Abstract
Advances in neuroimaging have enabled the mapping of white matter connections across the entire brain, allowing for a more thorough examination of the extent of white matter disconnection after stroke. To assess how cortical disconnection contributes to motor impairments, we examined the relationship between structural brain connectivity and upper and lower extremity motor function in individuals with chronic stroke. Forty‐three participants [mean age: 59.7 (±11.2) years; time poststroke: 64.4 (±58.8) months] underwent clinical motor assessments and MRI scanning. Nonparametric correlation analyses were performed to examine the relationship between structural connectivity amid a subsection of the motor network and upper/lower extremity motor function. Standard multiple linear regression analyses were performed to examine the relationship between cortical necrosis and disconnection of three main cortical areas of motor control [primary motor cortex (M1), premotor cortex (PMC), and supplementary motor area (SMA)] and motor function. Anatomical connectivity between ipsilesional M1/SMA and the (1) cerebral peduncle, (2) thalamus, and (3) red nucleus were significantly correlated with upper and lower extremity motor performance (P ≤ 0.003). M1–M1 interhemispheric connectivity was also significantly correlated with gross manual dexterity of the affected upper extremity (P = 0.001). Regression models with M1 lesion load and M1 disconnection (adjusted for time poststroke) explained a significant amount of variance in upper extremity motor performance (R 2 = 0.36–0.46) and gait speed (R 2 = 0.46), with M1 disconnection an independent predictor of motor performance. Cortical disconnection, especially of ipsilesional M1, could significantly contribute to variability seen in locomotor and upper extremity motor function and recovery in chronic stroke. Hum Brain Mapp 39:120–132, 2018. © 2017 Wiley Periodicals, Inc.
Keywords: stroke, motor, diffusion tensor imaging, connectome, upper extremity, gait
INTRODUCTION
To improve prognosis of motor recovery following stroke and enhance targeting of therapeutic interventions, an increased understanding of the damage caused to both local and global neural networks poststroke is needed. Diffusion tensor imaging (DTI) enables the examination of the microstructural integrity and orientation of white matter in the brain in vivo by estimating the magnitude and directionality of water diffusion [Fung et al., 2011; Sundgren et al., 2004]. DTI can be used to visualize ischemic regions within motor tracts or to quantify remaining white matter integrity, thus offering important information for predicting motor impairment and recovery after stroke [Stinear et al., 2007]. Several studies using DTI techniques have demonstrated a correlation between upper extremity motor dysfunction and decreased integrity of the corticospinal tract (CST)—the major neural pathway for discrete voluntary movements, especially of the hands—in both acute [Feng et al., 2015; Jang et al., 2005a; Radlinska et al., 2010; Takenobu et al., 2014] and chronic [Chen and Schlaug, 2013; Lindenberg et al., 2010; Sterr et al., 2014; Zhu et al., 2010] stroke. However, more research needs to be performed to enhance our understanding of the relationship between the integrity of multiple motor tracts, not just the CST, and motor function in chronic stroke.
Motor recovery of the affected upper extremity has been highly studied, whereas less is known about motor recovery mechanisms involved with lower extremity and locomotor function after stroke. Human locomotion is a complex movement that requires a dynamic interplay between spinal central pattern generators and supraspinal locomotor centers. The extended brain network involved in locomotor control includes cortical (e.g., primary sensorimotor area, supplementary motor area, premotor cortex, anterior cingulate cortex, cingulate motor area, occipital visual areas), subcortical (e.g., basal ganglia, thalamus, midbrain tegmentum, cerebellum), and brainstem regions, highlighting the complex neuronal circuitry involved in locomotion [Jaeger et al., 2014; la Fougere et al., 2010; Takakusaki, 2017]. Some stroke survivors display locomotor ability despite complete lateral CST injury in the affected hemisphere [Ahn et al., 2006]. Indeed, while the CST is necessary for fine movements of the hands [Davidoff, 1990; Stinear et al., 2007], locomotion and motor function of the legs is less dependent on the CST [Dawes et al., 2008; Jang et al., 2005b]. Other descending neural pathways such as the reticulospinal, rubrospinal, and vestibulospinal tracts also contribute to locomotor function.
Upper and lower extremity motor dysfunction and recovery poststroke can be variable among individuals with similar lesion size and location, as the full extent of white‐matter injury may not be revealed by traditional structural MRI scans [Fridriksson et al., 2007; Thomalla et al., 2005]. Wallerian degeneration (WD), characterized by anterograde degeneration of the distal portion of axons after injury to the cell body and/or proximal nerve, commonly occurs after ischemic stroke. WD has been detected as early as within the first 2 weeks poststroke using DTI [Thomalla et al., 2004] and can eventually lead to fibrosis and atrophy of the fiber tracts. Several studies have examined WD of the CST in individuals with stroke and found that reduced fractional anisotropy (FA) and/or reduced signal (interpreted as a reflection of WD) along the CST were associated with increased motor impairment [Liang et al., 2008; Thomalla et al., 2004; Watanabe et al., 2001]. Wallerian degeneration may occur in other brain regions affected by the infarct, further impacting motor recovery after stroke.
In addition to altered white‐matter integrity in the lesioned area, brain regions and motor tracts beyond the infarction site may also exhibit structural abnormalities. These nonlesioned areas can be indirectly affected by the loss of connections resulting from a stroke and can become dysfunctional [Cramer and Nudo, 2010], contributing to behavioral deficits. Advances in neuroimaging have enabled the mapping of white‐matter connections across the entire brain (the brain connectome) [Bonilha et al., 2014a, 2014b] using diffusion MRI, allowing for a more thorough examination of the extent of white‐matter disconnection after stroke.
To date, the connectivity‐based approach developed by Bonilha et al. [2014b] has been used to examine overall structural disconnection in the lesioned hemisphere (vs the nonlesioned hemisphere) after ischemic stroke [Bonilha et al., 2014a] and to examine the relationship between language impairments and structural brain connectivity in individuals with chronic aphasia [Bonilha et al., 2014c]. Results showed intrahemispheric disconnection extending beyond the necrotic area in individuals with chronic stroke, highlighting that extent of reduced structural connectivity cannot be surmised based solely on necrotic tissue size or location [Bonilha et al., 2014a, 2014c]. Additionally, structural disconnection of Brodmann area 45 was independently associated with naming performance after controlling for necrotic tissue volume [Bonilha et al., 2014c]. This connectivity‐based approach, however, has not yet been used to examine the relationship between motor impairments and impaired cortical connectivity in chronic stroke. By examining neural connectivity using the brain connectome, the extent of cortical disconnection beyond the lesion site may be more fully revealed, expanding our understanding of motor impairment–brain structure relationships after stroke.
The objective of this study was to examine the relationship between upper and lower extremity motor impairment and structural brain connectivity in individuals with chronic stroke using the connectome‐mapping techniques recently developed by Bonilha et al. [2014c]. Selective disruption of corticofugal fibers from multiple cortical motor regions can impact functional reorganization and motor recovery following stroke [Newton et al., 2006]. Thus, we focused on cortico‐cortico and interhemispheric connectivity between three main cortical areas of motor control [primary motor cortex (M1), premotor cortex (PMC), and supplementary motor area (SMA)]. Additionally, we examined cortico‐subcortical connectivity between these three cortical areas and several important subcortical regions in motor control (e.g., cerebral peduncle, red nucleus, thalamus). Fibers from the CST originate from multiple cortical regions (mainly primary motor and premotor cortices) and course through the cerebral peduncle of the midbrain [Davidoff, 1990]. Neuroplastic changes in the red nucleus of the affected hemisphere have also been found poststroke [Dong et al., 2007; Yeo and Jang, 2010], which may indicate a compensatory role of this brain region in motor recovery. The thalamus is involved in the integration of sensory information and is closely linked to the cerebral cortex; it is a key relay station for sensory‐motor neuronal loops involving the cerebellum and basal nuclei, which are important for voluntary movement [Afifi and Bergman, 1998]. We hypothesized that (1) structural connectivity between cortical/subcortical motor‐relevant brain regions would be positively associated with upper and lower extremity motor performance, and (2) poorer performance on gait and upper and lower extremity motor measures would be associated with residual cortical necrosis and/or overall disconnection, or loss of connectivity, of ipsilesional cortical motor areas (i.e., M1, PMC, and SMA).
