Abstract
CD8+ T lymphocytes mediate potent immune responses against tumor, but the role of human CD4+ T cell subsets in cancer immunotherapy remains ill-defined. Herein, we exhibit that CD26 identifies three T helper subsets with distinct immunological properties in both healthy individuals and cancer patients. Although CD26neg T cells possess a regulatory phenotype, CD26int T cells are mainly naive and CD26high T cells appear terminally differentiated and exhausted. Paradoxically, CD26high T cells persist in and regress multiple solid tumors following adoptive cell transfer. Further analysis revealed that CD26high cells have a rich chemokine receptor profile (including CCR2 and CCR5), profound cytotoxicity (Granzyme B and CD107A), resistance to apoptosis (c-KIT and Bcl2), and enhanced stemness (β-catenin and Lef1). These properties license CD26high T cells with a natural capacity to traffic to, regress and survive in solid tumors. Collectively, these findings identify CD4+ T cell subsets with properties critical for improving cancer immunotherapy.
The role of human CD4+ T cell subsets in cancer immunotherapy is still unclear. Here, the authors show that CD26 identifies three CD4+ T cell subsets with distinct immunological properties in both healthy individuals and cancer patients.
Introduction
Cancer patients have been treated with various therapies and until recently, many with poor outcomes. The discovery of cell-intrinsic inhibitory pathways and cancer-specific antigens has allowed for the advancement of immune checkpoint blockades1, 2 and a cellular therapy called adoptive cell transfer (ACT), respectively. ACT is an innovative therapy that entails the acquisition, expansion and infusion of autologous T cells back into the patient to eradicate tumors3. The ability to engineer T cells with T cell receptors (TCRs4, 5) or chimeric antigen receptors (CARs6, 7) has made this therapy available to more individuals. Despite the impressive results of CAR-T therapy in patients with blood-based malignancies, it has yielded poor results in patients with solid tumors thus far8, 9.
Although tumor-infiltrating lymphocytes (TILs10, 11) or immune checkpoint modulators12, 13 regress malignancies in some patients bearing immunogenic solid tumors, these approaches have been ineffective at treating poorly immunogenic tumors such as mesothelioma and pancreatic cancer14, 15. Though several factors could have a role in why these therapies fail, two possible characteristics crucial for effective tumor clearance include the ability of T cells to traffic to16, 17 and persist in the tumor18, 19. Although CD8+ T cells have shown clinical promise20 and the capacity to repopulate21, human CD4+ T cell subsets that exhibit properties of stemness and natural migration to the tumor have yet to be identified.
Previous work on CD4+ T cells has shown that cells polarized to a type 17 phenotype—Th17 cells—exhibit stem cell-like qualities and yield greater tumor regression and persistence in vivo than other traditional T helper subsets22, 23. However, the expansive culture conditions required to generate these cells in vitro has inhibited their transition to the clinic. Recently, Bengsch et al.24 reported that human T cells with a high expression of CD26 on their cell surface—termed CD26high T cells—produce large amounts of the Th17 hallmark cytokine, IL-17. CD26 is an enzymatically active, multi-functional protein shown to have a role in T cell costimulation as well as the binding of extracellular matrix proteins/adenosine deaminase25. Despite being well studied in autoimmune diseases such as diabetes26, the role of CD26 and its enzymatic activity in cancer has yet to be fully explored. Given the substantial IL-17 production from CD26high T cells, we postulated that CD26 expression on CD4+ T cells might correlate with a more stem cell-like lymphocyte with enhanced tumor regression.
Herein, we report that CD26 distinguishes three distinct human CD4+ subsets with varying responses to human tumors: one with regulatory characteristics (CD26neg), one with a naive phenotype (CD26int), and one with properties of durable memory and stemness (CD26high). CD26high T cells persist and regress/control tumors to a far greater extent than CD26neg T cells and surprisingly, slightly better than naive CD26int T cells. Our data reveal that CD26high T cells have enhanced multi-functionality (IL-17A, IFNγ, IL-2, TNFα, and IL-22), stemness properties (elevated β-catenin and Lef1), memory (long-term persistence and Bcl2 expression), and a rich profile of chemokine receptors (including CCR2 and CCR5), thereby enabling them to traffic to, regress mesothelioma and inhibit the growth of pancreatic tumors. Furthermore, better antitumor responses correlate with an increased presence of CD26+ T cells in the tumor. Collectively, our findings provide new insight into CD26 for the advancement of T cell-based cancer immunotherapies in the clinic.
