10x DMEM, and 1 volume of filtered FBS. The mixture was plated onto 24-well plates coated with diluted collagen type I. Gels were allowed to equilibrate with 1 ml of 1x DMEM overnight at 37°C. 5 x 105 cells PCa cells and prostate stromal cells (50:50) were then seeded on top of the matrix. Gel rafts were placed onto collagen-coated nylon sheets and lifted using a sterile supporting steel mesh to set up a raised air-liquid culture. Normal medium was changed in alternate days and organotypic cultures were allowed to grow for 14 days. Afterwards, organotypic gels were harvested, fixed in 10% neutral buffered formalin, bisected and embedded in paraffin. H&E stained sections were analyzed with a Zeiss light microscope supplemented with Axiovision40 software. Quantification of the invasion assays was performed as described previously (Valencia et al., 2014) using ImageProPlus software.
Immunofluorescence assays
WPMY-1 cells were plated on coverslips, fixed in 4% paraformaldehyde, blocked and permeabilized (0.3% BSA and 0.1% Triton X-100. Fixed cells on cover slips, or sections from mouse prostate samples, previously deparaffinized, were blocked for one hour in blocking buffer (0.3% BSA in PBS), and then incubated with primary antibody and corresponding Alexa-conjugated secondary antibodies (Life Technologies). Slides were mounted on Mowiol and examined with a Zeiss LSM 710 NLO Confocal Microscope. Macropinosomes were marked utilizing a high molecular weight FITC-dextran (D1820, Invitrogen) uptake assay wherein FITC-dextran was added to glutamine-free medium at a final concentration of 1 mg/mL for 30 minutes at 37°C. At the end of the incubation period, cells were rinsed five times in cold PBS and immediately fixed in 3.7% formaldehyde. Cells were DAPI-treated to stain nuclei and coverslips mounted onto slides using DAKO Mounting Media (DAKO). Images were analyzed using the ‘Analyze Particles’ feature in ImageJ (NIH). The total particle area per cell was determined from at least 5 fields that were randomly selected from different regions across the entirety of each sample. For quantification of GFP-LC3 puncta, cells displaying > 10 brightly fluorescent GFP-LC3 puncta were counted as positive. For mCherry-GFP-LC3 cells, simultaneous images in the Green and Red channel were acquired for the same field of cells followed by evaluation of the digitally merged image to estimate autophagosome and autolysosome number.
Flow cytometry
Cells were glutamine starved and collected using trypsin–EDTA. The cells were resuspended in annexin buffer and incubated for 15 min with Annexin V (BD Biosciences), or 7-AAD (eBioscience) according to the manufacturer’s protocol. The apoptotic cells were analyzed using a FACScan flow cytometer (BD Biosciences). FACS analyses were reproduced by three independent experiments.
Isotopic labeling
For isotopic labeling experiments of fibroblasts, cells were cultured in DMEM medium supplemented with 25 mM [U-13C6] or [3-13C]glucose (Cambridge Isotopes Inc), and 10% (v/v) dialyzed FBS for 48 hr prior to metabolite extraction. Metabolites abundance is shown as mole percent enrichment (MPE) time metabolite abundance per cell. MPE was calculated as the percent of all atoms within the metabolite pool that are labeled: where n is the number of carbon atoms in the metabolite and Mi is the relative abundance of the ith mass isotopomer. To measure the relative abundance of M1 labeled metabolites from [3-13C]glucose. PC3 cells were cultured in DMEM medium supplemented with 25 mM [U-13C6]glucose (Cambridge Isotopes Inc) and 5% (v/v) dialyzed FBS as well as 0.5 mM glutamine, 0.5 mM asparagine, 0.1 mM free ammonium or without a nitrogen source for 4 days prior to metabolite extraction. For 15N labeling experiments PC3 cells were cultured in DMEM medium supplemented with 25 mM glucose and 5% (v/v) dialyzed FBS as well as 0.5 mM [γ-15N]glutamine (Cambridge Isotopes Inc), 0.5 mM [γ-15N]asparagine (Sigma) or 0.1 mM [15N]NH4+ (Sigma) for 4 days prior to metabolite extraction.
