Abstract
Purpose
To quantify and correlate ellipsoid zone and photoreceptor outer segment changes with visual acuity in Stargardt disease.
Methods
An IRB-approved study of 32 eyes with Stargardt disease was performed. Following spectral domain optical coherence tomography, the macular cube was exported into a novel analysis tool and volumetric assessment from the ellipsoid zone to the retinal pigment epithelium (RPE) was performed. Utilizing this information, mapping was completed with en face representation of the height between the ellipsoid zone and RPE. This analysis provided quantification of ellipsoid zone and photoreceptor outer segments, including atrophy (ellipsoid zone to RPE thickness = 0 microns) and attenuation (ellipsoid zone to RPE thickness < 20 microns). These parameters were compared to visual acuity and to controls (n = 12 eyes).
Results
Visual acuity ranged from 20/30 to 20/250. Central foveal B-scan area of ellipsoid and photoreceptor outer segments was significantly less than controls (0.13 +/− 0.05mm2 vs 0.17 +/− 0.03 mm2, respectively p=0.0074). Central foveal B-scan mean thickness measured 22.52 +/− 9.0 um in Stargardt versus 30.0 +/− 5.08 um (p=0.0096). Atrophy and attenuation were significantly higher in Stargardt patients (22% vs. 1%, p=0.005 and 43% vs 1%, p=0.0002). Visual acuity directly correlated with ellipsoid zone/outer segment volume (R = 0.57, p < 0.005), and inversely correlated with attenuation and atrophy (R= −0.53 and −0.57; p < 0.005 for all).
Conclusion
Eyes with Stargardt disease frequently have significant disruption of the ellipsoid zone and outer segments. This degenerative change was successfully quantified with a novel assessment platform and identified correlates with visual function. This software provides the opportunity for quantitative assessment and possible longitudinal surveillance.
Keywords: Stargardt disease, OCT, optical coherence tomography, ellipsoid zone, ellipsoid zone mapping
Introduction
Karl Stargardt first described Stargardt disease in 1909.1 Characterized by macular atrophy, deep retinal yellow flecks, and progressive vision loss, it is the most common inherited juvenile macular dystrophy, occurring in 1 out of 10, 000 individuals.2, 3 Most commonly, the disease follows an autosomal recessive inheritance pattern, with mutations in the gene ABCA4, which codes for a retina specific ATP binding cassette transporter, ABCR. This transporting molecule is integral to movement of all-trans-retinol produced in rod outer segments. Dysfunction of this molecule leads to the toxic accumulation of lipofuscin in RPE cells. 4
Interestingly, visual acuity in these patients is highly variable, ranging from 20/20 to light perception.5 A number of studies have linked the decrease in visual acuity to inner and outer retinal changes.5–7 Investigations into retinal structure to explain the decline in vision have revealed loss of foveal photoreceptor and retinal pigment epithelium (RPE) layers, as well as disruptions in photoreceptor quantity.8–14 In particular, various analyses reveal a decrease in the foveal ellipsoid zone and photoreceptor outer segments in Stargardt patients, indicated by ellipsoid zone disruption on spectral domain optical coherence tomography (SD-OCT).9, 10 While these studies have demonstrated a qualitative decrease in these retinal layers, quantitative assessment of ellipsoid zone and outer segment loss remains uninvestigated. Ellipsoid zone mapping has been previously described in other macular disorders, including macular degeneration, hydroxychloroquine retinopathy, and ocriplasmin maculopathy.15, 16 The purpose of the present study was to utilize a novel ellipsoid zone mapping platform to quantify and characterize the ellipsoidal and photoreceptor outer segment changes noted in Stargardt disease.
Methods
This IRB approved retrospective case series included 32 eyes of 17 patients diagnosed with Stargardt disease between October 2012 to November 2014. Demographic and clinical data were collected through patient charts, and included gender, age at presentation, visual acuity (VA, Snellen visual acuity was converted to logmar for the purposes of statistical analysis) and visible macular pathology, including flecks, atrophy, and pigmentary changes. Inclusion criteria included an underlying diagnosis of Stargardt disease and SD-OCT macular cube data of sufficient quality for analysis.