Results
CD26high T cells are activated and regress established tumors
CD26 is expressed on effector and memory, but not regulatory (Tregs), CD4+ T cells27, 28. Yet, it remains unknown whether CD26 correlates with these opposing subsets in cancer therapy. To address this question, we flow-sorted murine TRP-1 CD4+ T cells, which express a transgenic TCR specific for tyrosinase on melanoma, via CD26 expression. This strategy enriched CD4+ T cells into two groups: CD26neg and CD26high. Strikingly, a mere 50,000 CD26high T cells were more effective at clearing B16F10 melanoma tumor than 50,000 CD26neg T cells when infused into lymphodepleted mice (Supplementary Fig. 1a, b). Moreover, half of the mice treated with CD26high T cells experienced a curative response (Supplementary Fig. 1c).
We next determined the antitumor activity of human CD26high T cells in a CAR-T model. To do this, we used the strategy depicted in Fig. 1a. First, CD4+ T cells were isolated from the peripheral blood of a healthy individual and sorted by CD26 expression. Following bead activation, CD26neg and CD26high T cells were transduced to express a chimeric antigen receptor that targets mesothelin and signals CD3ζ (MesoCAR; ~98% CAR-specific; Supplementary Fig. 1d). Similar to murine cells, human CD26high T cells ablated large, human mesothelioma tumors in NSG mice to a greater extent than CD26neg T cells (Fig. 1b). Our findings show that both murine and human T cells that express high levels of CD26 can effectively regress solid tumors in vivo.
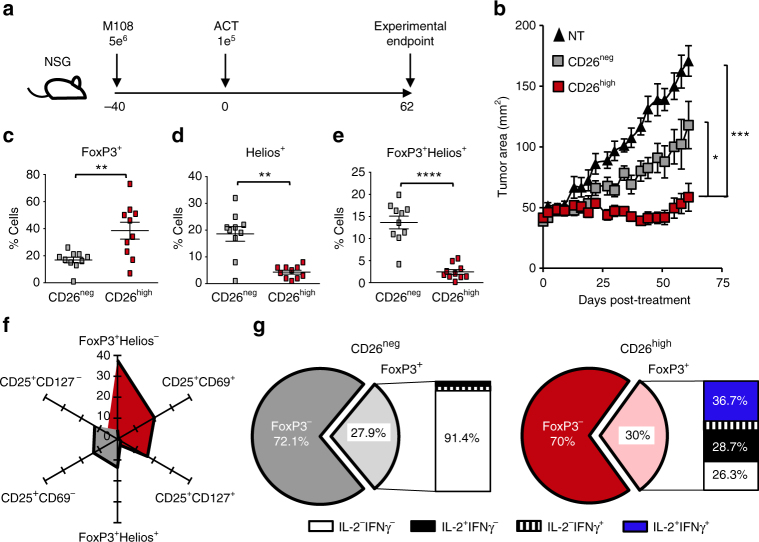
Fig. 1.
