Abstract
INTRODUCTION
In North Africa and the Middle East (EMRO), studies about dementia prevalence are scarce. The Arabic validated 10/66 Dementia Research Group diagnostic assessment was used to assess the prevalence of dementia in Lebanon in a population-based study. The study also examined care arrangement and access to care.
METHODS
A random sample of 502 persons older than 65 years and their informant were recruited from Beirut and Mount Lebanon governorates though multi-stage cluster sampling.
RESULTS
The crude dementia prevalence was 7.4% and age-standardized dementia prevalence was 9.0%. People with dementia were mainly cared for by relatives at home. Access to formal care was very limited.
DISCUSSION
Dementia prevalence in Lebanon ranks high within the global range of estimates. These first evidence-based data about disease burden and barriers to care serve to raise awareness and call for social and health care reform to tackle the dementia epidemic in Lebanon and EMRO.
Keywords: Dementia, prevalence, Lebanon, dementia care
BACKGROUND
In the North Africa and the Middle East (EMRO), due to very rapid demographic aging, the estimated number of people with dementia is expected to grow exponentially, 2 million people in 2015 rising to 4 million in 2030 and 10 million in 2050 [1], an increase of 329% from 2015 through to 2050, the second fastest in the world. Currently, EMRO is estimated to have the highest age-standardized prevalence globally [1]. Due to lack of studies, these are best estimates based on consensus judgment of an international panel of experts [2] and studies from Egypt and Turkey [1]. More prevalence studies in the region are needed to assess disease burden, raise awareness, and provide evidence-based data to develop health promotion and disease prevention strategies.
One great challenge has been the lack of well-validated education- and culture-fair screening and diagnostic instrument. Illiteracy among the older generations in EMRO is high. For example, it was estimated in 2009 that about 50% of people 65 years and older in Lebanon were illiterate [3]. Therefore, prior to this study, we had validated the Arabic versions of the one-stage 10/66 Dementia Research Group (DRG) diagnostic assessment for dementia [4] and two brief screening instruments, the Rowland Universal Dementia Assessment Scale (RUDAS) [5] and the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) [6]. All three instruments were designed to minimize the effect of education and culture on cognitive assessment and extensively validated across languages and cultures. In the Arabic language, educational, and cultural context, we had demonstrated that the 10/66 DRG diagnostic assessment has excellent psychometric properties to diagnose dementia [7]. Still, it is time-consuming and the subtype classification has not been validated. Alternatively, a two-stage case ascertainment strategy can be used, first with a brief screening instrument to be followed by a comprehensive diagnostic evaluation to diagnose dementia and determine subtypes for those who are screened positive. For this purpose, we had also demonstrated that the Arabic version of the RUDAS and the IQCODE possess very good discriminatory ability to screen for dementia, and the DemeGraph harnessing the RUDAS and IQCODE has better discriminatory ability than either alone [8,9,10].
We aimed to conduct a pilot study in two governorates of Lebanon, using the Arabic validated 10/66 DRG diagnostic assessment for case ascertainment, to field test this one-stage diagnostic procedure, assess the feasibility of a subsequent nationwide cohort study, generate preliminary data about dementia prevalence, and gather data about care need, arrangement and access to care. To have an alternative strategy in case this one-stage diagnostic procedure failed, we also applied the Arabic validated RUDAS, IQCODE, and DemeGraph to the same study population and validated these screening instruments against the 10/66 DRG dementia diagnosis.
METHODS
1. Sample size and sampling frame
The long-term goal was to establish a nationwide community-based cohort of individuals older than 65 years randomly selected from all regions of Lebanon to provide a precise estimate of dementia prevalence and incidence in the country. Based on the estimated prevalence of 5% for EMRO [11] available at the start of the study in 2011, the estimated required sample size was 2500 persons to achieve a maximal error of ±1% with 95% confidence interval and account for 25% non-response rate. The targeted number of study participants would be distributed across the 25 districts within the six governorates of Lebanon according to the proportions of people older than 65 years in each district.
Given the aims of this pilot study, we collected data in two governorates: Beirut and Mount Lebanon (Chouf and Aley districts). We computed the sample size for the pilot study nested within the sample size of the national cohort described above (2500 persons), with Beirut represented 13.4% of the total sample, Aley 3.2%, and Chouf 4.3%. These percentages corresponded to the national distribution of people older than 65 years living in each area. Multiplying these percentages with 2500, the targeted numbers of participants were 335 for Beirut, 108 for Chouf and 80 for Aley, yielding a total targeted sample size of 523 participants (Figure 1). The rationale behind the nested design was to extend data collection to the rest of the country and complete the national cohort immediately after the pilot study. Furthermore, a minimum sample size of 500 persons was large enough to allow a maximal error of ± 2 with a 95% confidence interval based on the available estimated prevalence of 5% for EMRO at the start of the study.
Figure 1. Sampling frame for data collection.

The percentages represent the national distribution of people over 65 years old in Lebanon
A multi-stage cluster sampling was employed. For Beirut, an existing sampling frame designed for another survey was used in which Beirut governorate was divided into 594 clusters each containing 50 residential buildings with complete detailed household listing of 60 randomly chosen clusters [12]. For this pilot study, seven clusters were randomly selected from the 60 clusters with complete household listing. Within the selected clusters, the trained research workers, who were university graduates, systematically knocked on every door to recruit participants. In Chouf and Aley districts, since there was no existing sampling frame, a number of villages and towns was randomly chosen and weighted according to their respective sizes. The research workers door-knocked the selected households and interviewed any person who was 65 years old and above and one informant. The informant was defined as the person who knew best the selected older person. If the selected older person needed care and support, the main caregiver was chosen as the informant. If there was a paid caregiver, the informant was the person who organized and supervised the paid care.
