Abstract
Background
Often the clinician is faced with a diagnostic and therapeutic dilemma in patients with concomitant traumatic brain injury (TBI) and hemorrhagic shock (HS), as rapid deterioration from either can be fatal. Knowledge about outcomes following concomitant TBI and HS may help prioritize the emergent management of these patients. We hypothesized that patients with concomitant TBI and HS (TBI+HS) had worse outcomes and required more intensive care compared to patients with only one of these injuries.
Methods
This is a post-hoc analysis of the Pragmatic, Randomized Optimal Platelets and Plasma Ratios (PROPPR) trial. TBI was defined by a head abbreviated injury scale >2. HS was defined as a base excess ≤ −4 and/or shock index ≥ 0.9. The primary outcome for this analysis was mortality at 30 days. Logistic regression, using generalized estimating equations (GEE), was used to model categorical outcomes.
Results
670 patients were included. Patients with TBI+HS had significantly higher lactate (median 6.3; IQR 4.7,9.2) compared to the TBI group (median 3.3; IQR 2.3,4). TBI+HS patients had higher activated prothrombin times and lower platelet counts. Unadjusted mortality was higher in the TBI+HS (51.6%) and TBI (50%) groups compared to the HS (17.5%) and neither group (7.7%). Adjusted odds of death in the TBI and TBI+HS groups were 8.2 (95% CI, 3.4–19.5) and 10.6 (95% CI, 4.8–23.2) times higher, respectively. Ventilator, ICU- and hospital-free days were lower in the TBI and TBI+HS groups compared to the other groups. Patients with TBI+HS or TBI had significantly greater odds of developing a respiratory complication compared to the neither group.
Conclusions
The addition of TBI to HS is associated with worse coagulopathy prior to resuscitation, and increased mortality. When controlling for multiple known confounders, the diagnosis of TBI alone or TBI+HS was associated with significantly greater odds of developing respiratory complications.
Keywords: traumatic brain injury, hemorrhagic shock, resuscitation, trauma
BACKGROUND
Despite advances in trauma resuscitation over the past two decades, hemorrhage and traumatic brain injury (TBI) remain leading causes of death in both civilian and military populations.1–3 TBI is the leading cause of morbidity and mortality in patients following injury, and hemorrhage is the second— and most preventable—cause of death.1–3 When these injuries occur concurrently, the clinician is faced with a diagnostic and therapeutic dilemma, as rapid deterioration of a patient from either TBI or hemorrhage can be lethal. There is currently a paucity of data to help guide decision-making in these circumstances, and knowledge about the physiological phenotype following concomitant TBI and hemorrhagic shock (HS) may help prioritize the emergent management of these injuries.
The contemporary damage-control resuscitation (DCR) paradigm supports actively bleeding trauma patients until hemorrhage control is achieved.4–7 Primary objectives include resolution of immediate life threating injuries followed by optimization of physiological status in the perioperative period.4,6 The principles of DCR center on early hemorrhage control and limiting ongoing blood loss by adopting strategies that limit crystalloid fluid administration, reduce blood pressure targets, and maintain hemostasis through balanced transfusion strategies.4,7 A central tenet of the DCR paradigm involves early administration of blood products in a balanced ratio; the Pragmatic Randomized Optimal Platelets and Plasma Ratios (PROPPR) trial was the first and largest prospective study to examine two different balanced blood product ratios in patients predicted to receive a massive transfusion.8 Application of DCR strategies has reduced mortality from HS and also seems to reduce the incidence and severity of complications such as organ failure and infection.5,6,9 However, very little is known about the clinical implications of DCR for patients with TBI and HS, especially considering the coagulopathy that often accompanies TBI.1,10–15 Additionally, there is virtually no data on how DCR affects neurologic status and outcomes with concomitant TBI.
More research is urgently required to help guide decision-making in cases of TBI and HS. The primary objective of this post hoc study was to examine the impact of combined TBI and HS on outcomes and intensive care requirements. We hypothesized that patients with concomitant TBI and HS (TBI+HS) had worse outcomes and required more intensive care compared to patients with only one of these injuries. We also hypothesized that a high incidence of coagulopathy was present in patients with both TBI and HS.
METHODS
This is a post-hoc analysis of the Pragmatic Randomized Optimal Platelets and Plasma Ratios (PROPPR) trial.8 The PROPPR trial was performed under Exception from Informed Consent (EFIC) guidelines and approved by all institutional review boards at participating hospitals as well as the US Army Human Research Protection Office, the Secretary of the Army, and the US Food and Drug Administration. The details of the PROPPR trial have been published previously.8,16,17 Briefly, the trial included severely injured patients predicted to receive a massive transfusion and admitted to 12 North American Level I trauma centers. Patients were randomized to a 1:1:1 ratio of plasma: platelets: red blood cells (RBC) versus a 1:1:2 ratio. In this analysis, TBI was defined by a head abbreviated injury scale >2. HS was defined as admission base excess ≤ −418–21 and/or admission shock index ≥ 0.9. Ten patients out of the original 680 were not included in this analysis because they were missing base deficit and heart rate or blood pressure values on admission. The primary outcome for this analysis was mortality at 30 days.
Differences among groups were assessed for statistical significance, and 95% confidence intervals were calculated using robust variance estimates of general estimating equations (GEE) to accommodate non-constant variance. All group comparisons were adjusted for heterogeneity. Additional analyses were conducted with adjustment for baseline covariates, including age, sex, race, ethnicity, any blunt or penetrating injury, pre-randomized total blood products, pre-randomized total crystalloids or colloids, time to randomization, and ABC score ≥ 2. Variables with more than 10% missing were not included as covariates in the regression models. Logistic regression, using GEE to control for clustering by study center, was used to model categorical outcomes. Treatment assignment and HS/TBI group variables with a random effect for site were included in all models. Other covariates were chosen using purposeful selection and a p-value <0.10. A GEE model with an independent correlation structure was chosen and multiple regression diagnostics were performed on the final model to assess model adequacy (i.e., goodness of fit testing, residual plots, Cook’s D, and DFBETA plots). A subgroup analysis was performed modeling the Glasgow Outcome Score-Extended (GOSE) dichotomized to a variable indicating a poor outcome (i.e., a GOSE of 6 or less). Statistical analyses were performed using SAS/STAT software, Version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Demographic and physiological data are presented in Table 1. 670 patients were included. Patients in the TBI only group were the most likely to have a blunt injury and be in the 1:1:1 group compared to all other categories. There were clinically significant differences in admission vital signs among the groups. Patients with TBI+HS had the lowest median systolic blood pressure (SBP) and highest heart rate (HR) compared to all other groups. Patients with TBI+HS had higher lactate (median 6.3; interquartile range [IQR] 4.7, 9.2) compared to the TBI only group (median 3.3; IQR 2.3, 4), but not the HS only group (median 7.0; IQR 4.7, 10.7). TBI+HS patients had higher activated prothrombin times and lower platelet counts compared to all other groups at admission (Table 1). Compared to the TBI only group, patients with TBI+HS had significantly smaller median maximum amplitude and lower G-value; no other thromboelastography results were statistically different between the two groups.