SD-OCT images were reviewed for qualitative features including epiretinal membrane, atrophy, and fluid. In addition to qualitative review, macular SD-OCT cubes were exported for each patient into a novel ellipsoid zone and retinal layer mapping tool, as previously described.15, 16 This mapping tool allows for en face analysis and volumetric evaluation of the ellipsoid zone and the underlying photoreceptor outer segments as defined with OCT reflectivity patterns. The inner segmentation boundary was the ellipsoid zone and the outer segmentation boundary was the RPE. The automated mapping tool provides multiple quantitative outputs. The specific parameters examined in this report included central foveal B-scan ellipsoid zone to RPE mean thickness (defined as the mean distance between the ellipsoid zone to RPE on the horizontal B-scan that included the foveal pit), central foveal B-scan ellipsoid zone to RPE (defined as area occupied by the ellipsoid zone and photoreceptor outer segments on the 6 mm horizontal B-scan that included the foveal pit), and the ellipsoid and photoreceptor outer segment macular volume (defined as the volume across the entire macular scan occupied by the space from the ellipsoid zone to the RPE). In addition, the en face ellipsoid zone map provided calculations related to percentage of ellipsoid zone atrophy (i.e., complete loss of ellipsoid zone with measurement value of ellipsoid zone to the RPE height of 0 microns) and attenuation (i.e., ellipsoid zone to RPE distance of <20 microns). These measurements were then compared to the normal ellipsoid zone and underlying photoreceptor outer segment measurements of 12 controls, as previously described.15
Results
Thirty-two eyes of 17 patients were included in this analysis. Ten of the 17 patients were males (59%). The mean age at presentation was 35.8 years (median, 36 years; range 11 to 65 years). Twenty eyes (63%) had macular flecks and 19 (59%) had clinical macular atrophy. Visual acuity ranged from 20/30 to 20/250.
Qualitative assessment of the OCT scans identified epiretinal membranes in 3 of the 32 (9%) although one of these was very slight. Four eyes had intraretinal fluid (13%), and 2 had lamellar macular holes (6%). All 32 patients had subfoveal ellipsoid zone disruption, while 8 had extrafoveal atrophy (25%). Figure 1 displays fundus photographs, autofluorescence, and SD-OCT displaying foveal ellipsoid zone and photoreceptor outer segment loss in a representative case.
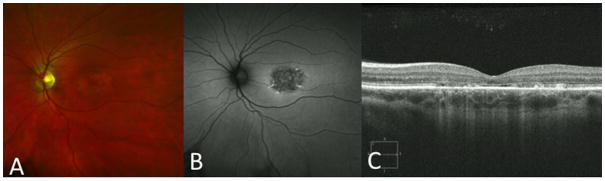
Figure 1.
Composite image of spectral domain optical coherence tomography (SD-OCT), fundus photograph and fundus auto-fluorescence (FAF). (A) Fundus photograph and (B) FAF with areas of macular atrophy. (C) SD-OCT displaying foveal ellipsoid zone loss.
In the Stargardt disease cohort, ellipsoid zone and photoreceptor outer segment central foveal B-scan area measured 0.13 +/− 0.05 mm2 compared to 0.17 +/− 0.03 mm2 (p=0.0074) in the control group. Corresponding central foveal B-scan mean ellipsoid zone to RPE thickness was 22.52 +/− 9.0 um compared to 30.0 +/− 5.08 um (p=0.0096) in controls. En face ellipsoid zone to RPE thickness maps were generated across the macular cube. These maps facilitated visualization of overall alterations, regional location, and severity of loss (Figures 2 and 3). The mean percentage of macular ellipsoid zone and outer segment attenuation was significantly higher in Stargardt disease eyes (43%) vs normal eyes (< 1%, p=0.0002). In addition, atrophy was significantly higher in Stargardt eyes compared to controls (22% vs <1%, p = 0.0005). Visual acuity was inversely correlated with EZ attenuation and atrophy (correlation coefficient = −0.53, p=0.00061 and correlation coefficient −0.57, p=0.00162 respectively). Additionally, ellipsoid zone and photoreceptor outer segment volume was directly correlated with visual acuity (correlation coefficient 0.57, p=0.00064).