Human CD26high T cells are activated and display antitumor activity. CD4+ lymphocytes were isolated from healthy donor PBMCs, sorted by CD26 expression and stimulated with magnetic beads coated with CD3 and ICOS agonists (cultured at a ratio of 1 bead to every 5 T cells). T cells were transduced 36 h post-activation with a lentiviral vector encoding a first generation chimeric antigen receptor that recognizes mesothelin and stimulates the CD3ζ domain. These cells were expanded for 10 days with IL-2 (100 IU/ml). a, b NSG mice were subcutaneously injected with 5e6 M108 mesothelioma cells. Forty days post-M108 establishment, mice were intravenously infused with 1e5 human CD26neg or CD26high T cells redirected to express MesoCAR. Tumors were measured bi-weekly (N = 10 mice per group). P values for the tumor curve were calculated by one-way ANOVA with a Kruskal-Wallis comparison using final tumor measurements from day 62. c–g Graphical representations of transcription factors (c–f N = 10) and cytokine production (g N = 3) by sorted T cells isolated from multiple healthy individuals prior to bead stimulation. In g, the frequency of FoxP3+ cells from the enriched CD26neg or CD26high cultures secreting inflammatory cytokines were assayed by flow cytometry. P values for c-e were calculated using a Mann-Whitney U Test. Data with error bars represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001
As FoxP3+ Treg cells express nominal CD2628, we suspected that CD26high T cells exhibited greater antitumor activity because they were not suppressive (i.e., FoxP3−/low). Surprisingly, CD26high T cells express more FoxP3 than CD26neg T cells (Fig. 1c). However, FoxP3+CD26high T cells did not co-express Helios, a transcription factor expressed on thymus-derived Tregs (Fig. 1d, e; Supplementary Fig. 1e). In confirmation of these findings, very few CD4+FoxP3+Helios− T cells found in the bulk CD4+ population lacked CD26 expression (~19%), whereas ~88% of CD4+FoxP3+Helios+ Tregs were CD26 negative (Supplementary Fig. 1f). Given that human T cells upregulate FoxP3 following activation29, we posited that CD26high T cells express FoxP3 because they exist in an activated state post-enrichment, but prior to ex vivo bead activation. As expected, CD26high T cells had a ten-fold higher expression of the activation markers CD25 and CD69 (~20%) than CD26neg T cells (~2%) (Supplementary Fig. 1g, h). CD26neg T cells also displayed a higher frequency of CD25+CD127− cells (CD26neg ~12%; CD26high ~5%), an extracellular phenotype associated with Tregs30, 31 (Fig. 1f). Finally, we discovered that ~74% of FoxP3+CD26high T cells secreted the cytokines IL-2 and/or IFNγ (Fig. 1g). Conversely, <9% of CD26neg T cells were capable of cytokine production. Collectively, our data reveal that a portion of CD26high T cells are activated post-enrichment and clear tumors when redirected with a CAR.
Naive and memory CD4+ T cells have variant CD26 expression
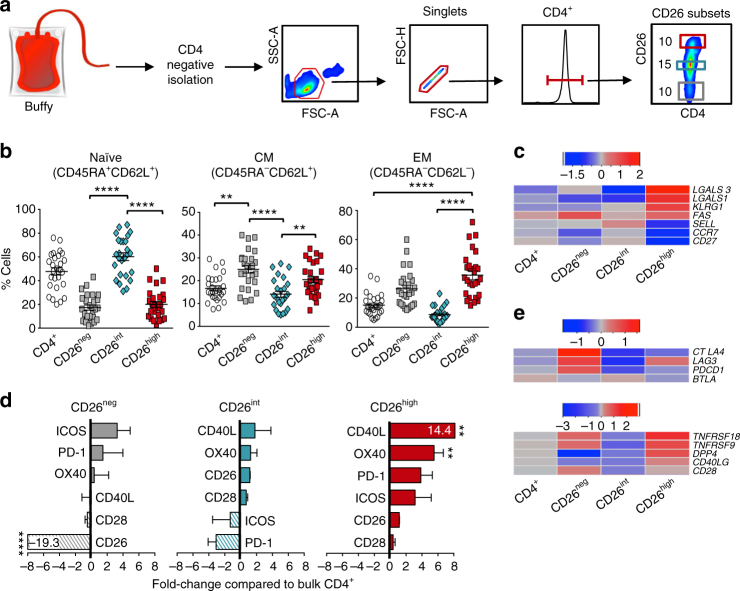
Although human CD26high T cells are more effective at clearing tumor than CD26neg cells, these cells are differentiated and might not be ideal for ACT. As CD26int cells have briefly been described as naive (CCR7+CD45RA+)24, we hypothesized that they would elicit superior antitumor immunity compared to CD26neg or CD26high T cells. To test this, we first performed a detailed characterization of their phenotype in vitro. CD4+ T cells were enriched from healthy donors and FACS-sorted into bulk CD4+, CD26neg, CD26int or CD26high subsets (sorting purity >80%—Fig. 2a; Supplementary Fig. 2a). As expected, ~65% of CD26int cells were naive (CD45RA+CD62L+), whereas more than half of CD26neg and CD26high T cells possessed a more differentiated central or effector memory phenotype (Fig. 2b; Supplementary Fig. 2b). Furthermore, we found a heightened expression of CD95 on CD26high T cells (Supplementary Fig. 2c). Gene array analysis corroborated our findings, showing both CD26neg and CD26high T cells expressed less CCR7, CD27 and CD62L than CD26int T cells (Fig. 2c). Furthermore, CD26high cells expressed heightened levels of markers associated with effector memory T cells (LGALS1, LGALS3) 32 as well as the senescence marker KLRG1 and FAS death ligand. Importantly, the differentiated phenotype of CD26neg and CD26high T cells and naive characteristics of CD26int T cells were reproducibly observed in more than 25 healthy donors.