Table 1.
Demographic and physiological data.
| Characteristic | Neither (N=130) |
HS Only (N=388) |
TBI Only (N=28) |
HS and TBI (N-124) |
All (N=670) |
|---|---|---|---|---|---|
|
| |||||
| Age in years | 37.5 (27,51) | 32 (24, 46.5) | 55 (38, 69.5) | 35.5 (23,56) | 34.5 (25,51) |
|
| |||||
| Treatment Group (%) | |||||
| 1:1:1 | 67 (51.5) | 197 (50.8) | 20 (71.4) | 51 (41.1) | 335 (50) |
| 1:1:2 | 63 (48.5) | 191 (49.2) | 8 (28.6) | 73 (58.9) | 225 (50) |
|
| |||||
| Gender (%) | |||||
| Male | 111 (85.4) | 318 (82.0) | 18 (64.3) | 90 (72. 6) | 537 (80.1) |
| Female | 19 (14.6) | 70 (18.0) | 10 (35.7) | 34 (27.4) | 133 (19.9) |
|
| |||||
| Race (%) | |||||
| Caucasian | 73 (56.2) | 237 (61.1) | 22 (78.6) | 102 (82.3) | 434 (64.8) |
| African American | 49 (37.7) | 114 (29.4) | 1 (3.6) | 14 (11.3) | 178 (26.6) |
| Other Race | 9 6.9) | 37 (9.5) | 5 17.9) | 8 (6.5) | 59 (8.8) |
|
| |||||
| Mechanism of Injury | |||||
| Blunt injury only | 55 (42.3) | 164 (42.3) | 27 (96.4) | 104 (83.9) | 357 (53.3) |
| Penetrating injury only | 75 (57.7) | 220 (56.7) | 1 (3.6) | 17 (13.7) | 320 (47.8) |
| Both types of injury | 0 | 5 (1.0) | 0 | 3 (2.4) | |
|
| |||||
| Systolic blood pressure | 114 (88, 132) | 100 (80,124) | 104 (82, 131) | 98 (79,123) | 102 (80,126) |
|
| |||||
| Heart rate | 99 (82,120) | 118 (98,133) | 104 (80, 122) | 120 (98,142) | 114 (94,132) |
|
| |||||
| Respiratory rate | 20 (16,26) | 22 (18,28) | 20 (16.5,22) | 20 (16,24) | 20 (17,26) |
|
| |||||
| Glasgow Coma Scale | 15 (13,15) | 14 (5.5,15) | 3 (3,11.5) | 3 (3,8) | 14 (3,15) |
|
| |||||
| Head AIS scores | 0 | 0 | 5 (2.5,5) | 4 (3,5) | 0 (0,2) |
|
| |||||
| Prehospital intubation (%) | 20 (15.4) | 74 (19.1) | 13 (46.4) | 72 (58.1) | 179 (26.7) |
|
| |||||
| pH (median, IQR) | 7.3 (7.3, 7.4) | 7.2 (7.1,7.3) | 7.3 (7.3,7.4) | 7.2 (7.0,7.3) | 7.2 (7.1,7.3) |
|
| |||||
| Base excess | −1.5 (−3,1.1) | −10 (−14.3, −6.8) | −1 (−2.4,1.2) | −10.4 (−14, −7.6) | −8 (−12.7, −4) |
|
| |||||
| Lactate | 3.4 (2.5, 5.1) | 7 (4.7, 10.7) | 3.3 (2.3, 4.0) | 6.3 (4.7,9.2) | 6 (3.7, 9.2) |
|
| |||||
| Hematocrit | 37 (33.2, 41.6) | 34.7 (29.1, 39.4) | 34.2 (28.5, 40.8) | 34.1 (30.5, 38.6) | 35 (30.4, 39.5) |
|
| |||||
| PT | 14.8 (13.9, 16.1) | 15.6 (14.4, 17.3) | 15.3 (14.9, 17) | 17.6 (15.7, 20.2) | 15.7 (14.5, 17.6) |
|
| |||||
| aPTT | 27 (24, 30.3) | 28.6 (25.4, 33.8) | 32 (29,40) | 38.9 (30.4, 53) | 29.6 (25.5, 35.5) |
|
| |||||
| INR | 1.2 (1.1,1.3) | 1.3 (1.2, 1.5) | 1.3 (1.2, 1.6) | 1.5 (1.3, 1.7) | 1.3 (1.2, 1.5) |
|
| |||||
| Thromboelastography results* | |||||
|
| |||||
| R-time (minutes) | 3.3 (2.7,4.2) | 3.8 (2.8,4.7) | 3.6 (3,4.4) | 4.2 (3.2,5) | 3.8 (2.9,4.7) |
|
| |||||
| K-time (minutes) | 1.3 (1.1,1.7) | 1.4 (1.2,1.8) | 1.4 (1.2,2.2) | 1.8 (1.3,2.5) | 1.5 (1.2,1.9) |
|
| |||||
| Alpha angle (%) | 72.4 (67.4,75.3) | 70.7 (65.7,74.3) | 68.3 (61.3,73.4) | 65.1 (59,70.4) | 70.2 (64.2,74.3) |
|
| |||||
| Maximum amplitude (mm) | 61.2 (56,65.4) | 61.5 (55.4,66) | 61.2 (57.8,66.8) | 57.3 (46.3, 63.1) | 60.8 (54.2, 65.4) |
|
| |||||
| G-value (d/sc) | 7.9 (6.4, 9.5) | 8 (6.3, 9.7) | 7.9 (6.8, 10) | 6.7 (4.3, 8.6) | 7.8 (5.9, 9.4) |
|
| |||||
| Ly30 (%) | 0.6 (0, 1.9) | 0.3 (0, 1.9) | 0.1 (0,1) | 0.7 (0, 8) | 0.3 (0, 2.2) |
Thromboelastography data were not available for all patients. Data are presented as medians with an interquartile range (P25,P75).