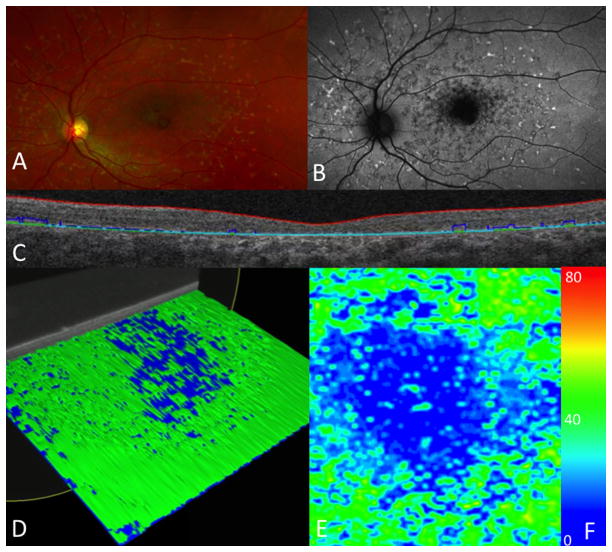
Figure 2.
Ellipsoid zone and photoreceptor outer segment mapping in a patient with moderate attenuation. (A) Fundus photograph with macular changes. (B) Fundus auto-fluorescence showing macular atrophy. (C) Ellipsoid and photoreceptor outer segment segmentation of B-scan. (D) Reconstruction of a three dimensional macular cube with moderate loss. (E) En face map with central areas of loss (blue) surrounded by normal thickness of the ellipsoid and photoreceptor outer segments (green). (F) Color scale depicting the thickness range (in microns) visualized on an en face map, from atrophy (0 microns, dark blue) to 80 microns (red). Normal ellipsoid zone and photoreceptor outer segment thickness (40 microns) is green.
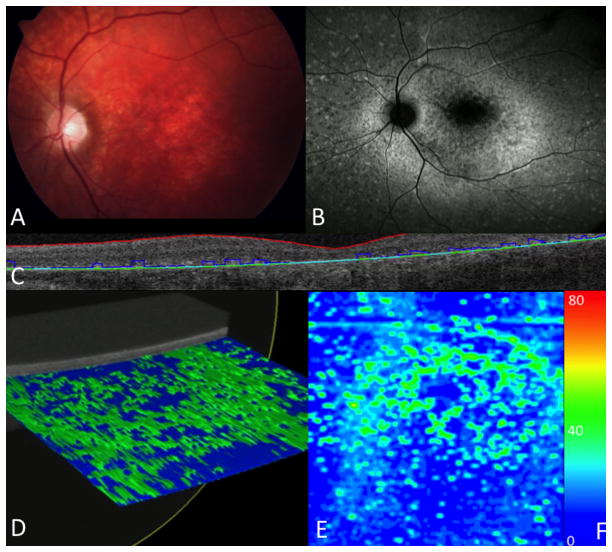
Figure 3.
Ellipsoid zone and photoreceptor outer segment mapping in a patient with severe attenuation. (A) Fundus photograph with macular changes. (B) Fundus auto-fluorescence showing macular atrophy. (C) Ellipsoid zone and photoreceptor outer segment segmentation of B scan. (D) Reconstruction of a three dimensional macular cube with severe loss. (E) En face map with larger central areas of loss (blue), with small areas of normal (green). (F) Color scale depicting the thickness range (in microns) visualized on an en face map, from atrophy (0 microns, dark blue) to 80 microns (red). Normal ellipsoid zone and photoreceptor and outer segment thickness (40 microns) is green.