METHODS
Participants
Behavioral assessments were performed on 52 participants. Nine participants were excluded from analyses due to incomplete MRI data. A summary of demographic, clinical, and imaging data for the remaining 43 participants is presented in Table 1. The mean age of participants (16 female, 27 male) was 59.7 years (range 31–80 years), with a mean time poststroke of 64.4 months (range 10–284 months). All participants gave written informed consent to participate in this study that was approved by the Institutional Review Board at the University of South Carolina. As this study was part of a larger study investigating a range of impairments—including speech and language processing—in individuals with chronic stroke, inclusion criteria consisted of (1) monolingual speaker of English (prestroke), (2) occurrence of a single left hemispheric ischemic or hemorrhagic stroke at least 6 months prior to study inclusion, (3) able to follow simple instructions, and (4) able to walk 8 m with or without an assistive device and no physical assistance. Exclusion criteria included contraindication for MRI (claustrophobia, pregnancy, metal implants, etc.), clinically reported history of dementia, alcohol abuse, psychiatric disorder, other neurological conditions (e.g., traumatic brain injury, Parkinson's Disease), or extensive visual acuity or visual‐spatial problems.
Table 1.
Summary of demographic, behavioral, and imaging data
| Age, years | 59.7 (11.2); 31–80 |
| Femalea | 16 (37.2) |
| Time since stroke, months | 64.4 (58.5); 10–284 |
| M1 lesion load, %b | 21.3 (23.3); 0–91.0 |
| PMC lesion load, %b | 15.3 (25.5); 0–98.4 |
| SMA lesion load, %b | 6.4 (14.8); 0–59.4 |
| Box & Block TestAff | 30.6 (21.7); 0–64 |
| GripAff, kg | 23.46 (15.72); 0–56.67 |
| Arm Motricity IndexAff | 74.5 (29.2); 0–99 |
| Leg Motricity IndexAff | 76.2 (22.1); 28–99 |
| Gait Speed, m/s | 0.94 (0.31); 0.21–1.46 |
Values are presented as mean (SD); range.
n (percentage).
Ipsilesional hemisphere.
Abbreviations: yr, years; mo, months; M1, primary motor cortex; PMC, premotor cortex; SMA, supplementary motor area; Aff, affected extremity; kg, kilograms; m/s, meters per second.
Motor Assessment
All participants underwent a comprehensive behavioral assessment of upper and lower extremity motor function, both on the affected (Aff) and unaffected sides, and gait performed by a licensed physical therapist. The Box and Block Test (BBT) [Chen et al., 2009; Desrosiers et al., 1994] was used to assess gross manual dexterity of the hand, and a handheld dynamometer was used to assess grip strength (average of 3 trials) [Bohannon, 1986; Riddle et al., 1989]. The Motricity Index (MI) [Collin and Wade, 1990; Demeurisse et al., 1980] was used to examine motor function/strength of the upper and lower extremities. Self‐selected walking speed was determined using the GAITRite [CIR Systems Inc., USA] electronic walkway (average of 3 trials) [Lewek and Randall, 2011; Stokic et al., 2009]. Assistive devices and/or orthotics commonly used with community ambulation were allowed for the walking speed assessment.
MRI Acquisition
All participants underwent scanning using a 3T Siemens Trio system with a 12‐element head coil at the McCausland Center for Brain Imaging (Columbia, SC) within 2 days of behavioral testing. High‐resolution 3D T1‐MRI scans [repetition time (TR) = 2,250 ms, inversion time (TI) 925 ms, echo time (TE) = 4.15 ms, flip angle = 9°, field of view (FOV) = 256 mm and voxel size = 1.0 × 1.0 × 1.0 mm] and 3D T2‐MRI scans [TR = 3,200 ms, TE = 212 ms, variable flip angle, FOV = 256 mm, and voxel size = 1.0 × 1.0 × 1.0 mm] were acquired for determination of lesion size and location. DTI was performed with a single shot gradient echo planar imaging (EPI) sequence using the following parameters: TR = 4,987 ms, TE = 79.2 ms, flip angle (α) = 90°, FOV = 207 mm, voxel size = 2.3 × 2.3 × 2.3 mm, slice thickness = 2.3 mm, 36 volumes with noncollinear diffusion directions at a b value of 1,000 s/mm2 and 5 volumes with a b value of 0, number of slices = 50. This diffusion sequence was acquired twice, with the second series reversing the phase‐encoding direction.
Image Preprocessing
Stroke lesions were manually outlined by a neurologist (Bonilha) on the T2 image, which was then coregistered to the T1 image. T1‐weighted images were normalized into standard MNI space utilizing enantiomorphic unified segmentation–normalization routines as part of the software Statistical Parametric Mapping (SPM) 12 [Rorden et al., 2012]. This step also provided probabilistic gray‐ and white‐matter maps in standard space [Ashburner and Friston, 2005]. The inverse transformation was then applied to a John Hopkins University (JHU) template and to the gray and white matter probabilistic maps to transform these maps/template onto native T1 space. The probabilistic gray‐matter map (now in T1 space) was then segmented into a map of cortical/subcortical JHU regions of interest (ROIs), excluding lesioned voxels. The percentage of necrotic lesion damage to each motor cortical ROI was calculated by overlaying the manually outlined lesion onto the segmented cortical map in native T1 space. These steps were performed through in‐house scripts written in MATLAB and available online (https://github.com/neurolabusc/nii_preprocess) [Bonilha et al., 2014c].
The diffusion images were undistorted using FSL's TOPUP and Eddy tools [Andersson et al., 2003; Andersson and Sotiropoulos, 2016] with excess scalp removed using the FSL BET tool. FSL's dtifit tool was used to compute a fractional anisotropy (FA) map. To improve registration between T1 and DTI spaces, the scalp‐stripped (based on segmentation estimates) T1 image was nonlinearly normalized (using SPM12's “old normalization” function) to match the undistorted FA image. The same transformation matrix was applied to the map of segmented cortical ROIs and the probabilistic white‐matter map (which were in T1 space) to transform these maps into DTI space.
Probabilistic DTI tractography was performed using FDT's bedpostx and probtrackx [Behrens et al., 2007; Hernandez et al., 2013; Hernandez‐Fernandez et al., 2016] (with 5,000 streamline samples) to determine the number of white matter streamlines connecting each JHU ROI. For each possible pair of cortical/subcortical ROIs i and j, the number of iterative streamlines connecting the pair was computed for the creation of a connectivity matrix A, where each Aij entry represented the weighted link between ROIs (arriving at j when i was seeded and vice versa, with the streamlines being adjusted by distance travelled and the total number of streamlines divided by the sum of the volumes of the ROIs i and j) [Bonilha et al., 2014c; Gleichgerrcht et al., 2017]. For our analyses, M1 corresponded to the JHU precentral gyrus ROI, PMC corresponded to the JHU middle frontal gyrus–posterior segment ROI, and SMA corresponded to the JHU superior frontal gyrus–posterior segment ROI. The weighted sum of all connections to these three cortical areas was computed to assess overall connectivity of these cortical regions (M1, PMC, and SMA). Loss of connectivity implies the weighted strength of connectivity of a specific node (brain region), irrespective of which other node each weighted link is connected to. The percentage of fibers compared to the homologous region in the unaffected hemisphere was calculated to assess fiber reduction in these motor areas in the affected hemisphere, and to normalize the final connectivity measure based on each subject's contralateral hemisphere.