Within the first 24 hours, patients in the TBI+HS group received more total RBCs, more FFP, and more platelets compared to all other groups (Figure 1). The total volume of crystalloids, colloids, and tranexamic acid did not differ between groups. Significantly more patients in the TBI+HS group required a massive transfusion, defined as10 units of RBCs within 24 hours, (60%) compared to all other groups (p<0.001). However the critical administration threshold (CAT), which is defined as 3 units of blood products within 1 hour and is less affected by survivor bias than MT, was not significantly different among the four groups (p=0.21). Time to hemostasis was longer in both the HS only group) and the TBI+HS group compared to the other groups. Fewer patients in the TBI+HS group achieved hemostasis (76.6%) compared to all other groups, although this result was not statistically significant (P=0.09).
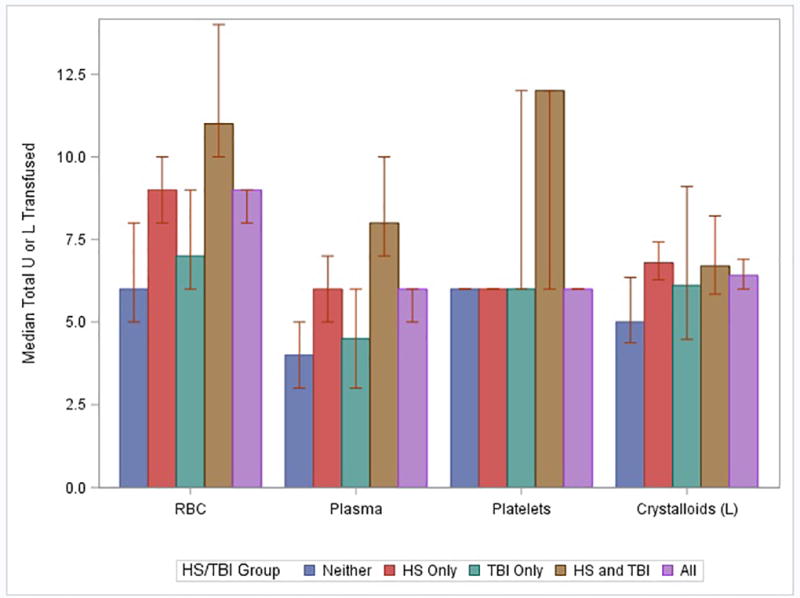
Hospital-free, ICU free and ventilator-free days were lower in the TBI only and TBI+HS groups compared to the other groups (Table 2). Significantly fewer TBI and TBI+HS patients were discharged home and both groups had a higher proportion of multiple organ failure as compared to the other groups. Unadjusted mortality was higher in the TBI+HS and TBI only groups.
Table 2.
Unadjusted outcomes and transfusion data.
| Outcome | Neither (N=130) | HS Only (N=388) | TBI Only (N=28) |
TBI + HS (N=124) | All (N=670) |
|---|---|---|---|---|---|
|
| |||||
| Hospital free days | 14.5 (0,21) | 7 (0,18.5) | 0 | 0 | 1 (0,17) |
|
| |||||
| ICU free days | 25 (15,28) | 22 (2,26) | 0 (0,12) | 0 (0,14) | 18 (0,26) |
|
| |||||
| Ventilator free days | 28 (21,29) | 26 (7,28) | 0 (0,14.5) | 0 (0,16) | 24 (0,28) |
|
| |||||
| Discharge GCS | 15 (15,15) | 15 (15,15) | 9.5 (8,13) | 14 (11,15) | 15 (15,15) |
|
| |||||
| Discharge GOSE | 6 (4,8) | 5 (3,7) | 1 (1,2) | 1 (1,3) | 1 (1,4) |
|
| |||||
| Disposition at 30 days (%) | |||||
| Hospitalized | 23 (17.7) | 95 (24.5) | 7 (25) | 34 (27.4) | 159 (23.7) |
| Home | 61 (46.9) | 153 (39.4) | 2 (7.1) | 5 (4) | 221 (33) |
| Long-term care facility | 3 (2.3) | 4 (1) | 3 (10.7) | 6 (4.8) | 16 (2.4) |
| Skilled nursing facility | 7 (5.4) | 17 (4.4) | 0 | 2 (1.6) | 26 (3.9) |
| Rehabilitation facility | 14 (10.8) | 19 (4.9) | 2 (7.1) | 10 (8.1) | 45 (6.7) |
| Acute care hospital | 2 (1.5) | 7 (1.8) | 0 | 2 (1.6) | 11 (1.6) |
| Other | 10 (7.7) | 25 (6.4) | 0 | 1 (0.8) | 36 (5.4) |
| Died | 10 (7.7) | 68 (17.5) | 14 (50) | 64 (51.6) | 156 (23.3) |
|
| |||||
| Multiple organ failure (%) | 2 (1.5) | 7 (1.8) | 2 (7.1) | 6 (4.8) | 17 (2.5) |
|
| |||||
| Transfusion data | |||||
|
| |||||
| Total RBCs | 6 (4,10) | 9 (6,15) | 7 (5,11.5) | 11 (7,18.5) | 9 (6,15) |
|
| |||||
| Total Plasma | 4 (2,8) | 6 (3,12) | 4.5 (3,8) | 8 (4,13) | 6 (3,11) |
|
| |||||
| Total Platelets | 6 (0,12) | 6 (6,18) | 6 (6,12) | 12 (6,18) | 6 (6,18) |
|
| |||||
| Total Crystalloids | 5 (3.4,8.5) | 6.8 (4,10.5) | 6.1 (3.6, 9.3) | 6.7 (3.7,10.4) | 6.4 (3.7, 9.9) |
|
| |||||
| Massive transfusion (%) | 39 (30) | 186 (47.9) | 8 (28.6) | 75 (60.5) | 308 (46) |
|
| |||||
| Time to anatomic hemostasis from randomization (minutes) | 86 (42,155) | 110 (62.5,180) | 93 (61,163) | 115 (72,252) | 103 (61,179) |
Continuous data are described with median and interquartile range. GCS-Glasgow coma scale, GOSE-Glasgow outcome scale. Transfusion data includes all blood products given in the prehospital environment up to the first 24 hours after admission.
In the adjusted analysis of 30 day mortality, odds of death in the HS only, TBI only and TBI+HS groups were 2.6 (95% CI, 1.2–5.4), 8.2 (95% CI, 3.4–19.5) and 10.6 (95% CI, 4.8–23.2) times higher than the neither group, respectively. Covariates in this model included treatment group, age, blunt injury, total blood products received prior to randomization and time to randomization. Using multiple logistic regression and excluding early deaths (<24 hours) because they were not at risk of developing a respiratory complication, patients in the TBI only and HS+TBI groups had significantly increased odds of developing a respiratory complication (acute respiratory distress syndrome, acute lung injury, or ventilator-associated pneumonia) compared to the neither group. Adjusted odds of developing a respiratory complication were 4.6 (95% CI, 1.6–13.1) and 2.9 (95% CI, 1.9–4.3 in the TBI only and HS+TBI groups respectively. The HS only group did not have significantly increased odds of respiratory complications compared to the neither group (OR 1.4; 95% CI, 0.89–2.19). This model adjusted for treatment group, sex, race, blunt injury, and time to randomization.