2. Instruments
2.1. Diagnostic instrument for dementia
One stage 10/66 DRG diagnostic assessment: Both the older persona and the informant participated [4,7]
The Arabic validated 10/66 DRG diagnostic assessment has demonstrated excellent discriminatory ability to diagnose dementia among older people with low education: 92.0% sensitivity, 95.1% specificity, and 92.9% positive predictive value (PPV) [7]. This diagnostic assessment consists of 1) cognitive test battery: The Community Screening Instrument of Dementia (CSI-D) [13] and the Consortium to Establish a Registry of Alzheimer’s Disease (CERAD) animal naming tests and modified 10 world list learning [14], 2) the Geriatric Mental State (GMS), which applies a computerized algorithm to identify organic brain syndrome (dementia), schizophrenia, neurotic and psychotic depression, and anxiety neuroses [15], 3) physical assessment and brief neurological examination (NEUROEX) [16], and 4) informant interview on cognitive and functional decline (CSI-D informant interview) [13]. 10/66 DRG dementia diagnosis is defined as scoring above a cutoff point of predicted probability of DSM-IV dementia syndrome from the logistic regression equation using coefficients from the CSI-D, GMS, and modified CERAD 10-word list learning [4]. It takes about one hour to administer.
2.2. Screening instruments for dementia
2.2.a. Rowland Universal Dementia Assessment Scale (RUDAS): Administered to the older person [5]
The RUDAS contains six items: Body orientation, praxis, drawing, judgment, memory and language. It takes 10-15 minutes to administer with scores ranging from 0 to 30; higher scores indicate better cognitive function. The Arabic validated RUDAS has demonstrated good psychometric properties to screen for dementia: sensitivity 83%, specificity 85%, and PPV 79% at the cutoff score ≤ 22 [8]. However, there was one important drawback. Half of the cognitively intact participants with no formal education in this validation study [8 could not complete the visuoconstructional item (drawing a cube), since they had never learned to draw or even to hold a pen (data unpublished). Among those who tried to draw the cube, the three-dimensional figure became a meaningless juxtaposition of two-dimensional figures and the angles became rounded (data unpublished). There specific findings were corroborated by another study on visuoconstructional abilities among illiterate Turkish immigrants in Denmark [17. Keeping this in mind, we examined the performance of the RUDAS in a population-based setting and being administered by trained lay research workers.
2.2.b. 16-item Informant Questionnaire on Cognitive Decline in the Elderly: Administered to the informant [6]
The 16-item IQCODE contains 16 questions rating changes in cognitive function over a specified time frame. It takes 10-15 minutes to administer. Each question is rated on a 5-point scale from 1 (much improved) to 5 (much worse) with 3 representing no change. The sum of scores is averaged over the 16 questions. At the cut-off average score > 3.34, the Arabic validated IQCODE has demonstrated excellent psychometric properties: 92.5% sensitivity, 94.4% specificity, and 91.5 % PPV [9] to screen for dementia.
2.2.c. DemeGraph combining RUDAS and IQCODE
Using a logistic regression equation to combine RUDAS and IQCODE at the cut-off scores defined in the validation studies [8,9], the specificity to screen for dementia was improved to 97.4 % while a good sensitivity of 86.0% was maintained, and PPV was increased to 96% [10]. The overall predictive ability of the 10/66 DRG diagnostic assessment and the DemeGraph were comparable, both having AUC of 0.97 [10,7]. Although the IQCODE alone was proven to be an excellent screening instrument in the validation study with 40% of informants being health professionals caring for the participants residing in nursing homes, its excellent psychometric properties were most likely not reproducible in a population-based study where informants were essentially lay persons [9]. Previous studies have shown that although the IQCODE minimizes educational bias, it is biased by informant characteristics such as depression and anxiety, burden of care, and the quality of the relationship with older person [6]. Furthermore, direct cognitive assessment is essential in the diagnostic process for dementia. The DemeGraph thus provides a brief (20-30 minutes) alternative screening instrument that harnesses a cognitive assessment and an informant interview to yield very good psychometric properties.
3. Assessment of care need, care arrangement, and care burden
We used the 10/66 DRG survey questionnaires with adaptation to local contexts to collect data about demographics (age, sex, and marital status), socioeconomic status (education, income, and occupation), and care (needs, arrangement, and participation of informal caregivers) [18]. To evaluate access to care, the older participants were asked about their health seeking behavior, medical insurance, health care costs, source of ongoing care, and care provider [18]. The interview burden was about one hour.
5. Data analysis
Data was analyzed using IBM SPSS Statistics version 22. Crude, age- and gender-specific prevalences of dementia were calculated. Age-standardized prevalence of dementia was computed using the Lebanese age-specific prevalence and the Western European population structure in 2013 [19]. Using 10/66 DRG diagnosis as a gold standard, the psychometric properties of IQCODE, RUDAS, and DemeGraph to screen for dementia in a population-based setting were computed, using the cut-off scores from the validation studies [8,10,9]. Chi-square test was used to determine whether there was a significant association between categorical variables, while Fisher’s exact test was employed when the expected observation was less than five. Independent t-test was used to determine if there was a statistically significant difference between the means in two groups Significance level was set at p ≤ 0.05.
6. Ethics
The Institutional Review Board of the American University of Beirut, Lebanon, approved the study. All participants gave written informed consent, or relatives gave consent on behalf of participants with impaired decision making capacity. Decision making capacity was assessed in all participants through a checklist to ensure their complete comprehension about the nature of the study and the protection of their right.
RESULTS
1. Feasibility
Data collection started in May 2013 and concluded in January 2014. The response rate was 63.3%. Households with high economic status were not admissible to the research workers, consistent with experience from previous 10/66 DRG studies in developing countries. The interview length and content (two hours minimum) was acceptable to the participants. Thus, applying the 10/66 DRG one-stage diagnostic assessment and survey questionnaires in a population-based study proved to be feasible in Lebanon.