To demonstrate the anatomical location of the most important white‐matter connections, we reconstructed subcortical pathways in 59 healthy controls (45 female, mean age 54.7 ± 8.3 years) with a similar age distribution as the stroke survivors included in this study (DTI parameters: twice‐refocused echo‐planar imaging b = 0, 1,000, 30 diffusion encoding directions, TR = 8,500 ms, TE = 98 ms, FOV = 222 × 222 mm2, matrix = 74 × 74, 3 mm slice thickness, and 40 axial slices; DSI studio: fiber reconstruction using Q‐Space Diffeomorphic Reconstruction [Yeh and Tseng, 2011], 1.25 diffusion length sampling ratio, 2 mm output resolution). The white‐matter tract was transferred to standard space using 7, 9, and 7 transformation parameters (Fourier basis) and then combined across subjects. The resulting bundle was interpolated into 100 segments and the center of mass for each segment was calculated. The streamlines whose segment‐wise deviation from the center of mass was >0.5 standard deviations were removed.
Statistical Analyses
Two‐tailed paired t tests (or Wilcoxon signed rank tests if data were not normally distributed) were used to evaluate differences in motor scores between the affected and unaffected extremities, and hemispheric differences in anatomical connectivity among a subsection of the motor network (Fig. 1). Spearman's correlation analyses were performed to examine the relationship between structural connectivity amid these a priori ROIs (including interhemispheric connectivity between homologous regions) and motor function. Correlations were interpreted as poor (<0.25), fair (0.25–<0.5), moderate (0.5–0.75), and strong (>0.75) [Portney and Watkins, 2000]. Significance level was set at P < 0.05, corrected for multiple comparisons (corrected P ≤ 0.003).
Figure 1.
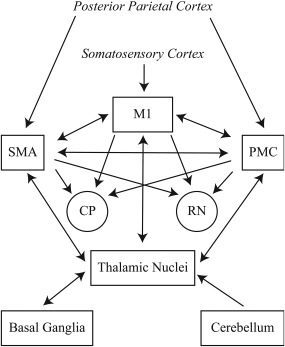
Subsection of the motor network. SMA, supplementary motor area; M1, primary motor cortex; PMC, premotor cortex; CP, cerebral peduncle; RN, red nucleus.
Standard multiple linear regression analyses were performed to examine the relationship between cortical necrosis and disconnection of M1, PMC, and SMA and upper/lower extremity motor performance. To normalize data for regression analyses, participants who scored 0 on BBT were removed as the BBT has a known floor effect [Chen et al., 2009; Desrosiers et al., 1994]. Similarly, participants who scored 99 on the MI for the affected upper/lower extremity were removed due to a ceiling effect [Collin and Wade, 1990; Demeurisse et al., 1980]. A reflect and square root transformation was then used with Arm MIAff data to obtain a normal distribution. Separate multiple linear regressions were performed for each behavioral measure, and included the clinical score as the dependent variable with the following independent variables: (1) percentage of necrotic lesion damage to each motor area and (2) percentage fiber number of each motor area. Each cortical motor area was analyzed separately. Significance level was set at P < 0.05, corrected based on the number (n = 5) of behavioral measures assessed (corrected P ≤ 0.01).
RESULTS
Behavioral Measures
Individuals showed reduced gross manual dexterity of the affected hand [median BBTAff score = 34.5 (interquartile range (IQR), 7.25–46.5), median BBTUnaffected score = 51.0 (IQR, 44.0–56.25), P < 0.001], decreased grip strength (GripAff = 23.46 ± 15.72 kg, GripUnaffected = 34.64 ± 10.51 kg, P < 0.001), and impaired upper extremity motor function [median Arm MIAff score = 88 (IQR, 56.5–100), median Arm MIUnaffected score = 100 (IQR, 100–100), P < 0.001]. The affected lower extremity also exhibited significant residual motor impairment [median Leg MIAff score = 79 (IQR, 59–100), median Leg MIUnaffected score = 100 (IQR, 100–100), P < 0.001]. Average gait speed was 0.94 ± 0.31 m/s.
For the subgroup of participants with normalized data for the regression analyses, average BBTAff score was 37.8 ± 17.4 (n = 34), average Arm MIAff score was 59.8 ± 28.1 (n = 25), and average Leg MIAff score was 64.9 ± 18.4 (n = 28).
Lesion Location
All participants exhibited a cortical/subcortical lesion in the left hemisphere, broadly distributed within the territory of the middle cerebral artery. For one participant, the lesion was too small to adequately outline; all other lesions were clearly visible on T2‐weighted images. Locations of maximal lesion overlap were the extranuclear and subgyral areas (Fig. 2).
Figure 2.
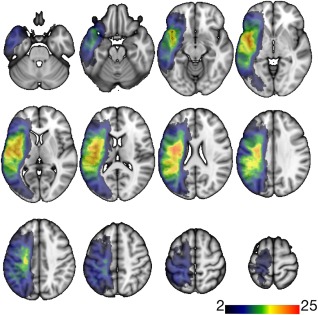
Lesion overlay. This figure demonstrates the overlay of the lesions in standard space from all subjects included in this study. The colored areas indicate the number of subjects whose lesions encompassed that voxel.
Lesion Size and Motor Impairment
Total lesion volume was not significantly correlated (Spearman's rho) with motor function of the affected extremity on BBT (r = −0.17, P = 0.29), grip strength (r = 0.10, P = 0.54), arm MI (r = −0.15, P = 0.37), leg MI (r = −0.06, P = 0.71), or with gait speed (r = 0.15, P = 0.35).
Cortical/Subcortical Connectivity and Motor Impairment
Anatomical reciprocal connectivity within the lesioned hemisphere between the following a priori ROIs was decreased overall compared with the homologous connectivity in the nonlesioned hemisphere: M1 ↔ thalamus (P = 0.002); PMC ↔ thalamus (P = 0.001); SMA ↔ to thalamus (P < 0.001); M1 ↔ cerebral peduncle (P = 0.002); SMA ↔ cerebral peduncle (P = 0.001); M1 ↔ PMC (P < 0.001); and caudate nucleus ↔ thalamus (P < 0.001). Connectivity between the remaining ROIs was not significantly different between hemispheres (P > 0.003).
Correlations between motor function and structural connectivity of a priori ROIs (lesioned hemisphere and interhemispheric) are presented in Table 2. Anatomical connectivity between ROIs corresponding to two cortical motor areas (M1 and SMA) and the (1) cerebral peduncle and (2) thalamus were significantly correlated with motor performance across all behavioral measures (P ≤ 0.003), with BBTAff exhibiting the strongest correlations. Structural connectivity between M1/SMA and the RN was significantly correlated with motor performance across all upper extremity measures and one lower extremity measure (Leg MIAff). In terms of basal ganglia connectivity, anatomical connectivity between the putamen and thalamus was significantly correlated with walking function (gait speed, r = 0.43, P = 0.003). Cortico‐cortico connectivity analyses revealed a significant correlation between M1 ↔ SMA connectivity and grip strength (r = 0.46, P = 0.001) and BBTAff (r = 0.42, P = 0.003). Interhemispheric connectivity between M1 ↔ M1 was also significantly correlated with gross manual dexterity of the affected upper extremity (BBTAff, r = 0.46, P = 0.001). Using a normative nonstroke database with a similar age distribution as our stroke sample, Figure 3 depicts the anatomical location of the white‐matter tracts most significantly associated with motor function abnormalities.
Table 2.