Gas chromatography-Mass spectrometry (GC-MS) sample preparation and analysis
Cells were washed with 1 ml saline solution and quenched with 0.5 ml −20 °C methanol. After adding 0.2 ml 4 °C cold water, cells were collected with a cell scraper and transferred in tubes containing 0.5 ml −20 °C chloroform. The extracts were vortexed at 1,400 rpm for 20 min at 4 °C and centrifuged at 16,000×g for 5 min at 4 °C. 0.35 ml of the upper aqueous phase w as evaporated under vacuum at −4°C using a refrigerated CentriVap Concentrator (Labconco). To determine the enrichment of nucleobases (adenine, guanine, cytosine, thymine) and glucosamine the interface was centrifuged with 1 ml −20 °C methanol at 16,000×g for 5 min at 4 °C, the pellet was hydrolyzed with 0.4 ml 6N HCl for 2 hr at 80°C, centrifuged at 16,000×g for 5 min and the supernatant was dried at 60 °C under airflow. Metabolite derivatization was performed using a Gerstel MPS. Dried metabolites were dissolved in 15 μl of 2% (w/v) methoxyamine hydrochloride (Thermo Scientific) in pyridine and incubated for 60 min at 45°C. An equal volume of 2,2,2-trifluoro-N-methyl-N-trimethylsilyl-acetamide (MSTFA) (nucleobases and glycans) or N-tertbutyldimethylsilyl-N-methyltrifluoroacetamide (MTBSTFA) (polar metabolites) with 1% tert-butyldimethylchlorosilane (Regis Technologies) was added and incubated further for 30 min at 45 °C. After derivatization, MSTFA and MTBSTFA derivatized samples were analyzed by GC-MS using a DB-35MS column (30 m x 0.25 i.d. x 0.25 μm, Agilent J&W Scientific) installed in an Agilent 7890A gas chromatograph (GC) interfaced with an Agilent 5975C mass spectrometer (MS) operating under electron impact ionization at 70 eV. The MS source was held at 230 °C and the quadrupole at 150 °C. 1μL of derivatized sample was injected in splitless mode and helium was used as carrier gas at a flow rate of 1 ml/min. For MSTFA derivatized samples, the GC oven temperature was held at 80 °C for 6 min, increased to 300 °C at 6 °C/min and after 10 m in increased to 325 °C at 10 °C/min for 4 min. The run time for one sample was 59 min. For MTBSTFA derivatized samples, the GC oven was held at 100 °C for 1 min, increased to 255 °C at 3.5 °C min/min, increased to 320 °C at 15 °C min/min and held at 320 °C for 3 min. The total run time for one sample was 54.6 min.
Gene-expression analyses
After quantification using a Nanodrop 1000 spectrophotometer (Thermo Scientific), 1 μg of RNA was reverse- transcribed using random primers and MultiScribe Reverse Transcriptase (Applied Biosystems). Gene expression was analyzed by amplifying 50 ng of the complementary DNA using the CFX96 Real Time PCR Detection System with SYBR Green Master Mix (BioRad). The amplification parameters were set at 95 °C for 30 s, 58 °C for 30 s, and 72 °C for 30 s (40 cycles total). Gene expression values for each sample were normalized to the 18S RNA. RNASeq studies were performed in the Genomics Core at SBP Medical Discovery Institute. Briefly, total RNA was extracted from WPMY p62-KO (sgp62) and control (sgC) cells (n = 3, biological replicates) and from prostate tissue from 3 independent p62f/f and p62fspKO mice (n = 3 mice per genotype). PolyA RNA was isolated using the NEBNext® Poly(A) mRNA Magnetic Isolation Module and barcoded libraries were made using the NEBNext® Ultra™ Directional RNA Library Prep Kit for Illumina® (NEB, Ipswich MA). Libraries were pooled and single end sequenced (1X75) on the Illumina NextSeq 500 using the High output V2 kit (Illumina Inc., San Diego CA). Sequencing Fastq files were uploaded to BaseSpace and processed with RNAexpress App (Illumina) to obtain raw reads counts for each gene.
Bioinformatic Analysis
Gene matrix files generated with RNAexpress app (Illumina) were used as input file for Gene set enrichment analysis. GSEA was performed using GSEA v2.0.14 software (http://www.broadinstitute.org/gsea/index.jsp) with 5000 gene-set permutations using the gene-ranking metric T-test with Hallmark MSigDb collection. Heat-map representation of gene expression was generated using Morpheus (Broad Institute). Differentially expressed genes using Deseq software (q value<0.05) were uploaded to IPA to be analyzed using “Core Pathway Analysis”. For correlation studies in the human prostate tumor stroma, raw data probe values were obtained from GEO (GSE34312) and collapsed to gene symbols using CollapseDataset module from GenePattern (median values). Gene expression values for SQSTM1 and ASNS were plotted to perform Pearson correlation analysis and linear regression. ATF4 Chip-seq data (54129_treat.bg) was downloaded from cistrome.org and plotted in the UCSC genome browser together with UCSC default layered H3K27Ac track for the shown genes. Transcription Factor motif analysis of PC and ASNS promoters was performed using JASPAR (http://jaspar.genereg.net/).