Discussion
Stargardt disease is most commonly characterized by juvenile onset macular degeneration, linked to RPE toxicity from lipofuscin accumulation due to a defect in the gene ABCA4.4 Histopathologic analysis confirms lipofuscin deposits in the foveal RPE with relative sparing of the periphery.14 Early reports established the relationship between macular atrophy and vision loss, while later investigations identified the importance of ellipsoid zone preservation in maintaining visual acuity. 8–10, 12, 13 Confirmation of photoreceptor loss has been achieved with infrared scanning laser ophthalmoscopy and OCT. 5–10, 12 Degeneration of the ellipsoid zone has been identified as a key feature of Stargardt disease in previous reports, but quantitative assessment of these outer retinal alterations in Stargardt disease has not been assessed. We utilized a novel imaging analysis technique to provide both an en face representation of the ellipsoid zone and photoreceptor outer segments alterations and volumetric assessment of these layers in eyes of patients with Stargardt disease.
Greenstein et al established a pattern of progressive of retinal atrophy that progressed outward from central foveal lesions in Stargardt disease.9 An additional report assessed the presence or absence of hyperreflective bands of the outer retina in Stargardt disease with OCT. The hyperreflective ellipsoid zone was uniformly present outside of the fovea, but only present in the fovea of 12% of the patients. 10 The current report not only confirms the high frequency of ellipsoid zone and photoreceptor outer segment loss in Stargardt disease, but also quantifies the degeneration. Furthermore, the analysis platform provided en face mapping of the thickness between the ellipsoid zone and the RPE that includes the photoreceptor outer segments that allowed for a unique visualization of atrophy and attenuation.
The association between visual acuity and outer retinal integrity has been well-documented in Stargardt disease. One previous report compared the preservation of the foveal photoreceptors in a patient with 20/20 vision and the paucity of the same layer in another patient with 20/100 vision.5 Other studies have correlated transverse photoreceptor loss with visual acuity.6 Similarly, the current report demonstrated the relationship between visual acuity and ellipsoid zone status. Specifically, visual acuity was directly correlated with ellipsoid zone/photoreceptor outer segment volume and inversely correlated with en face ellipsoid zone loss/atrophy and attenuation of the photoreceptor outer segments/ellipsoid zone.
This study does have important limitations that should be mentioned. This was retrospective and cross-sectional data. This study does not include assessment of longitudinal outer retinal changes. The majority of the patients included in this study had fairly advanced disease, increasing the probability of ellipsoid and photoreceptor outer segment loss. Observing these patients, as well as eyes with earlier disease, over a longer period with serial OCTs would allow for prospective analysis of the longitudinal dynamics that occur.
This novel imaging analysis technique may be instrumental in facilitating identification and surveillance of the outer retinal loss that occurs in Stargardt disease. Higher order quantitative assessment of these outer retinal changes provides a unique opportunity for disease monitoring and future clinical trial assessments. Future studies should include longitudinal assessment of quantitative metrics and evaluation of less severe phenotypes.
Summary Statement.
A novel ellipsoid zone mapping tool allows for quantitative assessment and en face visualization of the ellipsoid zone and photoreceptor outer segment changes in Stargardt disease.