2. Sociodemographic
508 older persons underwent the interview. Six persons had no informant and were therefore excluded, leaving 502 older person-informant dyads for data analysis. The socio-demographic characteristics of the study population are presented in Table 1. The mean age of the older persons was 72.5 years (SD 7.2). There were slightly more females (56.2%). One in five older persons never had a formal education, which was lower than the national average for the age group above 65 years old (about 50 %) [3]. About 30% still worked after retirement age (64 years old in Lebanon); 42.3% had never worked, predominantly women (98.4%). Only 6.1% had had a well-paid job as manager or professional, while the rest (48.6%) had held low paying jobs (Table 1). About one quarter (26.7%) did not have enough income to cover their basic needs (Table 1), consistent with the estimated 27% of the Lebanese population currently living in poverty [20].
Table 1.
Socio-demographic characteristics of the study population
| Persons older than 65 years old | N | % | |
|---|---|---|---|
| Gender | Male | 220 | 43.8 |
| N=502 | Female | 282 | 56.2 |
| Age | Mean age (SD) | 72.5 (7.2) | |
| N=502 | |||
| Education | No formal education | 101 | 20.1 |
| N=502 | Primary school (completed grade 5) | 162 | 32.3 |
| Intermediate (completed grade 9) | 117 | 23.3 | |
| Secondary (completed grade 12) | 90 | 17.9 | |
| Vocational training /University | 32 | 6.4 | |
| Marital status | Married | 315 | 62.7 |
| N = 502 | Single/widowed/divorced/separated | 187 | 37.3 |
| Still working | Yes | 150 | 30.5 |
| N= 491 | No | 341 | 69.5 |
| Highest job ever held* | Never worked | 186 | 42.3 |
| N= 440 | Managers | 11 | 2.5 |
| Professionals | 16 | 3.6 | |
| Technicians and associate professionals | 13 | 3.0 | |
| Clerical support workers | 33 | 7.5 | |
| Service and sales workers | 99 | 22.5 | |
| Craft and related trades workers | 51 | 11.6 | |
| Plant and machine operators and assemblers | 7 | 1.6 | |
| Elementary workers (cleaners, laborers, unskilled farmers) | 14 | 3.2 | |
| Armed forces occupations | 10 | 2.2 | |
| Sufficient income for basic needs | Yes | 356 | 73.3 |
| N=486 | No | 130 | 26.7 |
| Monthly income | < 500,000 Lebanese pound (333 USD) | 38 | 9.7 |
| N=392 | 500,000 – 999,000 Lebanese pound (333-666 USD) | 175 | 44.6 |
| 1,000,000-3,000,000 Lebanese pound (666-2000 USD) | 152 | 38.8 | |
| >3,000,000 Lebanese pound (2000 USD) | 27 | 6.9 | |
| Informants for the older persons | |||
| Relationship to the older person | Spouse | 152 | 30.2 |
| N=502 | Children/children in law | 245 | 48.8 |
| Other relatives | 61 | 12.2 | |
| Other (friends, neighbors, etc.) | 44 | 8.8 | |
| Gender | Male | 160 | 31.7 |
| N=502 | Female | 343 | 68.3 |
| Age | Mean age (SD) | 49.5 (17.7) | |
| N=502 | |||
| Education | No formal education | 49 | 9.8 |
| N=502 | Primary school (completed grade 5) | 78 | 15.5 |
| Intermediate (completed grade 9) | 83 | 16.5 | |
| Secondary (completed grade 12) | 154 | 30.7 | |
| Vocational training / University | 138 | 27.5 | |
| Marital status | Married | 351 | 69.9 |
| N=502 | Single/widowed/divorced/separated | 151 | 30.1 |
| Sufficient income for basic needs | Yes | 396 | 79.5 |
| N=498 | No | 102 | 20.5 |
International Labor Organization classification ISCO-88
3. Dementia prevalence
Thirty seven older participants were diagnosed with dementia, yielding a crude dementia prevalence of 7.4% (95% CI 5.4% to 10.0%). As shown in Figure 2, age-specific prevalence estimates for dementia increased steeply with age, comparable to the latest estimates by Alzheimer’s Disease International for EMRO [1]. After the age of 85 years, dementia prevalence levelled off, probably due to the very small number of persons in that age category (34 persons). Gender-specific prevalence was 11.0% for women and 2.7% for men (p<0.0001). Prevalence of dementia was 15.8% among those with no formal education and 5.2% among those with formal education (primary school and above, p = 0.001). The age-standardized prevalence of dementia was 9.0%, comparable to the 8.7% estimated for people above 60 years old in EMRO [1]. Based on the crude prevalence of 7.4 % and the estimated total number of Lebanese older than 65 years in 2013 (404,274 persons) [21], the estimated number of people with dementia in Lebanon was 29,916 persons.
Figure 2. Age-specific dementia prevalence in Lebanon compared to estimates for EMRO.