One hundred nineteen patients had information regarding the Glasgow Outcomes Scale Extended (GOSE). Unadjusted differences in GOSE were not statistically significantly different between the TBI only group (median GOSE 1.0: IQR; 2,4) and the TBI+HS group (median GOSE 1: IQR; 1,4). In the final logistic regression model, treatment with a 1:1:1 ratio was associated with an increased odds of having a worse GOSE (OR 1.92: 95% CI; 1.11, 3.31), but time to randomization was associated with an improved GOSE (OR 0.97: 95% CI; 0.96,0.98); no other variables were independently associated with worse neurological outcomes as assessed by GOSE.
DISCUSSION
TBI and HS remain the predominant predictors of death following severe injury. The combination of TBI and HS is associated with worse coagulopathy prior to resuscitation and increased morbidity and mortality compared to the HS or neither groups. When controlling for multiple known confounders, the diagnosis of TBI alone or TBI+HS was associated with significantly greater odds of developing respiratory complications.
The worse outcomes observed in the TBI+HS group are not surprising considering well-established principles of pathophysiology‥ In models of penetrating brain injury and HS, regional cerebral blood flow has been shown to decrease by more than 70% from baseline and is associated with slower cortical spreading depolarizations and reductions in brain tissue oxygen tension (P(bt)O2).22 Combined hypoxemia and hemorrhagic shock in animal models of TBI + HS have been associated with transient intracerebral reductions in brain glucose, pyruvate, and prolonged elevations of lactate.23,24 Energy-related neurochemical dysregulation in TBI + HS is likely a major factor contributing to worse neurologic outcomes.25 Therefore, principles of damage-control resuscitation, which include permissive hypotension,4,5,9 require further study in patients with TBI+HS, since a higher mean arterial pressure (>70-mm Hg) may result in better cerebral blood flow and cerebral mitochondrial function.26 Speed of resuscitation may also be imperative for protecting brain function; animal models using a stepwise ratio-guided protocol with FFP (compared to a bolus protocol) have demonstrated a neuroprotective effect.27 Additional pharmacologic modalities, such as valproic acid28,29 and hypertonic saline30 continue to be studied for the prevention of cerebral metabolic derangements and excitotoxicity in patients with TBI + HS.
In this analysis, patients with concomitant TBI and HS had worse coagulopathy, as evidenced by higher activated prothrombin times and lower platelet counts compared to all other groups at admission and upon termination of the study protocol. This finding is of great importance for trauma surgeons, intensivists, and all providers charged with caring for patients with these injuries. Whereas brain injury has been shown to cause altered vascular compensation in response to HS, 31 questions remain about the relationship between coagulopathy and the size of brain lesions.15,22,30,32,33 Ratio-guided resuscitation with fresh frozen plasma (FFP) may have beneficial effects for patients with TBI + HS,15 including fewer inflammatory complications.5,6,9 In experimental large animal studies, early administration of FFP reduced the size of brain lesions, decreased cerebral edema, and substantially attenuated the degree of neurological impairment.32,34 Benefits of FFP resuscitation may be related to gene expression. Recent work has shown that FFP resuscitation was positively associated with two distinct gene clusters, with up-regulation of genes involved in metabolic and platelet signaling as well as collagen formation, and down regulation of inflammatory pathway genes.35 Further work in this area is indicated to define the pathophysiological consequences of TBI + HS in humans, and the effects of interventions, such as ratio-guided resuscitation with blood components.
The presence of TBI only and TBI + HS was associated with greater odds of developing respiratory complications. This finding has significant implications for perioperative care of these patients. Pulmonary complications are known to be prevalent in patients with TBI. Acute lung injury, acute respiratory distress syndrome, pneumonia, pleural effusions, pulmonary edema, and pulmonary emboli are frequently encountered in this population.36 Timing of tracheostomy,37 early application of early lung protective strategies,38 and timely mobilization39 merit special consideration in this population.
There are a number of limitations with this work. Most studies of TBI and HS have been focused on neuronal death,33 neurochemical changes,23 or other physiological mechanisms,22,24 and none have been done in humans. While the number of patients in this post hoc analysis of patients previously randomized in PROPPR yielded a modest number of patients with TBI and HS, to our knowledge, this represents the largest study population with these two conditions conducted to date. The patients enrolled in PROPPR were selected because they were thought to be massively bleeding by the Assessment of Blood Consumption score or physician gestalt. For this reason, the results may not be applicable to a more general population of trauma patients with HS and/or TBI.40. Coagulopathy in this study was assessed with conventional tests such as PT, aPTT, and INR. Analyses did not fully control for additional variables such as thromboelastography since these results were not universally available for all patients in PROPPR. Although the role of thromboelastography and viscoelastic monitoring continues to evolve in trauma patients,41–43 it is possible that the degree of coagulopathy may have been underestimated in this study population since a growing body of literature suggests that early trauma-induced coagulopathy may be detected with these methods before changes are observed with conventional coagulation tests.44 Finally, longer term outcomes data were not available in this post hoc study; GOSE was studied in a small proportion (n=119) of patients where these data were available. Considering the relatively small number of patients with GOSE, it is difficult to draw firm conclusions regarding transfusion ratios and time to treatment. Given that this is a post hoc secondary analysis of prospectively collected data, it is impossible to make causal inferences; future work in this area, including additional multi-center studies that are appropriately powered to investigate the clinical impact of TBI with concomitant HS are warranted to elucidate physiological mechanisms and potentially beneficial resuscitation strategies.
Future studies in this area will be difficult to design and execute, but more work in this area is necessary considering the findings from our analysis. In this study, definitions of HS and TBI were based on the data available in PROPPR, which was not designed to prospectively identify these groups. In future studies, better definitions for TBI will be required. These definitions will likely require biomarkers, advanced monitors, or other biological measures of neuronal injury that can be readily measured. Future studies examining interventions for TBI+HS will need to utilize innovative designs and methodologies such as competing risks analyses, Bayesian analyses, and adaptive trial designs; broader study endpoints will also need to be considered, including indices of morbidity.45
CONCLUSION
The combination of TBI and HS was common (18%) and associated with worse coagulopathy prior to resuscitation, increased mortality at 30 days, and increased odds for developing a respiratory complication. Additional work is required in this area to better understand the pathophysiological consequences and potentially beneficial therapeutic interventions for patient with TBI and concomitant HS.