Nonparametric correlations between motor function and structural connectivity of a priori ROIs
| Behavioral measures | |||||
|---|---|---|---|---|---|
| Structural connectivity of a priori ROIs | BBTAff (n = 42) | GripAff (n = 42) | Arm MIAff (n = 40) | Leg MIAff (n = 42) | Gait speed (n = 40) |
| Ipsilesional Hemisphere | |||||
| M1 ↔ PMC | 0.35 | 0.24 | 0.28 | 0.17 | 0.10 |
| M1 ↔ SMA | 0.42* | 0.46* | 0.41 | 0.38 | 0.25 |
| PMC ↔ SMA | 0.31 | 0.22 | 0.22 | 0.14 | −0.05 |
| M1 ↔ CP | 0.74* | 0.67* | 0.67* | 0.63* | 0.55* |
| PMC ↔ CP | 0.45* | 0.30 | 0.45* | 0.41 | 0.34 |
| SMA ↔ CP | 0.66* | 0.56* | 0.63* | 0.63* | 0.54* |
| M1 ↔ RN | 0.53* | 0.49* | 0.49* | 0.43* | 0.24 |
| PMC ↔ RN | 0.35 | 0.28 | 0.35 | 0.34 | 0.23 |
| SMA ↔ RN | 0.52* | 0.42* | 0.46* | 0.45* | 0.38 |
| M1 ↔ Thalamus | 0.65* | 0.56* | 0.61* | 0.66* | 0.54* |
| PMC ↔ Thalamus | 0.39 | 0.21 | 0.37 | 0.37 | 0.23 |
| SMA ↔ Thalamus | 0.65* | 0.57* | 0.63* | 0.64* | 0.46* |
| Basal Ganglia & Cerebellum | |||||
| Caudate ↔ Thalamus | 0.05 | −0.07 | 0.10 | 0.10 | −0.09 |
| Putamen ↔ Thalamus | 0.37 | 0.36 | 0.27 | 0.40 | 0.43* |
| GP ↔ Thalamus | 0.23 | 0.29 | 0.25 | 0.33 | 0.36 |
| Cerebellum ↔ Thalamus | 0.50* | 0.51* | 0.43* | 0.48* | 0.39 |
| Interhemispheric Connectivity | |||||
| M1 ↔ M1 | 0.46* | 0.30 | 0.31 | 0.30 | 0.14 |
| PMC ↔ PMC | 0.24 | 0.01 | 0.16 | 0.08 | 0.06 |
| SMA ↔ SMA | 0.35 | 0.14 | 0.22 | 0.21 | 0.23 |
Values in the table are Spearman's coefficients (r).
Abbreviations: ROIs, regions of interest; BBTAff, Box and Block Test (affected extremity); GripAff, grip strength (affected extremity); Arm MIAff, Arm Motricity Index score (affected extremity); Leg MIAff, Leg Motricity Index score (affected extremity); M1, primary motor cortex; PMC, premotor cortex; SMA, supplementary motor area; CP, cerebral peduncle; RN, red nucleus; GP, globus pallidus.
*Significant at corrected P ≤ 0.003.
Figure 3.
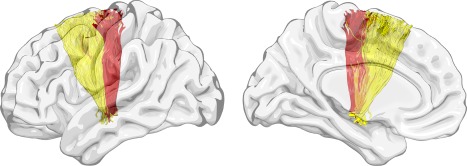
Tracts. Using reconstructed pathways from a normative nonstroke database of 59 healthy controls, this figure demonstrates the anatomical location of the white‐matter tracts most significantly associated with upper and lower extremity motor function abnormalities (e.g., BBTAff, gait speed). The tracts colored red are the connections from M1 (precentral gyrus) to the CP, those in orange from M1 (precentral gyrus) to the RN, and those in yellow from SMA (superior frontal gyrus) to the CP. BBTAff, Box and Block Test (affected extremity); M1, primary motor cortex; CP, cerebral peduncle; RN, red nucleus; SMA, supplementary motor area.
Multiple Regression Analyses
Results of the regression analyses are summarized in Table 3. For upper extremity motor performance, a model composed of M1 lesion load and M1 disconnection (adjusted for time poststroke) explained a significant amount of variance in BBTAff score [R 2 = 0.46, F(3,29) = 8.25, P < 0.001; β LL = −0.085, P = 0.57.; β disconnection = 0.531, P = 0.001], GripAff strength [R 2 = 0.36, F(3,37) = 7.04, P = 0.001; β LL = 0.051, P = 0.76; β disconnection = 0.611, P < 0.001], and arm MIAff score [R 2 = 0.43, F(3,20) = 5.02, P = 0.009; β LL = 0.031, P = 0.88; β disconnection = −0.560, P = 0.007]. Whereas M1 disconnection was an independent predictor of upper extremity motor performance across all three measures, M1 lesion load was not independently associated with upper extremity motor performance. Moreover, the R 2 for M1 disconnection was higher than those for most connections between M1 and relevant motor regions reported in the previous section. We also observed a trend toward significance between BBTAff score and a model composed of SMA lesion load and SMA disconnection [R 2 = 0.31, F(3,27) = 4.09, P = 0.016; β LL = −0.203, P = 0.38; β disconnection = 0.253, P = 0.167]; however, neither SMA lesion load or disconnection was an independent predictor of BBTAff score. Upper extremity motor performance was not associated with a model composed of PMC lesion load and disconnection.
Table 3.
Multiple regression analyses of cortical necrosis and disconnection and upper/lower extremity motor performance (adjusted for time post‐stroke)
| Behavioral measure | Predictors | R 2 | F statistic | P value for F | β LL | β disconnection |
|---|---|---|---|---|---|---|
| BBTAff (n = 33) | ||||||
| M1 | LLM1, % fiber number M1 | 0.46 | 8.25 | 0.000 | −0.085 | 0.531** |
| PMCb | LLPMC, % fiber number PMC | 0.11 | 1.13 | 0.353 | 0.085 | −0.019 |
| SMAc | LLSMA, % fiber number SMA | 0.31 | 4.09 | 0.016 | −0.203 | 0.253 |
| GripAff (n = 41) | ||||||
| M1 | LLM1, % fiber number M1 | 0.36 | 7.04 | 0.001 | 0.051 | 0.611** |
| PMCd | LLPMC, % fiber number PMC | 0.08 | 1.00 | 0.404 | 0.240 | 0.127 |
| SMA | LLSMA, % fiber number MA | 0.19 | 2.88 | 0.049 | −0.045 | 0.388* |
| Arm MIAff (n = 24)a | ||||||
| M1 | LLM1, % fiber number M1 | 0.43 | 5.02 | 0.009 | 0.031 | −0.560** |
| PMCb | LLPMC, % fiber number PMC | 0.32 | 2.99 | 0.057 | 0.500 | 0.455 |
| SMA | LLSMA, % fiber number SMA | 0.35 | 3.62 | 0.031 | 0.365 | −0.254 |
| Leg MIAff (n=27) | ||||||
| M1 | LLM1, % fiber number M1 | 0.18 | 1.63 | 0.209 | 0.112 | 0.464* |
| PMC | LLPMC, % fiber number PMC | 0.02 | 0.15 | 0.929 | 0.043 | 0.041 |
| SMA | LLSMA, % fiber number SMA | 0.07 | 0.58 | 0.634 | −0.167 | 0.151 |
| Gait speed (n = 39) | ||||||
| M1 | LLM1, % fiber number M1 | 0.46 | 9.75 | 0.000† | 0.398* | 0.557** |
| PMC | LLPMC, % fiber number PMC | 0.23 | 3.43 | 0.027 | 0.408 | 0.099 |
| SMAc | LLSMA, % fiber number SMA | 0.19 | 2.65 | 0.065 | −0.273 | 0.163 |
Abbreviations: BBTAff, Box and Block Test (affected extremity); GripAff, grip strength (affected extremity); Arm MIAff, Arm Motricity Index score (affected extremity); Leg MIAff, Leg Motricity Index score (affected extremity); M1, primary motor cortex; PMC, premotor cortex; SMA, supplementary motor area; LL, lesion load.
†Significant at corrected P ≤ 0.01; **P ≤ 0.01; *P ≤ 0.05.
Arm MIAff behavioral scores reflect and square root transformed to normalize behavioral data.
One outlier removed.
Two outliers removed.
Four outliers removed.
In terms of lower extremity motor performance, a significant relationship was found between gait speed and a model composed of M1 lesion load and M1 disconnection (adjusted for time poststroke) [R 2 = 0.46, F(3,35) = 9.75, P < 0.001; β LL = 0.398, P = 0.011; β disconnection = 0.557, P < 0.001]. Both lesion load and disconnection were independent predictors of gait speed. Necrosis and disconnection of PMC or SMA were not associated with lower extremity motor performance.