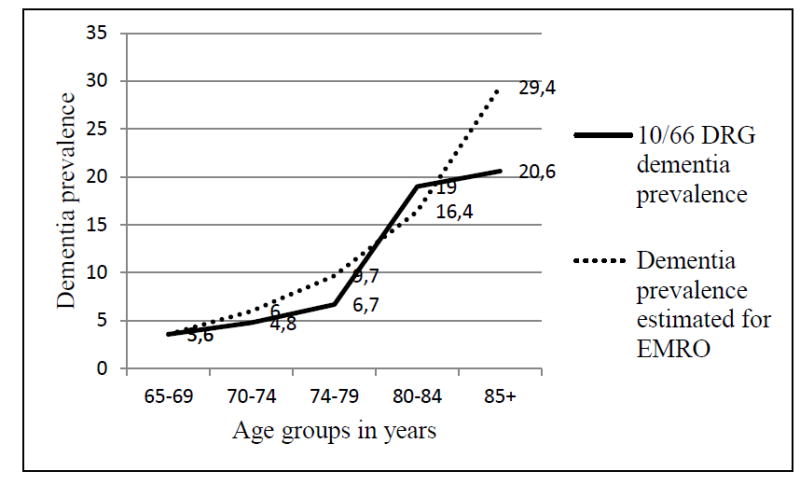
10/66 DRG: 10/66 Dementia Research Group
4. RUDAS, IQCODE, and DemeGraph as screening instruments for dementia
The percentages of people screened positive for dementia by IQCODE were 14.7% (95% CI 11.9% to 18.1%) and by RUDAS 39.5% (95% CI 35.3% to 43.9%). Since the 10/66 DRG one stage diagnostic assessment worked well in this study, there was no need to pursue the alternative strategies. Instead, RUDAS, IQCODE, and DemeGraph could be validated against 10/66 DRG diagnoses. The psychometrics properties of IQCODE, RUDAS, and DemeGraph are reported in Table 2. As predicted, the IQCODE did not demonstrate the same excellent psychometric properties in a population-based context compared to the validation study [9]. However, it performed much better the RUDAS as it had done during the validation study. The RUDAS demonstrated excellent sensitivity but low specificity and very poor PPV. The DemeGraph performed marginally better than the IQCODE, but it did correct for the low specificity and predictive value of the RUDAS, consistent with the finding in the validation study [10].
Table 2.
Psychometric properties of IQCODE, RUDAS, and DemeGraph against 10/66 DRG dementia diagnosis
| Screening Instruments N=494 | Sensitivity | Specificity | Youden index | PPV | NPV | Correctly identified |
|---|---|---|---|---|---|---|
| IQCODE Cut off > 3.34 | 86.5 | 91.1 | 0.78 | 43.8 | 98.8 | 90.7 |
| RUDAS Cut off ≤ 22 | 94.6 | 64.6 | 0.59 | 17.8 | 99.3 | 66.9 |
| DemeGraph | 89.2 | 91.9 | 0.81 | 47.1 | 99.1 | 91.7 |
PPV: Positive Predictive Value
NPV: Negative Predictive Value
Youden index is a single statistic that captures the performance of a dichotomous diagnostic test: J = (Sensitivity + Specificity) − 1
Care need and care arrangement
Of the total sample, 64 older persons needed care, 19 with dementia and 45 without. The two groups did not differ significantly regarding age, gender distribution, and co-morbidities. Among them, there was a high prevalence of hypertension (77%), heart diseases (34%), stroke (11%), diabetes (51%), and other chronic diseases (50%). To various extents, they needed assistance in different basic and instrumental activities of daily living. Slightly more than half (51.4%) of the older persons with dementia needed care compared to 9.7 % of those without dementia (p <0.0001). Among those with care need, 42.1% of those with dementia required care most of the time compared to 11.1% of those without dementia (p = 0.01). More persons with dementia than those without required assistance in communicating (31.5% versus 8.9%), eating (26.3% versus 4.4%), bathing (36.9% versus 11.1%), toileting (21.1% versus 2.2%), grooming (36.8% versus 8.9%), and travelling (10.6% versus 4.4%). Except for travelling, the differences were statistically significant (≤ 0.05).
Care arrangement for those with and without dementia was similar (Table 3). Although the extended family structure still prevailed, 15.6% older persons with care needs lived alone and 6.3 % lived with a maid (Table 3). The caregivers were mostly women (75.0%), mainly wives, daughters, or other female relatives. Although most caregivers did not have to take time off paid work to care for the older persons, approximately one-third of caregivers never worked outside the house; most of them (94.4%) were women (Table 3). The typical caregiver was thus a female relative, who had most likely never worked outside the home. About a fourth of the households had live-in maids; migrant workers who were an integral part of caregiving on a regular basis (Table 3).
Table 3.
Care arrangement for the 64 older persons with care need
| Older persons with care need | With dementia | Without dementia | p | |||||
|---|---|---|---|---|---|---|---|---|
| N=64 | % | N=19 | % | N=45 | % | |||
| Informant was | Main hands on caregiver | 23 | 35.9 | 9 | 47.4 | 14 | 31.1 | 0.4 |
| Main organizational caregiver | 28 | 43.8 | 6 | 31.6 | 22 | 48.9 | ||
| Slightly involvement in care | 13 | 20.3 | 4 | 21.1 | 9 | 20.0 | ||
| Available care provider | Family member(s) | 54 | 84.4 | 17 | 89.5 | 37 | 82.2 | 0.9 |
| Unpaid friend(s)/neighbor(s) | 2 | 3.1 | 0 | 0 | 2 | 4.4 | ||
| Paid caregiver(s) | 8 | 12.5 | 2 | 10.5 | 6 | 13.4 | ||
| Time taken from work to provide care | Gave up work | 1 | 1.6 | 1 | 5.3 | 0 | 0 | 0.2 |
| Continued to work | 41 | 64.1 | 10 | 52.6 | 31 | 68.9 | ||
| Never worked | 18 | 28.1 | 6 | 31.6 | 12 | 26.7 | ||
| Not applicable | 4 | 6.2 | 2 | 10.5 | 2 | 4.4 | ||
| Who lives with the older person | Spouse | 16 | 25.0 | 5 | 26.3 | 11 | 24.4 | 0.4 |
| Extended family structure* | 34 | 53.1 | 11 | 57.9 | 23 | 51.1 | ||
| Live-in maid | 4 | 6.3 | 2 | 10.5 | 2 | 4.4 | ||
| No one | 10 | 15.6 | 1 | 5.3 | 9 | 20.1 | ||
| Regular additional help from relatives or friends with care | Yes | 24 | 37.5 | 7 | 36.8 | 17 | 37.8 | 0.9 |
| No | 40 | 62.5 | 12 | 63.2 | 28 | 62.2 | ||
| Live-in maid | Yes | 15 | 23.4 | 5 | 26.3 | 10 | 22.2 | 0.8 |
| No | 49 | 76.6 | 14 | 73.7 | 35 | 77.8 | ||
| Live-in maid involvement in caregiving | Sometimes | 2 | 13.3 | 0 | 0 | 2 | 20.0 | 0.8 |
| Regularly | 9 | 60.0 | 4 | 80.0 | 5 | 50.0 | ||
| Constantly | 4 | 26.7 | 1 | 20.0 | 3 | 30.0 | ||
| Caregiver during the day beside the live-in maids | Yes | 5 | 7.8 | 3 | 15.8 | 2 | 4.4 | 0.2 |
| No | 59 | 92.2 | 16 | 84.2 | 43 | 95.6 | ||
Extended family structure: cohabitation of spouses, children, grandchildren, and siblings
5. Access to care
The rate of help seeking for memory problems was very low. In all, 165 older persons had subjective memory complaints, 36 (21.8%) of whom were diagnosed with 10/66 DRG dementia. Among the 337 older persons with no subjective memory complaints, 1 (0.3%) had 10/66 DRG dementia diagnosis. Only nine (5.5%) among the 165 older persons with subjective memory complaints sought help from a medical doctor or another health professional. Of the 37 older persons with dementia, only six sought help (16.2%), and four were prescribed medication for their memory problems. Thus, the treatment rate was only 10.8%.