Acknowledgments
Financial Support: NIH/NEI K23-EY022947-01A1 (JPE); Ohio Department of Development TECH-13-059 (JPE); Research to Prevent Blindness (Cole Eye Institutional)
Footnotes
Financial Disclosures:
SA: None; EIT: Sanofi (C), Retrophin (C), Sparks Therapeutics (C); JPE: Bioptigen (C, P), Thrombogenics (C, R), Synergetics (P), Genentech (R), Regeneron (R), Leica (C), Zeiss (C), Alcon (C, R), Santen (C);
The funders had no role in the design and conduct of the study, in the collection, analysis and interpretation of the data, and in the preparation, review or approval of the manuscript. Justis P. Ehlers, M.D. has had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Stargardt K. Uber familiar progressive degeneration in der makulagegend des auges. Albrecht von Graefes Arch Klin Exp Ophthalmol. 1909;71:534–50. [Google Scholar]
- 2.Bither PP, Berns LA. Stargardt’s disease: a review of the literature. J Am Optom Assoc. 1988;59:106–11. [PubMed] [Google Scholar]
- 3.Fishman GA. Fundus flavimaculatus. A clinical classification. Arch Ophthalmol. 1976;94:2061–7. doi: 10.1001/archopht.1976.03910040721003. [DOI] [PubMed] [Google Scholar]
- 4.Haji AS, Hirose T. Stargardt-fundus flavimaculatus: recent advancements and treatment. Semin Ophthalmol. 2013;28(5–6):372–6. doi: 10.3109/08820538.2013.825286. [DOI] [PubMed] [Google Scholar]
- 5.Wirtutscg MG, Ergun E, Hermann B, et al. Ultrahigh resolution optical coherence tomography in macular dystrophy. Am J Ophthalmol. 2005;140:976–83. doi: 10.1016/j.ajo.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 6.Ergun E, Hermann B, Wirtitisch M, et al. Assesment of central visual function in Stargardt’s disease / fundus flavimaculatus with ultra high-resoution optical coherence tomography. Invest Ophthalmol Vis Sci. 2005;46:310–6. doi: 10.1167/iovs.04-0212. [DOI] [PubMed] [Google Scholar]
- 7.Querques G, Prato R, Iaculli C, et al. Correlation of visual function impairment and OCT findings in patients with Stargardt disease and fundus flavimaculatus. Eur J Ophthalmol. 2008;18:239–47. doi: 10.1177/112067210801800212. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasan VJ, Wotjkowski M, Witkin AJ, et al. High- definition and 3-dimensional imaging of macular pathologies with high speed ultrahigh-resolution optical coherence tomography. Ophthalmology. 2006;113:2054. doi: 10.1016/j.ophtha.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenstein VC, Schuman AD, Lee W, et al. Near-infared autofluorescence: its relationship to short wavelength autofluorescence and optical coherence tomography in recessive stargardt disease. Invest Ophthalmol Vis Sci. 2015;56:3226–34. doi: 10.1167/iovs.14-16050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JC, Collison FT, Fishman GA, et al. Objective Analysis of Hyperreflectiv Outer Retinal Bands Imaged by Optical Coherence Tomography in Patients with Stargardt Disease. Invest Ophthalmol Vis Sci. 2015;56:4662–7. doi: 10.1167/iovs.15-16955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujinami K, Zernant J, Chana RK, et al. Clinical and Molecular Characteristics of Childhood-Onset Stargardt Disease. Opthalmology. 2015;122(2):326–4. doi: 10.1016/j.ophtha.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anastasakis A, Fishman GA, Lindeman M, et al. Infared scanning laser ophthalmoscopy imaging of the macula and its correlation with functional loss and structural changes in patients with stargardt disease. Retina. 2011;31:949–58. doi: 10.1097/IAE.0b013e3181f441f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song H, Rossi EA, Latchney L, et al. Cone and rod Loss in Stargardt disease revealed by Adaptive Optics Scanning Light Ophthalmoscopy. JAMA Ophthalmology. 2015;133(10):1198–1203. doi: 10.1001/jamaophthalmol.2015.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonilha VL, Rayborn ME, Bell BA, et al. Retinal histopathology in eyes from a patient with Stargardt disease caused by compound heterozygous ABCA4 mutations. Ophthalmic Genet. 2015 Aug; doi: 10.3109/13816810.2014.958861. 21–11 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 15.Itoh Y, Vasanji A, Ehlers JP. Volumetric ellipsoid zone mapping for enhanced visualization of the outer retinal integrity with optical coherence tomography. Br J Ophthalmol. doi: 10.1136/bjophthalmol-2015-307105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellipsoid zone mapping and outer retinal characterization following intravitreal ocriplasmin. Retina. 2016 Dec;36(12):2290–2296. doi: 10.1097/IAE.0000000000001110. [DOI] [PMC free article] [PubMed] [Google Scholar]