DISCUSSION
Preservation of the integrity of the CST and additional corticofugal fibers is important for motor recovery in chronic stroke [Lindenberg et al., 2010; Sterr et al., 2014; Ward et al., 2006]. In addition to direct disruption of these tracts by lesioned necrotic tissue, broader structural changes have been shown to occur in the nonlesioned motor network following stroke [Lindenberg et al., 2012; Liu et al., 2015; Schaechter et al., 2009]. In this study, we demonstrated that both upper and lower extremity motor performance in chronic stroke was positively associated with structural connectivity between several ipsilesional brain regions involved in motor control (e.g., M1/SMA and the cerebral peduncle, red nucleus, and thalamus). Furthermore, cortical disconnection of M1 was independently associated with motor function of the affected upper extremity and gait speed. This study is novel in that it used innovative methodology to examine cortical/subcortical disconnection, and showed that cortical disconnection (controlling for extent of necrosis) contributes to both upper and lower extremity motor impairments in individuals with chronic stroke.
Structural Connectivity of Ipsilesional Motor Network
Anatomical reciprocal connectivity within the lesioned hemisphere was significantly reduced (vs the nonlesioned hemisphere) between cortical motor areas and the cerebral peduncle and thalamus. There were no significant hemispheric differences, however, in connectivity between cortical regions and the red nucleus, as well as between SMA and M1/PMC. This may reflect adaptive changes in structural connectivity between these three cortical regions and the red nucleus, as well as changes in cortico‐cortico connectivity in the lesioned hemisphere following stroke. The red nucleus is the origin of the rubrospinal tract, which is less defined, but still present, in humans compared to nonhuman primates [Mamata et al., 2002; Yang et al., 2011]. Afferent input to the red nucleus includes fibers from the cerebral cortex and cerebellum, and it is believed the rubrospinal tract is an indirect pathway by which the cortex and cerebellum can influence motor neurons in the spinal cord [Snell, 2001; Yeo and Jang, 2010]. Previous studies in both nonhuman primates [Belhaj‐Saif and Cheney, 2000; Sinkjaer et al., 1995] and humans [Ruber et al., 2012; Takenobu et al., 2014] suggest that remodeling and neuroplastic changes occur in the red nucleus during the early stages of stroke (and possibly later) and are still evident in the chronic stage. These changes may indicate compensation for CST injury and contribute to motor recovery. Our results showed that variability in upper and lower extremity motor function was associated with variability in structural connectivity between ipsilesional M1/SMA and the red nucleus, suggesting that connectivity between these cortical regions and midbrain nucleus may play a role in motor recovery in chronic stroke.
Correlation With Motor Status
Connectivity between ipsilesional M1/SMA and the cerebral peduncle positively correlated with motor function across all upper/lower extremity behavioral measures, while connectivity between PMC and the cerebral peduncle only correlated with upper extremity motor function (BBTAff and Arm MIAff). These results are consistent with previous studies demonstrating selective disruption of corticofugal fibers from multiple motor regions can impact functional reorganization and motor recovery following stroke [Newton et al., 2006]. Microstructural integrity of CST pathways originating from primary and secondary cortical motor areas in the affected hemisphere have been shown to be reduced in chronic stroke, with grip strength strongly related to the integrity of fibers originating from the primary motor and (to a lesser extent) dorsal premotor cortices [Schulz et al., 2012]. Additionally, we found structural connectivity between ipsilesional M1/SMA and the thalamus was significantly correlated with motor function across all behavioral measures. This finding is not surprising, as the thalamus is a main relay station for sensory‐motor neuronal loops and is closely connected to the cerebral cortex [Afifi and Bergman, 1998].
Investigation of the influence of basal nuclei connectivity on motor performance revealed a significant correlation only between gait speed and structural connectivity between the ipsilesional putamen and thalamus, indicating a potential role of the basal ganglia in lower extremity motor recovery following stroke. These nuclei have extensive connections with many different regions of the brain and are known to play an important role in postural control and voluntary movement [Afifi and Bergman, 1998]. Previous research has demonstrated increased putamen activation during imagined standing and walking in healthy adults [Jahn et al., 2004]. Moreover, damage to the putamen has been associated with temporal gait asymmetry [Alexander et al., 2009] and decreased walking speed [Reynolds et al., 2014] in individuals with chronic stroke, highlighting basal ganglia involvement in modulation of gait.
The findings of this study provide further support to the current knowledge of brain regions involved in locomotor control. Afferent input converges on subcortical (e.g., thalamus) and cortical regions and is then transmitted to motor regions (e.g., SMA) to produce or modify motor programs [Takakusaki, 2017]. Cortical locomotor commands occur primarily from the primary motor cortex, while other brains regions such as prefrontal supplementary motor areas and basal ganglia may provide an indirect pathway for planning and modulation of locomotion [la Fougere et al., 2010]. Thus, structural connectivity between these brain regions is important for supraspinal control of locomotion.
Interhemispheric structural connectivity between M1s was also significantly and positively correlated with upper extremity dexterity (BBTAff score), suggesting that communication between primary motor cortices is important for upper extremity movement and dexterity of the hand post‐stroke. Structural connectivity between M1s is crucial for interhemispheric inhibition, a process in which activity in M1 of one hemisphere inhibits activity in the homologous region of the opposite hemisphere to execute unimanual movements and coordinated bimanual tasks [Duque et al., 2005; Johansen‐Berg et al., 2007; Perez and Cohen, 2009]. Previous studies have found chronic upper extremity motor impairment is associated with reduced white‐matter integrity of transcallosal fibers [Chen and Schlaug, 2013; Lindenberg et al., 2012]. Functional neuroimaging studies have also demonstrated decreased functional connectivity [Carter et al., 2010; Wang et al., 2012] and impaired interhemispheric inhibition [Murase et al., 2004; Perez and Cohen, 2009] between motor cortices is associated with upper extremity motor impairment poststroke. Our results could also suggest a role for contralesional M1 involvement in upper extremity motor recovery, which is supported by previous studies investigating functional connectivity and cortical reorganization following stroke [Lotze et al., 2012; Schaechter et al., 2008].
Cortical Disconnection and Motor Performance
Our findings reveal that cortical disconnection occurs alongside cortical necrosis, and that cortical disconnection is independently associated with upper/lower extremity motor performance. We observed that grip strength and motor function (BBT and MI) of the affected upper extremity was associated with preservation of cortical integrity and connectivity of ipsilesional M1; gross manual dexterity (BBT) of the affected hand also trended toward an association with preservation of SMA cortical integrity and connectivity. Furthermore, preserved cortical connectivity of M1 was an independent predictor of upper extremity motor performance across all measures, after controlling for lesion load. As the CST is the major neural pathway for skilled, discrete voluntary movements (especially for fine movements of the hands) [Davidoff, 1990], preservation of white‐matter fibers supporting ipsilesional M1 is important as approximately one‐third of CST fibers arise from M1 [Snell, 2001]. The SMA is connected directly via bidirectional pathways with the ipsilateral primary motor, premotor, and somatosensory cortices and indirectly receives subcortical input mainly from the basal ganglia via corticothalamic pathways [Afifi and Bergman, 1998]. It has direct connections with spinal motor neurons which innervate the hand [Dum and Strick, 2002], and therefore can play an augmented functional role in producing simple hand movements poststroke. The SMA is important in the temporal/sequential organization of movement and becomes more significant in the execution of simple motor tasks if the primary motor cortex is injured [Afifi and Bergman, 1998; Mintzopoulos et al., 2009].
Previous data are mixed concerning the relationship between lesion volume and motor impairment and function. Several studies have found motor impairment after stroke is associated with lesion size, [Saver et al., 1999; Schiemanck et al., 2006] while others have not found a significant correlation [Page et al., 2013; Sterr et al., 2014]. Degree of lesion overlap with the CST is often a much better predictor of motor impairment and function than overall lesion volume [Pineiro et al., 2000; Zhu et al., 2010]. There is great variability, however, in clinical manifestation and recovery from stroke in the chronic phase. Our results found that lesion size alone was not correlated with chronic motor impairment, and that cortical disconnection of motor regions, especially of ipsilesional M1, could significantly contribute to the variability seen in upper extremity motor function/recovery. The extent of brain damage poststroke may therefore be underestimated by examination of the necrotic tissue alone if more salient, global effects on the motor network are not taken into account.