Looking at the use of health services within the past three months, older participants with dementia seemed to use health services (primary care and private doctors) more often than those without dementia. Access to health services was available within thirty minutes of travel time (Table 4). Private doctors were consulted much more frequently than the public primary care and hospital-based physicians (Table 4). Approximately 40% of the older persons had health care insurance (Table 4). The costs of health care were high compared to the income level stated in Table 1, thus a major prohibiting factor to access to care.
Table 4.
Use of health services during the last three months
| Total study population N=502 | With dementia | Without dementia | |||
|---|---|---|---|---|---|
| N = 37 | N=465 | ||||
| Primary care | N | % | N | % | p |
| Yes | 3 | 8.1 | 9 | 1.9 | 0.05 |
| Travel time in in minutes: Mean (SD) | 25 (9) | 16 (13) | 0.3 | ||
| Travel cost in USD: mean (SD) | 17 (7) | 10 (7) | 0.5 | ||
| Consultation cost in USD: mean (SD) | 10 (0) | 21 (18) | 0.4 | ||
| Government hospital doctor (N=502) | |||||
| Yes | 2 | 5.4 | 12 | 2.6 | 0.3 |
| Travel time in in minutes: Mean (SD) | 30 (0) | 21 (8) | 0.1 | ||
| Travel costs in USD: mean (SD) | 1 (0) | 6 (3) | 0.1 | ||
| Consultation cost in USD: mean (SD) | 33 (0) | 16 (4) | 0.006 | ||
| Private doctor | |||||
| Yes | 14 | 37.8 | 100 | 21.5 | 0.04 |
| Travel time in minutes: mean (SD) | 33 (36) | 27 (25) | 0.6 | ||
| Travel cost in in USD: mean (SD) | 6 (5) | 10 (14) | 0.6 | ||
| Consultation cost in USD: mean (SD) | 32 (12) | 38 (16) | 0.3 | ||
| Hospital admission for at least 3 nights | |||||
| Yes | 5 | 13.5 | 29 | 6.2 | 0.2 |
| Cost of the admission in USD: mean (SD) | 1200 (0) | 1102 (2168) | 0.9 | ||
| Medicine | |||||
| Yes | 29 | 78.4 | 326 | 70.1 | 0.4 |
| Medicine cost in USD | 156 (152) | 134 (445) | 0.8 | ||
| Health insurance | |||||
| Yes | 15 | 40.5 | 196 | 42.9 | 0.9 |
DISCUSSION
For the first time, a reliable estimate of dementia prevalence in Lebanon is provided. With a crude prevalence of 7.4% and an age-standardized prevalence of 9.0% for those above 65 years old, Lebanon fits within the higher end of the global range of dementia prevalence. This is consistent with the latest updates from the World Alzheimer’s Report 2015, in which EMRO and Latin America were estimated to have the highest age-standardized prevalence in the world, 8.7% and 8.4% respectively, and Central Europe the lowest (4.7%). Most regions fall within a narrow range of standardized prevalence, ranging from 5.6% to 7.6% [1]. Dementia prevalence has been reported to be as high as 27% in some indigenous populations, potentially related to cognitive reserve and poorly managed health conditions over the course of life, notably vascular risk factors and diseases [22].
Similarly, the most likely cause for the observed high dementia prevalence in Lebanon and EMRO is a combination of rapid population aging and rising prevalence of vascular risk factors. Lebanese are at particular high risk for familial hypercholesterolemia, a combined result of founder mutations and high incidence of consanguinity [23,24]. Furthermore, as it is happening elsewhere in EMRO, there is an epidemiological transition with increasing prevalence of smoking, sedentary lifestyle, and consumption of a diet high in carbohydrates, salt, and saturated fats [25,26,27,28]. Consequently, the prevalence of metabolic syndrome and cardiovascular diseases is on the rise, with disease projections exceeding those of other regions [28,29]. The problem is compounded by the lack of public health intervention programs to adequately address these vascular risk factors [28]. Furthermore, a high percentage of the older Lebanese generation had low level of education (50% with no formal education), another established risk factor for dementia [3].
There is a marked gender effect as dementia prevalence among women is about three times that of men in our study. This is very high compared to the gender-based discrepancies in dementia prevalence observed in other regions of the world, where the estimated prevalence for men was between 14% and 32% lower than that for women [1]. Gender affects the ability of women to gain access to resources such as nutrition and education, factors that affect the cognitive reserve. Lebanese women of this generation were much less educated than men, with about two-thirds of people with no formal education being women [3]. Furthermore, there may be a selective survival of older men who have lower risk of developing dementia. Nevertheless, due to the small sample size and the modest number of outcomes, this marked effect of gender on dementia prevalence should be interpreted with caution. A longitudinal is needed to determine if gender is indeed associated with dementia incidence in the Lebanese population.