Acknowledgments
Source of Funding/ Role of Sponsors: This work was sponsored by the U.S. National Heart, Lung, and Blood Institute (U01HL077863) and the U.S. Department of Defense, as well as Defence Research and Development Canada in partnership with the Canadian Institutes of Health Research (CIHR) - Institute of Circulatory and Respiratory Health (CRR-120612). No sponsors were involved in the collection, management, or analysis of the data; preparation of the manuscript; or the decision to submit the manuscript for publication. The content is the sole responsibility of the authors and is not to be construed as official or as reflecting the views of any sponsor.
Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) Study Group:
Clinical Coordinating Center, University of Texas Health Science Center at Houston: John B. Holcomb, MD; Charles E. Wade, PhD; Deborah J. del Junco, PhD; Erin E. Fox, PhD; Nena Matijevic, PhD (Laboratory Committee co-Chair); Jeanette M. Podbielski, RN; Angela M. Beeler, BS.
Data Coordinating Center, University of Texas Health Science Center at Houston: Barbara C. Tilley, PhD; Sarah Baraniuk, PhD; Stacia M. DeSantis, PhD; Hongjian Zhu, PhD; Joshua Nixon, MS; Roann Seay, MS; Savitri N. Appana, MS; Hui Yang, MS; Michael O. Gonzalez, MS.
Core Laboratory, University of Texas Health Science Center at Houston: Lisa Baer, MS; Yao-Wei Willa Wang, MD; Brittany S. Hula, MS; Elena Espino, BS; An Nguyen, BS; Nicholas Pawelczyk, BS; Kisha D. Arora-Nutall, BS; Rishika Sharma, MD; Jessica C. Cardenas, PhD; Elaheh Rahbar, PhD; Tyrone Burnett, Jr., BS; David Clark, BS.
Resuscitation Outcomes Consortium, University of Washington: Gerald van Belle, PhD; Susanne May, PhD; Brian Leroux, PhD; David Hoyt, MD; Judy Powell, BSN, RN; Kellie Sheehan, BSN.
Systems Biology Committee, University of California, Berkeley: Alan Hubbard, PhD (co-Chair); Adam P. Arkin, PhD.
Transfusion Committee: John R. Hess, MD, MPH (co-Chair, University of Washington); Jeannie L. Callum, MD (co-Chair, Sunnybrook Health Sciences Centre)
Anesthesiology Committee: Jean-Francois Pittet, MD (Chair, University of Alabama at Birmingham)
Emergency Medicine Committee: Christopher N. Miller, MD (Chair, University of Cincinnati);
PROPPR Clinical Sites (listed in order of number of patients enrolled):
University of Texas Health Science Center at Houston: Bryan A. Cotton, MD, MPH; Laura Vincent, BSN, RN, CCRP; Timothy Welch; Tiffany Poole, DC; Evan G. Pivalizza, MD; Sam D. Gumbert, MD; Yu Bai, MD, PhD; James J. McCarthy, MD; Amy Noland, MD; Rhonda Hobbs, MT(ASCP)SBB.
University of Washington: Eileen M. Bulger, MD; Patricia Klotz, RN; Lindsay Cattin, BA; Keir J. Warner, BS; Angela Wilson, BA; David Boman, BA; Nathan White, MD, MS; Andreas Grabinsky, MD; Jennifer A. Daniel-Johnson, MBBS.
University of California, San Francisco: Mitchell Jay Cohen, MD (Systems Biology and Laboratory Committees co-Chair); Rachael A. Callcut, MD, MSPH; Mary Nelson, RN, MPA; Brittney Redick, BA; Amanda Conroy, BA; Marc P. Steurer, MD, DESA; Preston C. Maxim, MD; Eberhard Fiebig, MD; Joanne Moore; Eireen Mallari, MT.
University of Cincinnati: Peter Muskat, MD; Jay A. Johannigman, MD; Bryce R. H. Robinson, MD; Richard D. Branson, MSc, RRT; Dina Gomaa, BS, RRT; Christopher Barczak, BS, MT(ASCP); Suzanne Bennett, MD; Patricia M. Carey, MD; Helen Hancock, BS, MT(ASCP); Carolina Rodriguez, BA.
University of Southern California: Kenji Inaba, MD; Jay G. Zhu, MD; Monica D. Wong, MS; Michael Menchine, MD, MPH; Kelly Katzberg, MD, FACEP; Sean O. Henderson, MD; Rodney McKeever, MD; Ira A. Shulman, MD; Janice M. Nelson, MD; Christopher W. Tuma, BA, MT(ASCP), SBB; Cheryl Y. Matsushita, BS, MT(ASCP).
Shock, Trauma and Anesthesiology Research - Organized Research Center (STAR-ORC), R Adams Cowley Shock Trauma Center, University of Maryland Medical Center: Thomas M. Scalea, MD; Deborah M. Stein, MD, MPH; Cynthia K. Shaffer, MS, MBA; Christine Wade, BA; Anthony V. Herrera, MS; Seeta Kallam, MBBS; Sarah E. Wade, BS; Samuel M. Galvagno, Jr, DO, PhD; Magali J. Fontaine, MD, PhD; Janice M. Hunt, BS, MT(ASCP) SBB; Rhonda K. Cooke, MD.
University of Tennessee Health Science Center, Memphis: Timothy C. Fabian, MD; Jordan A. Weinberg, MD; Martin A. Croce, MD; Suzanne Wilson, RN; Stephanie Panzer-Baggett, RN; Lynda Waddle-Smith, BSN; Sherri Flax, MD.
Medical College of Wisconsin: Karen J. Brasel, MD, MPH; Pamela Walsh, AS, CCRC; David Milia, MD; Allia Nelson, BS, BA; Olga Kaslow, MD, PhD; Tom P. Aufderheide, MD, MS; Jerome L. Gottschall, MD; Erica Carpenter, MLS(ASCP).
University of Arizona: Terence O’Keeffe, MBChB, MSPH; Laurel L. Rokowski, RN, BSN, MKT; Kurt R. Denninghoff, MD; Daniel T. Redford, MD; Deborah J. Novak, MD; Susan Knoll, MS, MT(ASCP) SBB.
University of Alabama at Birmingham: Jeffrey D. Kerby, MD, PhD; Patrick L. Bosarge, MD; Albert T. Pierce, MD; Carolyn R. Williams, RN, BSN, BSME; Shannon W. Stephens, EMTP; Henry E. Wang, MD, MS; Marisa B. Marques, MD.