Statistical Analysis
Statistical analyses for figures were performed using GraphPad Prism software (San Diego, CA). Data are presented as the mean ± SD. For qPCR experiments, Gaussian distribution was assumed and a Student’s t-test (two-tailed unpaired) was used to determine statistical significance. If the data did not meet this test, a Mann-Whitney test was used. Two-way ANOVA was used for in vitro experiments as indicated. All experiments were performed at least two or three times, unless otherwise noted. Gene expression correlation analyses were performed using Pearson’s correlation coefficients, with a two-tailed test. The significance level for statistical testing was set at p < 0.05.
Data and software availability
The accession number for the RNA-seq data reported in this paper is GSE94343 and GSE94367. Unprocessed original image data have been deposited to Mendely Data and are available at http://dx.doi.org/10.17632/7zwjzjtyd7.1.
Supplementary Material
Highlights.
Loss of p62 in the stroma reprograms metabolism to endure glutamine deprivation
Stromal loss of p62 upregulates ATF4 to sustain Asparagine-mediated tumor growth
p62 regulates ATF4 stability through ubiquitin-mediated proteasomal degradation
Fibroblast selective deletion of p62 activates the ATF4-ASNS axis in vivo
Acknowledgments
Research was funded by grants from NIH (R01CA192642, R01CA218245 to M.T.D.-M.; R01DK108743, R01CA172025 to J.M. and 5P30CA030199 to M.T.D.-M. and J.M.; R01CA188652, R01CA218245 to C.M.M.), NSF (CAREER Award #1454425 to C.M.M.), DOD (W81XWH-13-1-0105 to C.M.M.) and CCSPG Pilot Project Grant under award 5P30CA030199 to M.T.D.-M. M.R.C is supported by “La Caixa” fellowship for studies in North America. We thank Maryellen Daston for editing the manuscript and Sarah Gilmour, the personnel of Histology, Cell Imaging, Genomics, Animal Facility, Cancer Metabolism and Viral Vectors Shared Resources at SBP Medical Discovery Institute for technical assistance.
Footnotes
Author Contributions
M.T.D.-M., C.M.M. and J.M. devised and coordinated the project; J.F.L., T.C., A.D., M.R.-C., T. V., and C.S.A. performed all the experiments; E.A.C. provided pathologist expertise for histological analysis; M.T.D.-M., C.M.M. and J.M. designed the experiments and wrote the manuscript with help from J.F.L., T.C., A.D. and M.R.C.; M.T.D.-M., C.M.M. and J.M. provided funding.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn CS, Metallo CM. Mitochondria as biosynthetic factories for cancer proliferation. Cancer Metab. 2015;3:1. doi: 10.1186/s40170-015-0128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa-Tessmann IP, Chen C, Zhong C, Schuster SM, Nick HS, Kilberg MS. Activation of the unfolded protein response pathway induces human asparagine synthetase gene expression. J Biol Chem. 1999;274:31139–31144. doi: 10.1074/jbc.274.44.31139. [DOI] [PubMed] [Google Scholar]
- Barron DA, Rowley DR. The reactive stroma microenvironment and prostate cancer progression. Endocr Relat Cancer. 2012;19:R187–204. doi: 10.1530/ERC-12-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17:351–359. doi: 10.1038/ncb3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Sudderth J, Yang C, Mullen AR, Jin ES, Mates JM, DeBerardinis RJ. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proc Natl Acad Sci U S A. 2011;108:8674–8679. doi: 10.1073/pnas.1016627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DM, Won KJ, Nguyen N, Lazar MA, Chen CS, Steger DJ. ATF4 licenses C/EBPbeta activity in human mesenchymal stem cells primed for adipogenesis. Elife. 2015;4:e06821. doi: 10.7554/eLife.06821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNicola GM, Chen PH, Mullarky E, Sudderth JA, Hu Z, Wu D, Tang H, Xie Y, Asara JM, Huffman KE, et al. NRF2 regulates serine biosynthesis in non-small cell lung cancer. Nat Genet. 2015;47:1475–1481. doi: 10.1038/ng.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran A, Amanchy R, Linares JF, Joshi J, Abu-Baker S, Porollo A, Hansen M, Moscat J, Diaz-Meco MT. p62 Is a Key Regulator of Nutrient Sensing in the mTORC1 Pathway. Molecular Cell. 2011;44:134–146. doi: 10.1016/j.molcel.