Due to the unstable sociopolitical situation in Lebanon caused by the ongoing war in Syria since 2011, we were unable to extend data collection for the rest of the country and complete the national cohort of 2500 participants to provide a more precise estimate of dementia prevalence. However, a study population of 502 participants was large enough to provide a reliable estimate with fairly narrow 95% CI. Although the study sample was not national, it is noteworthy that half of the Lebanese population resides in the two selected regions, which represent the urban coastal strip (Beirut) and the peri-urban/rural central mountainous terrain (Mount Lebanon). The sociodemographic indicators such as the percentage of people working after the age of 65 years old, the distribution of job categories, the percentage of men and women who had worked, the distribution of income levels, and the percentage of health insurance coverage, are quite comparable to the national figures from the Lebanese Central Administration of Statistics [3,30,31]. Therefore, the study sample was fairly representative of the Lebanese population older than 65 years old.
The 10/66 DRG diagnostic assessment and the IQCODE provide researchers in Lebanon and EMRO with validated methods for case ascertainment and screening for dementia in epidemiological studies. The lack of validated instruments for cognitive assessment and reliable estimate of dementia prevalence in Lebanon was highlighted by a large population-based study carried out in 2011-2012 [22] in which Mini Mental State Examinations (MMSE) was used [33]. MMSE has not been properly validated in Arabic [34] and is not a suitable screening instrument because of the high rate of illiteracy among older people in Lebanon. The rate of cognitive dysfunction was unrealistically high (86.2%) among illiterate persons older than 65 years [22].
In a population-based study, the Arabic validated 10/66 DRG dementia diagnostic assessment proved to be an excellent tool for case ascertainment. The assessment burden was acceptable to the participants regarding both length and content. The trained lay research workers could administer the assessment without much difficulty. The validity of the assessment is clearly demonstrated by the fact that both the overall and age-specific 10/66 DRG dementia prevalence in Lebanon agree with the latest estimates for EMRO and follow the same age distribution of dementia prevalence globally.
Therefore, it was justifiable to use the 10/66 DRG dementia diagnosis as gold standard against which the RUDAS and IQCODE were validated in a population-based context. Given that the prevalence of dementia in this study population was only 7.4%, much lower than the 40 % in the previous validation studies [8,10,9], it was expected that the PPV would be lower. For the IQCODE, PPV was 43.2% compared to 91.5% from the validation study. For the RUDAS, PPV was only 17.7% compared to 79% from the validations study. Given the low specificity and poor PPV, using the RUDAS to screen for dementia in a population-based study is not cost-effective. The low specificity and PPV could partly be explained by the participants’ difficulty in carrying out the visuoconstructional item of the RUDAS. Furthermore, while the 10/66 DRG diagnostic assessment and the IQCODE can reliably be performed by trained lay research workers without any clinical expertise, this may not hold true for the RUDAS [10]. Therefore, the RUDAS may not be a suitable community-based screening test to be administered by trained lay persons to a population with a high percentage of people with no formal education. More research is needed to answer these questions. Harnessing the RUDAS and IQCODE together using the DemeGraph, a better balance was achieved. As predicted, the IQCODE did not demonstrate the same excellent psychometric properties in a population-based context compared to the validation study [9], but its performance was as good as the DemeGraph. It has been observed that caregiver report performed better than direct cognitive assessment in cultures where extended family structure still prevails [10,35]. Preferably, combining a direct cognitive assessment with an informant interview should be used for the reasons stated in the method section under the DemeGraph heading. However, if direct cognitive assessment is not possible, the IQCODE can be used alone as a valid screening instrument for dementia in this cultural context.
The results showed that older persons with dementia had more care needs than those without. In another paper, we have reported that caregivers for people with dementia in our study rated highest on Zarit Burden Interview compared to those caring for people without dementia [36]. The results showed that older persons with dementia had more care needs than those without. Support and care for the older persons with care needs rested mainly on their family members. Caregivers were mostly female relatives, a pattern recognized globally [37]. Although the extended family structure was still dominant, one in five older persons with care needs lived alone or with a maid, reflecting the reality that the extended family, the main form of social welfare in the country, is slowly eroding after decades of urbanization, emigration and participation of women in the work force [38]. As compensation, live-in maids are often employed to care for the elderly. Institutionalization is not a common option, as the number of nursing homes is limited, housing only 1.4% of the elderly Lebanese [39].
Seen from the results of this study, access to care was very limited, with a very low rate of help seeking help for cognitive complaints and treatment rate. The barriers to care include lack of awareness about dementia, lack of financial resources, high costs associated with health care, inadequate coverage of health care insurance, and a health care system dominated by private providers [38]. There is still no universal old-age pension plan in Lebanon. Older women are particularly vulnerable as most never worked and therefore have no pension or health insurance [38]. Currently, dementia is rarely addressed by social and health authorities in Lebanon. The percentage of people older than 65 years old in Lebanon was estimated as 9.7% in 2013 [21] and projected to increase to 14.1 in 2030 and 23.3% in 2050 [19]. Population aging is imminent [38] and as a major disease burden of aging, dementia threatens to become a public health crisis in Lebanon within the next 20 years. The knowledge about disease prevalence and barriers to care from this study serves as the first evidence-based data to raise awareness and advocate for reform in the socio-medical system to provide better diagnosis, treatment, and support for people with dementia in Lebanon and the region.
Highlights.
The crude dementia prevalence in Lebanon is 7.4% and age-standardized dementia prevalence is 9.0%.