Oregon Health and Science University: Martin A. Schreiber, MD ; Jennifer M. Watters, MD; Samantha J. Underwood, MS; Tahnee Groat, MPH; Craig Newgard, MD, MPH; Matthias Merkel, MD, PhD ; Richard M. Scanlan, MD; Beth Miller, MT(ASCP)SBB.
Sunnybrook Health Science Center: Sandro Rizoli, MD, PhD; Homer Tien, MD; Barto Nascimento, MD, MSc, CTBS; Sandy Trpcic; Skeeta Sobrian-Couroux, RN, CCRP, BHA; Marciano Reis; Adic Pérez, MD; Susan E. Belo, MD, PhD; Lisa Merkley, BA, MLT, CBTS; Connie Colavecchia, BSc, MLT.
Footnotes
Trial Registration: Clinicaltrials.gov, NCT01545232
Conflicts of Interest: No conflicts of interest have been declared by any author in regards to this manuscript.
Presentation: This study was presented at the 30th Annual Scientific Assembly of the Eastern Association for the Surgery of Trauma (EAST), January 10–14, 2017, in Hollywood, FL.
Author Contributions:
Study concept and design: Stein, Fox, Baraniuk, Holcomb, Galvagno
Acquisition of data: Stein, Bosarge, Bulger, Callcut, Cotton, Goodman, Inaba, O’Keeffe, Schreiber, Scalea, Holcomb, Galvagno
Analysis and interpretation of data: Fox, Appana, Galvagno, Stein, Baraniuk,
Drafting of the manuscript: Galvagno, Fox, Stein
Critical revision of the manuscript for important intellectual content: Galvagno, Fox, Appana, Stein, Baraniuk, Bosarge, Bulger, Callcut, Cotton, Goodman, Inaba, O’Keeffe, Schreiber, Wade, Scalea, Holcomb
Contributor Information
Samuel M. Galvagno, Jr, University of Maryland School of Medicine, Department of Anesthesiology, Chief, Division of Critical Care Medicine And Associate Director of Critical Care, University of Maryland Medical Center, Program in Trauma, R Adams Cowley Shock Trauma Center, 22 South Greene Street, T3N08, Shock Trauma Center, Baltimore, MD, 21201, sgalvagno@anes.umm.edu
Erin E. Fox, Assistant Professor, Department of Surgery, Division of Acute Care Surgery, Center for Translational Injury Research (CeTIR), University of Texas Health Science Center at Houston, Houston, TX, Erin.E.Fox@uth.tmc.edu
Savitri N. Appana, Senior Statistician, The University of Texas Health Sciences Center at Houston, School of Public Health, Department of Biostatistics, Houston, TX, Savitri.N.Appana@uth.tmc.edu
Sarah Baraniuk, Assistant Professor of Biostatistics, University of Texas-Houston Health Sciences Center School of Public Health, Houston, TX, sbaraniuk@gmail.com
Patrick L. Bosarge, Associate Professor, University of Alabama School of Medicine, Department of Surgery, Division of Acute Care Surgery, Birmingham, AL, pbosarge@uabmc.edu
Eileen M. Bulger, Professor, University of Washington Department of Surgery, Chief of Trauma, Harborview Medical Center, Seattle, WA, ebulger@u.washington.edu
Rachel A. Callcut, Associate Professor, Division of General Surgery, University of California San Francisco, San Francisco, CA, CallcutR@sfghsurg.ucsf.edu
Bryan A. Cotton, Professor, Department of Surgery, Division of Acute Care Surgery, University of Texas Health Science Center, Houston, TX, Bryan.A.Cotton@uth.tmc.edu
Michael Goodman, Assistant Professor, Department of Surgery, University of Cincinnati School of Medicine, Cincinnati, OH, goodmamd@ucmail.uc.edu
Kenji Inaba, Associate Professor, Department of Surgery, University of Southern California Keck School of Medicine, Los Angeles, CA, Kenji.Inaba@med.usc.edu
Terence O’Keeffe, Associate Professor, University of Arizona School of Medicine, Tucson, AZ, tokeeffe@surgery.arizona.edu
Martin A. Schreiber, Professor, Oregon Health & Science University School of Medicine, Portland, OR Chief, Division of Trauma, Critical Care, and Acute Care Surgery, schreibm@ohsu.edu.
Charles E. Wade, Professor, Department of Surgery, University of Texas Health Science Center, Houston, TX, Charles.E.Wade@uth.tmc.edu
Thomas M. Scalea, Professor, Director, Program in Trauma, Francis X. Kelly Professor of Trauma Surgery, Physician-in-Chief, R Adams Cowley Shock Trauma Center, Baltimore, MD, tscalea@umm.edu
John B. Holcomb, Professor, Department of Surgery, University of Texas Health Science Center, Houston, TX, John.Holcomb@uth.tmc.edu
Deborah M. Stein, R Adams Cowley Professor of Trauma, University of Maryland School of Medicine, Department of Surgery, Program in Trauma, Chief of Trauma and Director of Neurotrauma Critical Care, R Adams Cowley Shock Trauma Center, 22 South Greene Street, S4B04, Shock Trauma Center, Baltimore, MD, 21201, dstein@umm.edu
References
- 1.Dutton RP, Stansbury LG, Leone S, Kramer E, Hess JR, Scalea TM. Trauma mortality in mature trauma systems: are we doing better? An analysis of trauma mortality patterns, 1997–2008. J Trauma. 2010;69:620–6. doi: 10.1097/TA.0b013e3181bbfe2a. [DOI] [PubMed] [Google Scholar]
- 2.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73:S431–7. doi: 10.1097/TA.0b013e3182755dcc. [DOI] [PubMed] [Google Scholar]
- 3.Eastridge BJ, Hardin M, Cantrell J, Oetjen-Gerdes L, Zubko T, Mallak C, Wade CE, Simmons J, Mace J, Mabry R, et al. Died of wounds on the battlefield: causation and implications for improving combat casualty care. J Trauma. 2011;71:S4–8. doi: 10.1097/TA.0b013e318221147b. [DOI] [PubMed] [Google Scholar]
- 4.Bogert JN, Harvin JA, Cotton BA. Damage Control Resuscitation. J Intensive Care Med. 2016;31:177–86. doi: 10.1177/0885066614558018. [DOI] [PubMed] [Google Scholar]