Gait speed was also associated with preservation of cortical integrity and connectivity of ipsilesional M1. Both lesion load and connectivity of this brain region were independently associated with gait speed, with cortical disconnection being a stronger predictor. Motor recovery of the affected upper extremity has been highly studied, whereas less is known about the motor recovery mechanisms involved with lower extremity and locomotor function after stroke. While the CST is necessary for fine movements of the hands [Davidoff, 1990; Stinear et al., 2007], locomotion and motor function of the legs is less dependent on the CST [Ahn et al., 2006; Dawes et al., 2008; Jang et al., 2005b]. Some studies have suggested that the lateral CST does not play a central role in basic locomotor function in primates or humans [Ahn et al., 2006; Jang, 2010] but rather it is involved in “skilled walking,” or the adaption of gait kinematics to environmental demands [Jang, 2009], and may be more strongly associated with temporal parameters of gait [Capaday, 2002; Dawes et al., 2008]. Other descending neural pathways such as the corticoreticulospinal, rubrospinal, and vestibulospinal tracts could contribute to locomotor function [Barthelemy et al., 2015; Jang et al., 2013]. Furthermore, the neural control of upper and lower limb movements is not analogous, as spinal interneurons play a more important role in the central pattern generation of gait [Barbeau and Rossignol, 1987; Lovely et al., 1986] while fine hand movements are primarily under cerebral control. Cortical reorganization following stroke, therefore, is most likely different for lower limb function compared to what has been demonstrated with upper limb function. Our results suggest that while we might not know the precise pathways that play a role in locomotor recovery, preservation of white matter fibers supporting ipsilesional M1 is important for locomotor function in individuals with chronic stroke.
Overall, these results complement the findings of Bonilha et al. [2014c] related to language impairments and cortical disconnection after stroke, and further highlight the discrepancy of brain regions that appear intact on structural scans but actually exhibit reduced structural connectivity that may contribute to motor impairments poststroke. Improving our insight and understanding of the broader structural and functional changes that occur beyond the direct lesion can help improve motor recovery prognosis and target therapeutic interventions in a more tailored fashion for stroke survivors, thereby improving the clinical management of chronic mobility impairments in this patient population. More research needs to be performed to clarify the mechanisms that underlie changes in structural integrity and connectivity poststroke, and examine training‐induced changes in structural connectivity of motor networks in both subacute and chronic stroke.
This study is one of the first to incorporate a large sample size with left hemisphere necrotic damage when examining the relationship between motor function and structural brain connectivity in chronic stroke. Motor attention [Rushworth et al., 2003], action selection [Stewart et al., 2014], and task complexity [Haaland et al., 2004] are predominantly associated with activation in the left rather than right hemisphere. Left hemispheric lesions are particularly disruptive to more complex movement sequences [Haaland and Harrington, 1996] and selection of movements [Rushworth et al., 1998]. Therefore, our findings related to ipsilesional motor network integrity and connectivity and the relationship to motor function involving more complex tasks (e.g., Box and Block Test, locomotion) may not generalize to right hemispheric stroke. Examining structural brain connectivity and correlates of motor function in persons with right hemispheric stroke is also imperative. The results of our study, however, should be interpreted in the context of the limitations of DTI tractography (e.g., false tracking in low anisotropic areas and/or regions with fiber complexity and crossing) [Assaf and Pasternak, 2008; Tuch et al., 2002]. Additionally, our ROIs were defined by the atlas we used, thus limiting our ability to examine certain structures in more detail (e.g., dividing the thalamus into separate nuclei). Other studies have used alternative analysis techniques that incorporate different ROI parameters or evaluate different subsections of white matter tracts and/or brain regions [Lindenberg et al., 2010; Schaechter et al., 2009]. Lastly, as this study was part of a larger study investigating lesion‐impairment mapping of speech and language processing in individuals with chronic stroke, the majority of participants had lesions involving language regions of the brain resulting in a large number of subjects with minimal motor impairment. Future studies involving lesions primarily distributed in sensorimotor areas may provide a broader range of motor impairments and enable the examination of structural disconnection related to more direct necrotic lesion damage to the motor network.
CONCLUSIONS
Our findings demonstrate that ipsilesional structural connectivity between several brain regions involved in the motor network (particularly between M1/SMA and the cerebral peduncle, red nucleus, and thalamus) is associated with both upper and lower extremity motor performance in individuals with chronic stroke. Furthermore, upper extremity motor function of the affected extremity and gait speed is dependent on the preservation of cortical integrity and connectivity of ipsilesional M1, with cortical disconnection of M1 being an independent predictor of motor function. Our findings highlight the importance of examining structural changes and cortical disconnection in the broader motor network poststroke. Such insight could enhance our understanding of the underlying factors contributing to motor impairments after stroke. Future research examining treatment‐induced changes in structural integrity and connectivity may provide insight into more global patterns of structural brain plasticity that could help clinicians and researchers better target therapeutic interventions to enhance motor recovery potential.
ACKNOWLEDGMENTS
The funding sources played no further role in the present work other than financial support to conduct the research.
The authors have no conflicts of interest to declare.
REFERENCES
- Afifi AK, Bergman RA (1998): Functional Neuroanatomy. New York, NY: McGraw‐Hill. [Google Scholar]
- Ahn YH, Ahn SH, Kim H, Hong JH, Jang SH (2006): Can stroke patients walk after complete lateral corticospinal tract injury of the affected hemisphere? Neuroreport 17:987–990. [DOI] [PubMed] [Google Scholar]
- Alexander LD, Black SE, Patterson KK, Gao F, Danells CJ, McIlroy WE (2009): Association between gait asymmetry and brain lesion location in stroke patients. Stroke 40:537–544. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Skare S, Ashburner J (2003): How to correct susceptibility distortions in spin‐echo echo‐planar images: Application to diffusion tensor imaging. NeuroImage 20:870–888. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Sotiropoulos SN (2016): An integrated approach to correction for off‐resonance effects and subject movement in diffusion MR imaging. NeuroImage 125:1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. NeuroImage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Pasternak O (2008): Diffusion tensor imaging (DTI)‐based white matter mapping in brain research: A review. J Mol Neurosci 34:51–61. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S (1987): Recovery of locomotion after chronic spinalization in the adult cat. Brain Res 412:84–95. [DOI] [PubMed] [Google Scholar]
- Barthelemy D, Willerslev‐Olsen M, Lundell H, Biering‐Sorensen F, Nielsen JB (2015): Assessment of transmission in specific descending pathways in relation to gait and balance following spinal cord injury. Prog Brain Res 218:79–101. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW (2007): Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage 34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhaj‐Saif A, Cheney PD (2000): Plasticity in the distribution of the red nucleus output to forearm muscles after unilateral lesions of the pyramidal tract. J Neurophysiol 83:3147–3153. [DOI] [PubMed] [Google Scholar]