Age-specific prevalences are: 3.6 % for the age group 65-69 years old, 4.8 % for 70-74, 6.7% for 75-79, 19.0% for 80-84, and 20.6 % for ≥ 85.
The absolute number and the age distribution of dementia prevalence are consistent with the latest estimates for the North Africa and Middle East (EMRO) region from the World Alzheimer Report 2015.
The burden of caregiving rests mainly with the family and access to dementia care is very limited.
The findings provide the first evidence-base data to raise awareness and advocate for socio-medico policy reform in the country.
Research in context.
Systematic review: The authors reviewed the literature using PubMed. Since studies on dementia prevalence in North Africa and Middle East (EMRO) region are scarce, it has not been possible to precisely estimate dementia prevalence in the region through meta-analysis. One great challenge has been the lack of validated cognitive assessments.
Interpretation: The findings provides the first reliable estimate of dementia prevalence in Lebanon, showing that the age-standardized prevalence ranks high in the global range of dementia prevalence, corroborating the latest estimation from World Alzheimer Report 2015 that EMRO currently has the highest dementia prevalence in the world.
Future direction: More epidemiological studies to assess disease prevalence in the region are needed. The 10/66 Dementia Research Group one-stage diagnostic assessment and the Informant Questionnaire on Cognitive Decline in the Elderly used in this population-based study have been validated in the educational, linguistic, and cultural context of the region and are recommended to be used for case ascertainment and screening for dementia in future epidemiological studies. They can be reliably administered by trained lay research workers.
Acknowledgments
The study was funded by the Fogarty International Center, American National Institutes of Health and National Institute of Aging, grant No. 1R21AG039333-01, under the program ‘Brain Disorders in the Developing World: Research across the Lifespan (BRAIN)’. The content is solely the responsibility of the authors and does not represent the official views of the funding agencies. The funding agency had no role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report, and in the decision to submit the paper for publication. Associate Professor Sawsan Abdulrahim at the Department of Health Promotion and Community Health, Faculty of Health Sciences, American University of Beirut, Lebanon, provided the sampling frame for data collection in the Beirut governorate. The study was conducted at the Faculty of Health Sciences, American University of Beirut, Lebanon, in collaboration with the Department of Neurology at the American University of Beirut; and the Danish Dementia Research Center, Department of Neurology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark.
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alzheimer’s Disease International. World Alzheimer Report 2015: The Global Impact of Dementia. [5/4/2016]; https://www.alz.co.uk/research/world-report-2015.
- 2.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Central Administration of Statistics. Multiple Indicators Cluster Survey. [5/4/2016];2009 http://www.cas.gov.lb/index.php/demographic-and-social-en/education-en.
- 4.Prince M, Acosta D, Chiu H, et al. Dementia diagnosis in developing countries: a cross-cultural validation study. Lancet. 2003;361:909–917. doi: 10.1016/S0140-6736(03)12772-9. [DOI] [PubMed] [Google Scholar]
- 5.Storey JE, Rowland JT, Basic D, et al. The Rowland Universal Dementia Assessment Scale (RUDAS): a multicultural cognitive assessment scale. Int Psychogeriatr. 2004;16:13–31. doi: 10.1017/s1041610204000043. [DOI] [PubMed] [Google Scholar]
- 6.Jorm AF. The Informant Questionnaire on cognitive decline in the elderly (IQCODE): a review. Int Psychogeriatr. 2004;16:275–293. doi: 10.1017/s1041610204000390. [DOI] [PubMed] [Google Scholar]
- 7.Phung KT, Chaaya M, Waldemar G, et al. Validation of the 10/66 Dementia Research Group diagnostic assessment for dementia in Arabic: a study in Lebanon. J Geriatr Psychiatry Neurol. 2014;27:282–290. doi: 10.1177/0891988714532019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaaya M, Phung TK, El AK, et al. Validation of the Arabic Rowland Universal Dementia Assessment Scale (A-RUDAS) in elderly with mild and moderate dementia. Aging Ment Health. 2015:1–8. doi: 10.1080/13607863.2015.1043620. [DOI] [PubMed] [Google Scholar]
- 9.Phung TK, Chaaya M, Asmar K, et al. Performance of the 16-Item Informant Questionnaire on Cognitive Decline for the Elderly (IQCODE) in an Arabic-Speaking Older Population. Dement Geriatr Cogn Disord. 2015;40:276–289. doi: 10.1159/000437092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen TR, Phung TK, Chaaya M, et al. Combining the Rowland Universal Dementia Assessment Scale and the Informant Questionnaire on Cognitive Decline in the Elderly to Improve Detection of Dementia in an Arabic-Speaking Population. Dement Geriatr Cogn Disord. 2016;41:46–54. doi: 10.1159/000441649. [DOI] [PubMed] [Google Scholar]
- 11.Alzheimer’s Disease International. World Alzheimer Report. [5/4/2016];2009 http://www.alz.co.uk/research/files/WorldAlzheimerReport.pdf.
- 12.Abdulrahim S, Ajrouch KJ, Jammal A, et al. Survey methods and aging research in an Arab sociocultural context--a case study from Beirut, Lebanon. J Gerontol B Psychol Sci Soc Sci. 2012;67:775–782. doi: 10.1093/geronb/gbs083. [DOI] [PubMed] [Google Scholar]
- 13.Hall KS, Hendrie HH, Brittain HM, et al. The development of a dementia screening interview in two distinct languages. Int J Methods Psychiatric Res. 1993;3:1–28. [Google Scholar]
- 14.Ganguli M, Chandra V, Gilby JE, et al. Cognitive test performance in a community-based nondemented elderly sample in rural India: the Indo-U.S. Cross-National Dementia Epidemiology Study. Int Psychogeriatr. 1996;8:507–524. doi: 10.1017/s1041610296002852. [DOI] [PubMed] [Google Scholar]
- 15.Copeland JR, Dewey ME, Griffiths-Jones HM. A computerized psychiatric diagnostic system and case nomenclature for elderly subjects: GMS and AGECAT. Psychol Med. 1986;16:89–99. doi: 10.1017/s0033291700057779. [DOI] [PubMed] [Google Scholar]
- 16.Broe GA, Jorm AF, Creasey H, et al. Impact of chronic systemic and neurological disorders on disability, depression and life satisfaction. Int J Geriatr Psychiatry. 1998;13:667–673. doi: 10.1002/(sici)1099-1166(1998100)13:10<667::aid-gps839>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen TR, Jorgensen K. Visuoconstructional abilities in cognitively healthy illiterate Turkish immigrants: a quantitative and qualitative investigation. Clin Neuropsychol. 2013;27:681–692. doi: 10.1080/13854046.2013.767379. [DOI] [PubMed] [Google Scholar]
- 18.Alzheimer’s Disease International. 10/66 population-based study (prevalence phase) protocols. [5/30/2016]; https://www.alz.co.uk/1066/population_based_study_prevalence.php.