- 5.Cotton BA, Reddy N, Hatch QM, LeFebvre E, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Holcomb JB. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg. 2011;254:598–605. doi: 10.1097/SLA.0b013e318230089e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Undurraga VJ, Leroux B, Cook MR, Watson J, Fair K, Martin DT, Kerby JD, Williams C, Inaba K, Wade CE, et al. Damage-control resuscitation and emergency laparotomy: Findings from the PROPPR study. J Trauma Acute Care Surg. 2016;80:568–74. doi: 10.1097/TA.0000000000000960. discussion 74-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spinella PC, Holcomb JB. Resuscitation and transfusion principles for traumatic hemorrhagic shock. Blood Rev. 2009;23:231–40. doi: 10.1016/j.blre.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–82. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shrestha B, Holcomb JB, Camp EA, Del Junco DJ, Cotton BA, Albarado R, Gill BS, Kozar RA, Kao LS, McNutt MK, et al. Damage-control resuscitation increases successful nonoperative management rates and survival after severe blunt liver injury. J Trauma Acute Care Surg. 2015;78:336–41. doi: 10.1097/TA.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 10.Yuan Q, Sun YR, Wu X, Yu J, Li ZQ, Du ZY, Wu XH, Zhou LF, Hu J. Coagulopathy in Traumatic Brain Injury and Its Correlation with Progressive Hemorrhagic Injury: A Systematic Review and Meta-Analysis. J Neurotrauma. 2016;33:1279–91. doi: 10.1089/neu.2015.4205. [DOI] [PubMed] [Google Scholar]
- 11.Dekker SE, Duvekot A, de Vries HM, Geeraedts LM, Jr, Peerdeman SM, de Waard MC, Boer C, Schober P. Relationship between tissue perfusion and coagulopathy in traumatic brain injury. J Surg Res. 2016;205:147–54. doi: 10.1016/j.jss.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 12.Brasel KJ, Vercruysse G, Spinella PC, Wade CE, Blackbourne LH, Borgman MA, Zarzabal LA, Du F, Perkins JG, Maegele M, et al. The association of blood component use ratios with the survival of massively transfused trauma patients with and without severe brain injury. J Trauma. 2011;71:S343–52. doi: 10.1097/TA.0b013e318227ef2d. [DOI] [PubMed] [Google Scholar]
- 13.Peiniger S, Nienaber U, Lefering R, Braun M, Wafaisade A, Wutzler S, Borgmann M, Spinella PC, Maegele M Trauma Registry of the Deutsche Gesellschaft für Unfallchirurgie. Balanced massive transfusion ratios in multiple injury patients with traumatic brain injury. Crit Care. 2011;15:R68. doi: 10.1186/cc10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genét GF, Johansson PI, Meyer MA, Sølbeck S, Sørensen AM, Larsen CF, Welling KL, Windeløv NA, Rasmussen LS, Ostrowski SR. Trauma-induced coagulopathy: standard coagulation tests, biomarkers of coagulopathy, and endothelial damage in patients with traumatic brain injury. J Neurotrauma. 2013;30:301–6. doi: 10.1089/neu.2012.2612. [DOI] [PubMed] [Google Scholar]
- 15.Chang R, Folkerson LE, Sloan D, Tomasek JS, Kitagawa RS, Choi HA, Wade CE, Holcomb JB. Early plasma transfusion is associated with improved survival after isolated traumatic brain injury in patients with multifocal intracranial hemorrhage. Surgery. 2016 doi: 10.1016/j.surg.2016.08.023. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baraniuk S, Tilley BC, del Junco DJ, Fox EE, van Belle G, Wade CE, Podbielski JM, Beeler AM, Hess JR, Bulger EM, et al. Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) Trial: design, rationale and implementation. Injury. 2014;45:1287–95. doi: 10.1016/j.injury.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu H, Fox EE, Baraniuk S, Holcomb JB, Wade CE, Del Junco DJ, Tilley BC. Assessing protocol adherence in a clinical trial with ordered treatment regimens: Quantifying the pragmatic, randomized optimal platelet and plasma ratios (PROPPR) trial experience. Injury. 2016;47:2131–7. doi: 10.1016/j.injury.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singhal R, Coghill JE, Guy A, Bradbury AW, Adam DJ, Scriven JM. Serum lactate and base deficit as predictors of mortality after ruptured abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2005;30:263–6. doi: 10.1016/j.ejvs.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Caputo ND, Kanter M, Fraser R, Simon R. Comparing biomarkers of traumatic shock: the utility of anion gap, base excess, and serum lactate in the ED. Am J Emerg Med. 2015;33:1134–9. doi: 10.1016/j.ajem.2015.04.085. [DOI] [PubMed] [Google Scholar]
- 20.Park M, Taniguchi LU, Noritomi DT, Liborio AB, Maciel AT, Cruz-Neto LM. Clinical utility of standard base excess in the diagnosis and interpretation of metabolic acidosis in critically ill patients. Braz J Med Biol Res. 2008;41:241–9. doi: 10.1590/s0100-879x2006005000199. [DOI] [PubMed] [Google Scholar]
- 21.Yumoto T, Iida A, Hirayama T, Tsukahara K, Shiba N, Yamanouchi H, Sato K, Ugawa T, Ichiba S, Ujike Y. Immediate screening method for predicting ht enecessity of massive transfusions in trauma patients: a retrospective single-center study. J Intensive Care. 2014;2:54. doi: 10.1186/s40560-014-0054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung LY, Wei G, Shear DA, Tortella FC. The acute effects of hemorrhagic shock on cerebral blood flow, brain tissue oxygen tension, and spreading depolarization following penetrating ballistic-like brain injury. J Neurotrauma. 2013;30:1288–98. doi: 10.1089/neu.2012.2715. [DOI] [PubMed] [Google Scholar]
- 23.Leung LY, Deng-Bryant Y, Cardiff K, Winter M, Tortella F, Shear D. Neurochemical changes following combined hypoxemia and hemorrhagic shock in a rat model of penetrating ballistic-like brain injury: A microdialysis study. J Trauma Acute Care Surg. 2016;81:860–7. doi: 10.1097/TA.0000000000001206. [DOI] [PubMed] [Google Scholar]
- 24.Leung LY, Deng-Bryant Y, Shear D, Tortella F. Experimental Models Combining TBI, Hemorrhagic Shock, and Hypoxemia. Methods Mol Biol. 2016;1462:445–58. doi: 10.1007/978-1-4939-3816-2_25. [DOI] [PubMed] [Google Scholar]
- 25.Proctor JL, Scutella D, Pan Y, Vaughan J, Rosenthal RE, Puche A, Fiskum G. Hyperoxic resuscitation improves survival but worsens neurologic outcome in a rat polytrauma model of traumatic brain injury plus hemorrhagic shock. J Trauma Acute Care Surg. 2015;79:S101–9. doi: 10.1097/TA.0000000000000742. [DOI] [PubMed] [Google Scholar]