- Bohannon RW (1986): Test‐retest reliability of hand‐held dynamometry during a single session of strength assessment. Phys Ther 66:206–209. [DOI] [PubMed] [Google Scholar]
- Bonilha L, Nesland T, Rorden C, Fillmore P, Ratnayake RP, Fridriksson J (2014a): Mapping remote subcortical ramifications of injury after ischemic strokes. Behav Neurol 2014:215380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Nesland T, Rorden C, Fridriksson J (2014b): Asymmetry of the structural brain connectome in healthy older adults. Front Psychiatry 4:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Rorden C, Fridriksson J (2014c): Assessing the clinical effect of residual cortical disconnection after ischemic strokes. Stroke 45:988–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C (2002): The special nature of human walking and its neural control. Trends Neurosci 25:370–376. [DOI] [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, Pope DL, Shulman GL, Corbetta M (2010): Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol 67:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HM, Chen CC, Hsueh IP, Huang SL, Hsieh CL (2009): Test‐retest reproducibility and smallest real difference of 5 hand function tests in patients with stroke. Neurorehabil Neural Repair 23:435–440. [DOI] [PubMed] [Google Scholar]
- Chen JL, Schlaug G (2013): Resting state interhemispheric motor connectivity and white matter integrity correlate with motor impairment in chronic stroke. Front Neurol 4:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin C, Wade D (1990): Assessing motor impairment after stroke: A pilot reliability study. J Neurol Neurosurg Psychiatry 53:576–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC, Nudo RJ (2010): Brain Repair After Stroke. New York, NY: Cambridge University Press. [Google Scholar]
- Davidoff RA (1990): The pyramidal tract. Neurology 40:332–339. [DOI] [PubMed] [Google Scholar]
- Dawes H, Enzinger C, Johansen‐Berg H, Bogdanovic M, Guy C, Collett J, Izadi H, Stagg C, Wade D, Matthews PM (2008): Walking performance and its recovery in chronic stroke in relation to extent of lesion overlap with the descending motor tract. Exp Brain Res 186:325–333. [DOI] [PubMed] [Google Scholar]
- Demeurisse G, Demol O, Robaye E (1980): Motor evaluation in vascular hemiplegia. Eur Neurol 19:382–389. [DOI] [PubMed] [Google Scholar]
- Desrosiers J, Bravo G, Hebert R, Dutil E, Mercier L (1994): Validation of the Box and Block Test as a measure of dexterity of elderly people: Reliability, validity, and norms studies. Arch Phys Med Rehabil 75:751–755. [PubMed] [Google Scholar]
- Dong Y, Winstein CJ, Albistegui‐DuBois R, Dobkin BH (2007): Evolution of FMRI activation in the perilesional primary motor cortex and cerebellum with rehabilitation training‐related motor gains after stroke: A pilot study. Neurorehabil Neural Repair 21:412–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL (2002): Motor areas in the frontal lobe of the primate. Physiol Behav 77:677–682. [DOI] [PubMed] [Google Scholar]
- Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG (2005): Transcallosal inhibition in chronic subcortical stroke. NeuroImage 28:940–946. [DOI] [PubMed] [Google Scholar]
- Feng W, Wang J, Chhatbar PY, Doughty C, Landsittel D, Lioutas VA, Kautz SA, Schlaug G (2015): Corticospinal tract lesion load: An imaging biomarker for stroke motor outcomes. Ann Neurol 78:860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Bonilha L, Rorden C (2007): Severe Broca's aphasia without Broca's area damage. Behav Neurol 18:237–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SH, Roccatagliata L, Gonzalez RG, Schaefer PW (2011): MR diffusion imaging in ischemic stroke. Neuroimaging Clin N Am 21:345–377, xi. [DOI] [PubMed] [Google Scholar]
- Gleichgerrcht E, Fridriksson J, Rorden C, Bonilha L (2017): Connectome‐based lesion‐symptom mapping (CLSM): A novel approach to map neurological function. NeuroImage Clin 16:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaland KY, Elsinger CL, Mayer AR, Durgerian S, Rao SM (2004): Motor sequence complexity and performing hand produce differential patterns of hemispheric lateralization. J Cogn Neurosci 16:621–636. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL (1996): Hemispheric asymmetry of movement. Curr Opin Neurobiol 6:796–800. [DOI] [PubMed] [Google Scholar]
- Hernandez M, Guerrero GD, Cecilia JM, Garcia JM, Inuggi A, Jbabdi S, Behrens TE, Sotiropoulos SN (2013): Accelerating fibre orientation estimation from diffusion weighted magnetic resonance imaging using GPUs. PLoS One 8:e61892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez‐Fernandez M, Reguly I, Giles M, Ibabdi S, Smith SM, Sotiropoulos SN (2016): A fast and flexible toolbox for tracking brain connections in diffusion MRI datasets using GPUs. 22nd Annual Meeting of the Organization for Human Brain Mapping (OHBM), Geneva, Switzerland.
- Jaeger L, Marchal‐Crespo L, Wolf P, Riener R, Michels L, Kollias S (2014): Brain activation associated with active and passive lower limb stepping. Front Hum Neurosci 8:828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn K, Deutschlander A, Stephan T, Strupp M, Wiesmann M, Brandt T (2004): Brain activation patterns during imagined stance and locomotion in functional magnetic resonance imaging. NeuroImage 22:1722–1731. [DOI] [PubMed] [Google Scholar]
- Jang SH (2009): The role of the corticospinal tract in motor recovery in patients with a stroke: A review. NeuroRehabilitation 24:285–290. [DOI] [PubMed] [Google Scholar]
- Jang SH (2010): Prediction of motor outcome for hemiparetic stroke patients using diffusion tensor imaging: A review. NeuroRehabilitation 27:367–372. [DOI] [PubMed] [Google Scholar]
- Jang SH, Chang CH, Lee J, Kim CS, Seo JP, Yeo SS (2013): Functional role of the corticoreticular pathway in chronic stroke patients. Stroke 44:1099–1104. [DOI] [PubMed] [Google Scholar]
- Jang SH, Cho SH, Kim YH, Han BS, Byun WM, Son SM, Kim SH, Lee SJ (2005a): Diffusion anisotrophy in the early stages of stroke can predict motor outcome. Restor Neurol Neurosci 23:11–17. [PubMed] [Google Scholar]
- Jang SH, You SH, Kwon YH, Hallett M, Lee MY, Ahn SH (2005b): Cortical reorganization associated lower extremity motor recovery as evidenced by functional MRI and diffusion tensor tractography in a stroke patient. Restor Neurol Neurosci 23:325–329. [PubMed] [Google Scholar]
- Johansen‐Berg H, Della‐Maggiore V, Behrens TE, Smith SM, Paus T (2007): Integrity of white matter in the corpus callosum correlates with bimanual co‐ordination skills. NeuroImage 36: T16–T21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Fougere C, Zwergal A, Rominger A, Forster S, Fesl G, Dieterich M, Brandt T, Strupp M, Bartenstein P, Jahn K (2010): Real versus imagined locomotion: A [18F]‐FDG PET‐fMRI comparison. NeuroImage 50:1589–1598. [DOI] [PubMed] [Google Scholar]
- Lewek MD, Randall EP (2011): Reliability of spatiotemporal asymmetry during overground walking for individuals following chronic stroke. J Neurol Phys Ther 35:116–121. [DOI] [PubMed] [Google Scholar]
- Liang Z, Zeng J, Zhang C, Liu S, Ling X, Xu A, Ling L, Wang F, Pei Z (2008): Longitudinal investigations on the anterograde and retrograde degeneration in the pyramidal tract following pontine infarction with diffusion tensor imaging. Cerebrovasc Dis 25:209–216. [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Betzler F, Alsop D, Schlaug G (2010): Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology 74:280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg R, Zhu LL, Ruber T, Schlaug G (2012): Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Hum Brain Mapp 33:1040–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Qin W, Zhang J, Zhang X, Yu C (2015): Enhanced interhemispheric functional connectivity compensates for anatomical connection damages in subcortical stroke. Stroke 46:1045–1051. [DOI] [PubMed] [Google Scholar]
- Lotze M, Beutling W, Loibl M, Domin M, Platz T, Schminke U, Byblow WD (2012): Contralesional motor cortex activation depends on ipsilesional corticospinal tract integrity in well‐recovered subcortical stroke patients. Neurorehabil Neural Repair 26:594–603. [DOI] [PubMed] [Google Scholar]
- Lovely RG, Gregor RJ, Roy RR, Edgerton VR (1986): Effects of training on the recovery of full‐weight‐bearing stepping in the adult spinal cat. Exp Neurol 92:421–435. [DOI] [PubMed] [Google Scholar]