- 19.UNDP. World Population Prospects, the 2015 Revision. http://esa.un.org/undp/wpp/
- 20.Central Administration of Statistics. Poverty snapshot. [5/4/2016];2011-2012 http://www.cas.gov.lb/index.php/demographic-and-social-en/poverty-en.
- 21.Ministry of Public Health. Statistical bulletin. [5/30/2016];2013 http://www.moph.gov.lb/Statistics/Documents/StatisticalBulletin2013.pdf.
- 22.de Souza-Talarico JN, de Carvalho AP, Brucki SM, et al. Dementia and Cognitive Impairment Prevalence and Associated Factors in Indigenous Populations: A Systematic Review. Alzheimer Dis Assoc Disord. 2016;30:281–287. doi: 10.1097/WAD.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 23.Abifadel M, Rabes JP, Jambart S, et al. The molecular basis of familial hypercholesterolemia in Lebanon: spectrum of LDLR mutations and role of PCSK9 as a modifier gene. Hum Mutat. 2009;30:E682–E691. doi: 10.1002/humu.21002. [DOI] [PubMed] [Google Scholar]
- 24.Khachadurian AK, Uthman SM. Experiences with the homozygous cases of familial hypercholesterolemia. A report of 52 patients. Nutr Metab. 1973;15:132–140. doi: 10.1159/000175431. [DOI] [PubMed] [Google Scholar]
- 25.Tamim H, Al-Sahab B, Akkary G, et al. Cigarette and nargileh smoking practices among school students in Beirut, Lebanon. Am J Health Behav. 2007;31:56–63. doi: 10.5555/ajhb.2007.31.1.56. [DOI] [PubMed] [Google Scholar]
- 26.Sibai A, Tohme R, Mahfoud Z, et al. Non-Communicable Diseases and Behavioral Risk Factor Survey: Comparison of Estimates Based on Cell Phone Interviews with Face to Face Interviews. 2009 [Google Scholar]
- 27.Mallah C, Obeid O. Dietary lipid supply in Lebanon: Does it fit the Mediterranean diet profile? Lipid Technology. 2014;26:127–129. [Google Scholar]; Lipid Technology. 2014;26:127–129. [Google Scholar]
- 28.Ramahi TM. Cardiovascular disease in the Asia Middle East region: global trends and local implications. Asia Pac J Public Health. 2010;22:83S–89S. doi: 10.1177/1010539510373034. [DOI] [PubMed] [Google Scholar]
- 29.Zeidan RK, Farah R, Chahine MN, et al. Prevalence and correlates of coronary heart disease: first population-based study in Lebanon. Vasc Health Risk Manag. 2016;12:75–84. doi: 10.2147/VHRM.S97252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Central Administration of Statistics. The National Survey of Household Living Condition. [5/26/2016];2004 http://www.cas.gov.lb/index.php/demographic-and-social-en/population-en.
- 31.Central Administration of Statistics. The National Survey of Household Living Condition. [5/26/2016];2007 http://www.cas.gov.lb/index.php/demographic-and-social-en/population-en.
- 32.Boulos C, Salameh P, Barberger-Gateau P. The AMEL study, a cross sectional population-based survey on aging and malnutrition in 1200 elderly Lebanese living in rural settings: protocol and sample characteristics. BMC Public Health. 2013;13:573. doi: 10.1186/1471-2458-13-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 34.Al-Rajeh S, Ogynniyi A, Awada A, et al. Preliminary assessment of an Arabic version of the Mini-Mental state examination. Ann Saudi Med. 1999;19:150–152. doi: 10.5144/0256-4947.1999.150. [DOI] [PubMed] [Google Scholar]
- 35.Narasimhalu K, Lee J, Auchus AP, et al. Improving detection of dementia in Asian patients with low education: combining the Mini-Mental State Examination and the Informant Questionnaire on Cognitive Decline in the Elderly. Dement Geriatr Cogn Disord. 2008;25:17–22. doi: 10.1159/000111128. [DOI] [PubMed] [Google Scholar]
- 36.Chaaya M, Phung TK, Atweh S, et al. Dementia and family burden of care in Lebanon. BJPSYCH INTERNATIONAL. 2017;14:7–9. doi: 10.1192/s2056474000001574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prince M. Care arrangements for people with dementia in developing countries. Int J Geriatr Psychiatry. 2004;19:170–177. doi: 10.1002/gps.1046. [DOI] [PubMed] [Google Scholar]
- 38.Sibai AM, Sen K, Baydoun M, et al. Population ageing in Lebanon: current status, future prospects and implications for policy. Bull World Health Organ. 2004;82:219–225. [PMC free article] [PubMed] [Google Scholar]
- 39.Chemali Z, Chahine LM, Sibai AM. Older adult care in Lebanon: towards stronger and sustainable reforms. East Mediterr Health J. 2008;14:1466–1476. [PubMed] [Google Scholar]