- 26.Hu Y, Wu Y, Tian K, Lan D, Chen X, Xue M, Liu L, Li T. Identification of ideal resuscitation pressure with concurrent traumatic brain injury in a rat model of hemorrhagic shock. J Surg Res. 2015;195:284–93. doi: 10.1016/j.jss.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 27.Sillesen M, Jin G, Johansson PI, Alam HB. Resuscitation speed affects brain injury in a large animal model of traumatic brain injury and shock. Scand J Trauma Resusc Emerg Med. 2014;22:46. doi: 10.1186/s13049-014-0046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwabejire JO, Jin G, Imam AM, Duggan M, Sillesen M, Deperalta D, Jepsen CH, Lu J, Li Y, deMoya MA, et al. Pharmacologic modulation of cerebral metabolic derangement and excitotoxicity in a porcine model of traumatic brain injury and hemorrhagic shock. Surgery. 2013;154:234–43. doi: 10.1016/j.surg.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Jin G, Duggan M, Imam A, Demoya MA, Sillesen M, Hwabejire J, Jepsen CH, Liu B, Mejaddam AY, Lu J, et al. Pharmacologic resuscitation for hemorrhagic shock combined with traumatic brain injury. J Trauma Acute Care Surg. 2012;73:1461–70. doi: 10.1097/TA.0b013e3182782641. [DOI] [PubMed] [Google Scholar]
- 30.Pinto FC, Oliveira MF, Prist R, Silva MR, Silva LF, Capone Neto A. Effect of volume replacement during combined experimental hemorrhagic shock and traumatic brain injury in prostanoids, brain pathology and pupil status. Arq Neuropsiquiatr. 2015;73:499–505. doi: 10.1590/0004-282X20150039. [DOI] [PubMed] [Google Scholar]
- 31.Fulton RL, Flynn WJ, Mancino M, Bowles D, Cryer HM. Brain injury causes loss of cardiovascular response to hemorrhagic shock. J Invest Surg. 1993;6:117–31. doi: 10.3109/08941939309141603. [DOI] [PubMed] [Google Scholar]
- 32.Halaweish I, Bambakidis T, He W, Linzel D, Chang Z, Srinivasan A, Dekker SE, Liu B, Li Y, Alam HB. Early resuscitation with fresh frozen plasma for traumatic brain injury combined with hemorrhagic shock improves neurologic recovery. J Am Coll Surg. 2015;220:809–19. doi: 10.1016/j.jamcollsurg.2015.01.057. [DOI] [PubMed] [Google Scholar]
- 33.Dennis AM, Haselkorn ML, Vagni VA, Garman RH, Janesko-Feldman K, Bayir H, Clark RS, Jenkins LW, Dixon CE, Kochanek PM. Hemorrhagic shock after experimental traumatic brain injury in mice: effect on neuronal death. J Neurotrauma. 2009;26:889–99. doi: 10.1089/neu.2008.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin G, DeMoya MA, Duggan M, Knightly T, Mejaddam AY, Hwabejire J, Lu J, Smith WM, Kasotakis G, Velmahos GC, et al. Traumatic brain injury and hemorrhagic shock: evaluation of different resuscitation strategies in a large animal model of combined insults. Shock. 2012;38:49–56. doi: 10.1097/SHK.0b013e3182574778. [DOI] [PubMed] [Google Scholar]
- 35.Sillesen M, Bambakidis T, Dekker S, Li Y, Alam HB. Fresh Frozen Plasma Modulates Brain Gene Expression in a Swine Model of Traumatic Brain Injury and Shock: A Network Analysis. J Am Coll Surg. 2016 doi: 10.1016/j.jamcollsurg.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Lee K, Rincon F. Pulmonary complications in patients with severe brain injury. Critical Care Research and Practice. 2012;2012:1–8. doi: 10.1155/2012/207247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alali AS, Scales DC, Fowler RA, Mainprize TG, Ray JG, Kiss A, de Mestral C, Nathens AB. Traucheostomy timing in traumatic brain injury: a propensity-matched cohort study. J Trauma Acute Care Surg. 2014;76:70–6. doi: 10.1097/TA.0b013e3182a8fd6a. [DOI] [PubMed] [Google Scholar]
- 38.Arora S, Singh PM, Trikha A. Ventilatory strategies in trauma patients. Journal of Emergencies, Trauma, and Shock. 2014;7:25–31. doi: 10.4103/0974-2700.125635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark DE, Lowman JD, Reiff JD. Effectiveness of an early mobilzation protocol in a trauma and burns intensive care unit: a retrospective cohort study. Phys Ther. 2013;93:186–96. doi: 10.2522/ptj.20110417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nunez TC, Voskresensky IV, Dossett LA, Shinall R, Dutton WD, Cotton BA. Early prediction of massive transfusion in trauma: simple as ABC (assessment of blood consumption)? J Trauma. 2009;66:346–52. doi: 10.1097/TA.0b013e3181961c35. [DOI] [PubMed] [Google Scholar]
- 41.Da Luz LT, Nascimento B, Shankarakutty AK, Rizoli S, Adhikari NK. Effect of thromboelastography (TEG(R)) and rotational thromboelastometry (ROTEM(R)) on diagnosis of coagulopathy, transfusion guidance and mortality in trauma: descriptive systematic review. Crit Care. 2014;18:518. doi: 10.1186/s13054-014-0518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunt H, Stanworth S, Curry N, Woolley T, Cooper C, Ukoumunne O, Zhelev Z, Hyde C. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma induced coagulopathy in adult trauma patients with bleeding. Cochrane Database Syst Rev. 2015:CD010438. doi: 10.1002/14651858.CD010438.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whiting P, Al M, Westwood M, Ramos IC, Ryder S, Armstrong N, Misso K, Ross J, Severens J, Kleijnen J. Viscoelastic point-of-care testing to assist with the diagnosis, management and monitoring of haemostasis: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2015;19:1–228. v–vi. doi: 10.3310/hta19580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liou DZ, Shafi H, Bloom MB, Chung R, Ley EJ, Salim A, Tcherniantchouk O, Margulies DR. Defining early trauma-induced coagulopathy using thromboelastography. Am Surg. 2014;80:994–8. [PubMed] [Google Scholar]
- 45.Angus D, Mira J-P, Vincent J-L. Improving clinical trials in the critically ill. Crit Care Med. 2010;38:527–32. doi: 10.1097/CCM.0b013e3181c0259d. [DOI] [PubMed] [Google Scholar]