- Mamata H, Mamata Y, Westin CF, Shenton ME, Kikinis R, Jolesz FA, Maier SE (2002): High‐resolution line scan diffusion tensor MR imaging of white matter fiber tract anatomy. AJNR Am J Neuroradiol 23:67–75. [PMC free article] [PubMed] [Google Scholar]
- Mintzopoulos D, Astrakas LG, Khanicheh A, Konstas AA, Singhal A, Moskowitz MA, Rosen BR, Tzika AA (2009): Connectivity alterations assessed by combining fMRI and MR‐compatible hand robots in chronic stroke. NeuroImage 47: T90–T97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG (2004): Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 55:400–409. [DOI] [PubMed] [Google Scholar]
- Newton JM, Ward NS, Parker GJ, Deichmann R, Alexander DC, Friston KJ, Frackowiak RS (2006): Non‐invasive mapping of corticofugal fibres from multiple motor areas–relevance to stroke recovery. Brain 129:1844–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page SJ, Gauthier LV, White S (2013): Size doesn't matter: Cortical stroke lesion volume is not associated with upper extremity motor impairment and function in mild, chronic hemiparesis. Arch Phys Med Rehabil 94:817–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Cohen LG (2009): Interhemispheric inhibition between primary motor cortices: What have we learned? J Physiol 587:725–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineiro R, Pendlebury ST, Smith S, Flitney D, Blamire AM, Styles P, Matthews PM (2000): Relating MRI changes to motor deficit after ischemic stroke by segmentation of functional motor pathways. Stroke 31:672–679. [DOI] [PubMed] [Google Scholar]
- Portney LG, Watkins MP (2000): Correlation. Foundations of Clinical Research: Applications to Practice, 2nd ed Upper Saddle River, NJ: Prentice Hall; p 494. [Google Scholar]
- Radlinska B, Ghinani S, Leppert IR, Minuk J, Pike GB, Thiel A (2010): Diffusion tensor imaging, permanent pyramidal tract damage, and outcome in subcortical stroke. Neurology 75:1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AM, Peters DM, Vendemia JM, Smith LP, Sweet RC, Baylis GC, Krotish D, Fritz SL (2014): Neuronal injury in the motor cortex after chronic stroke and lower limb motor impairment: A voxel‐based lesion symptom mapping study. Neural Regen Res 9:766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DL, Finucane SD, Rothstein JM, Walker ML (1989): Intrasession and intersession reliability of hand‐held dynamometer measurements taken on brain‐damaged patients. Phys Ther 69:182–194. [DOI] [PubMed] [Google Scholar]
- Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO (2012): Age‐specific CT and MRI templates for spatial normalization. NeuroImage 61:957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruber T, Schlaug G, Lindenberg R (2012): Compensatory role of the cortico‐rubro‐spinal tract in motor recovery after stroke. Neurology 79:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Johansen‐Berg H, Gobel SM, Devlin JT (2003): The left parietal and premotor cortices: Motor attention and selection. NeuroImage 20: S89–100. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Nixon PD, Wade DT, Renowden S, Passingham RE (1998): The left hemisphere and the selection of learned actions. Neuropsychologia 36:11–24. [DOI] [PubMed] [Google Scholar]
- Saver JL, Johnston KC, Homer D, Wityk R, Koroshetz W, Truskowski LL, Haley EC (1999): Infarct volume as a surrogate or auxiliary outcome measure in ischemic stroke clinical trials. The RANTTAS Investigators. Stroke 30:293–298. [DOI] [PubMed] [Google Scholar]
- Schaechter JD, Fricker ZP, Perdue KL, Helmer KG, Vangel MG, Greve DN, Makris N (2009): Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum Brain Mapp 30:3461–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaechter JD, Perdue KL, Wang R (2008): Structural damage to the corticospinal tract correlates with bilateral sensorimotor cortex reorganization in stroke patients. NeuroImage 39:1370–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemanck SK, Kwakkel G, Post MW, Prevo AJ (2006): Predictive value of ischemic lesion volume assessed with magnetic resonance imaging for neurological deficits and functional outcome poststroke: A critical review of the literature. Neurorehabil Neural Repair 20:492–502. [DOI] [PubMed] [Google Scholar]
- Schulz R, Park CH, Boudrias MH, Gerloff C, Hummel FC, Ward NS (2012): Assessing the integrity of corticospinal pathways from primary and secondary cortical motor areas after stroke. Stroke 43:2248–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkjaer T, Miller L, Andersen T, Houk JC (1995): Synaptic linkages between red nucleus cells and limb muscles during a multi‐joint motor task. Exp Brain Res 102:546–550. [DOI] [PubMed] [Google Scholar]
- Snell RS (2001): Clinical Neuroanatomy for Medical Students. Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- Sterr A, Dean PJ, Szameitat AJ, Conforto AB, Shen S (2014): Corticospinal tract integrity and lesion volume play different roles in chronic hemiparesis and its improvement through motor practice. Neurorehabil Neural Repair 28:335–343. [DOI] [PubMed] [Google Scholar]
- Stewart JC, Tran X, Cramer SC (2014): Age‐related variability in performance of a motor action selection task is related to differences in brain function and structure among older adults. NeuroImage 86:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD (2007): Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain 130:170–180. [DOI] [PubMed] [Google Scholar]
- Stokic DS, Horn TS, Ramshur JM, Chow JW (2009): Agreement between temporospatial gait parameters of an electronic walkway and a motion capture system in healthy and chronic stroke populations. Am J Phys Med Rehabil 88:437–444. [DOI] [PubMed] [Google Scholar]
- Sundgren PC, Dong Q, Gomez‐Hassan D, Mukherji SK, Maly P, Welsh R (2004): Diffusion tensor imaging of the brain: Review of clinical applications. Neuroradiology 46:339–350. [DOI] [PubMed] [Google Scholar]
- Takakusaki K (2017): Functional neuroanatomy for posture and gait control. J Mov Disord 10:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenobu Y, Hayashi T, Moriwaki H, Nagatsuka K, Naritomi H, Fukuyama H (2014): Motor recovery and microstructural change in rubro‐spinal tract in subcortical stroke. NeuroImage Clin 4:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomalla G, Glauche V, Koch MA, Beaulieu C, Weiller C, Rother J (2004): Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. NeuroImage 22:1767–1774. [DOI] [PubMed] [Google Scholar]
- Thomalla G, Glauche V, Weiller C, Rother J (2005): Time course of wallerian degeneration after ischaemic stroke revealed by diffusion tensor imaging. J Neurol Neurosurg Psychiatry 76:266–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ (2002): High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med 48:577–582. [DOI] [PubMed] [Google Scholar]
- Wang LE, Tittgemeyer M, Imperati D, Diekhoff S, Ameli M, Fink GR, Grefkes C (2012): Degeneration of corpus callosum and recovery of motor function after stroke: A multimodal magnetic resonance imaging study. Hum Brain Mapp 33:2941–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Thompson AJ, Greenwood RJ, Rothwell JC, Frackowiak RS (2006): Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain 129:809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Honda Y, Fujii Y, Koyama M, Matsuzawa H, Tanaka R (2001): Three‐dimensional anisotropy contrast magnetic resonance axonography to predict the prognosis for motor function in patients suffering from stroke. J Neurosurg 94:955–960. [DOI] [PubMed] [Google Scholar]
- Yang HS, Kwon HG, Hong JH, Hong CP, Jang SH (2011): The rubrospinal tract in the human brain: Diffusion tensor imaging study. Neurosci Lett 504:45–48. [DOI] [PubMed] [Google Scholar]
- Yeh FC, Tseng WY (2011): NTU‐90: A high angular resolution brain atlas constructed by q‐space diffeomorphic reconstruction. NeuroImage 58:91–99. [DOI] [PubMed] [Google Scholar]
- Yeo SS, Jang SH (2010): Changes in red nucleus after pyramidal tract injury in patients with cerebral infarct. NeuroRehabilitation 27:373–377. [DOI] [PubMed] [Google Scholar]
- Zhu LL, Lindenberg R, Alexander MP, Schlaug G (2010): Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke 41:910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]