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran A, Hernandez ED, Reina-Campos M, Castilla EA, Subramaniam S, Raghunandan S, Roberts LR, Kisseleva T, Karin M, Diaz-Meco MT, et al. p62/SQSTM1 by Binding to Vitamin D Receptor Inhibits Hepatic Stellate Cell Activity, Fibrosis, and Liver Cancer. Cancer Cell. 2016;30:595–609. doi: 10.1016/j.ccell.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascard P, Tlsty TD. Carcinoma-associated fibroblasts: orchestrating the composition of malignancy. Genes Dev. 2016;30:1002–1019. doi: 10.1101/gad.279737.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenific CM, Debnath J. Cellular and metabolic functions for autophagy in cancer cells. Trends Cell Biol. 2015;25:37–45. doi: 10.1016/j.tcb.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall AS, Xu S, Graeber TG, Braas D, Christofk HR. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat Commun. 2016;7:11457. doi: 10.1038/ncomms11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassot I, Segeral E, Berlioz-Torrent C, Durand H, Groussin L, Hai T, Benarous R, Margottin-Goguet F. ATF4 degradation relies on a phosphorylation-dependent interaction with the SCF(betaTrCP) ubiquitin ligase. Mol Cell Biol. 2001;21:2192–2202. doi: 10.1128/MCB.21.6.2192-2202.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares JF, Duran A, Reina-Campos M, Aza-Blanc P, Campos A, Moscat J, Diaz-Meco MT. Amino Acid Activation of mTORC1 by a PB1-Domain-Driven Kinase Complex Cascade. Cell Rep. 2015;12:1339–1352. doi: 10.1016/j.celrep.2015.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009;137:1001–1004. doi: 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT. Feedback on fat: p62-mTORC1-autophagy connections. Cell. 2011;147:724–727. doi: 10.1016/j.cell.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Karin M, Diaz-Meco MT. p62 in Cancer: Signaling Adaptor Beyond Autophagy. Cell. 2016;167:606–609. doi: 10.1016/j.cell.2016.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing G, Li B, Vu A, Skuli N, Walton ZE, Liu X, Mayes PA, Wise DR, Thompson CB, Maris JM, et al. ATF4 regulates MYC-mediated neuroblastoma cell death upon glutamine deprivation. Cancer Cell. 2012;22:631–644. doi: 10.1016/j.ccr.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers K, Fox MP, Bousamra M, 2nd, Slone SP, Higashi RM, Miller DM, Wang Y, Yan J, Yuneva MO, Deshpande R, et al. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J Clin Invest. 2015;125:687–698. doi: 10.1172/JCI72873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, Kremer D, Hwang RF, Witkiewicz AK, Ying H, et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature. 2016;536:479–483. doi: 10.1038/nature19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura A, He F, Taniguchi K, Nakagawa H, Yamachika S, Font-Burgada J, Zhong Z, Subramaniam S, Raghunandan S, Duran A, et al. p62, Upregulated during Preneoplasia, Induces Hepatocellular Carcinogenesis by Maintaining Survival of Stressed HCC-Initiating Cells. Cancer Cell. 2016;29:935–948. doi: 10.1016/j.ccell.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacanti NM, Divakaruni AS, Green CR, Parker SJ, Henry RR, Ciaraldi TP, Murphy AN, Metallo CM. Regulation of substrate utilization by the mitochondrial pyruvate carrier. Mol Cell. 2014;56:425–435. doi: 10.1016/j.molcel.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia T, Kim JY, Abu-Baker S, Moscat-Pardos J, Ahn CS, Reina-Campos M, Duran A, Castilla EA, Metallo CM, Diaz-Meco MT, et al. Metabolic reprogramming of stromal fibroblasts through p62-mTORC1 signaling promotes inflammation and tumorigenesis. Cancer Cell. 2014;26:121–135. doi: 10.1016/j.ccr.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis DN, Bobrovnikova-Marjon E, Diehl JA, Ron D, Koumenis C. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 2010;29:2082–2096. doi: 10.1038/emboj.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Palm W, Peng M, King B, Lindsten T, Li MO, Koumenis C, Thompson CB. GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes Dev. 2015;29:2331–2336. doi: 10.1101/gad.269324.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Fan J, Venneti S, Cross JR, Takagi T, Bhinder B, Djaballah H, Kanai M, Cheng EH, Judkins AR, et al. Asparagine plays a critical role in regulating cellular adaptation to glutamine depletion. Mol Cell. 2014;56:205–218. doi: 10.1016/j.molcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.