Fig. 2.
CD26int T cells are naive, whereas CD26neg/CD26high T cells are differentiated. a Sorting strategy: CD4+ T cells were isolated from buffy coats from healthy individuals and FACS-sorted into bulk CD4+, CD26neg (bottom ~ 10%), CD26int (middle ~15%), and CD26high (top ~10%). b, c Memory phenotype for all subsets was determined using flow cytometry (b N = 26) and gene array analysis (c N = 3–5) prior to bead stimulation. For c, RNA was isolated and gene expression assessed by OneArray. Heat map displays (+/−) log2-fold change in memory-associated genes. d, e Graphical representation of co-stimulatory and co-inhibitory markers determined by flow cytometry (d N = 20–26) and gene array (e N = 3–5) prior to bead stimulation. Surface marker expression in d was calculated and graphed as a fold change of CD26neg, CD26int, and CD26high T cells compared to bulk CD4+. P values were calculated using a One-way ANOVA with a Kruskal Wallis comparison. Error bars represent mean ± SEM. **P < 0.01; ****P < 0.0001
Given that CD26int cells are naive, we posited that they would express less co-stimulatory and co-inhibitory receptors than CD26neg and CD26high T cells. As expected, CD26int T cells expressed less co-stimulatory and co-inhibitory markers than CD26high T cells (Fig. 2d; Supplementary Fig. 2d). Conversely, CD26high T cells expressed more PD-1, CD40L, and OX40 than the other subsets, whereas ICOS and PD-1 were the most prevalent markers on CD26neg. Furthermore, gene array analysis revealed an upregulation of CTLA4,LAG3, and PDCD1 in both CD26neg and CD26high T cells compared to CD26int (Fig. 2e, top). Despite the increased co-inhibitory markers on CD26high T cells, they also expressed many co-stimulatory markers, including CD40L (CD40LG), 41BB (TNFRSF9), and GITR (TNFRSF18) (Fig. 2e, bottom). Overall, these findings reveal that CD26int T cells possess a naive phenotype and express less co-stimulatory/inhibitory receptors compared to CD26neg and CD26high T cells.
CD26 expression correlates with specific CD4+ T cell subsets
Although CD26neg T cells contain a Treg population28 and CD26high cells have been published to exhibit a Th133 or Th1724 phenotype, CD26int cells have not yet been characterized. Given that CD26int T cells appear naive, we hypothesized that they would not express the chemokine receptors indicative of any particular subset, whereas CD26neg and CD26high T cells would exhibit their reported Treg and Th1/Th17 phenotypes. As expected, CD26high T cells were composed of Th1 (CXCR3+CCR6−) and Th17 (CCR4+CCR6+) cells, with the majority being hybrid Th1/Th17 (CXCR3+CCR6+) cells (Fig. 3a, b). These cells also exhibited heightened levels of CD161, which has recently been identified as a marker for long-lived antigen-specific memory T cells34 (Supplementary Fig. 3a). We also identified Th1, Treg (CD25+CD127−), and Th2 (CCR4+CCR6−) populations in the CD26neg subset. On the contrary and as expected, CD26int cultures were comprised of lower frequencies of helper memory subsets. As shown in Fig. 3c, gene array analysis confirmed that CD26neg cells expressed a Treg (i.e., TIGIT, TOX, CTLA4), Th1 (i.e., EOMES, GZMK, IL12RB2) and Th2 (CCR4, GATA3) signature. Conversely, CD26high T cells had high gene expression in the Th1 (i.e., GZMK, TBX21, EOMES), Th17 (i.e., CCL20, IL18RAP, PTPN13), and Th1/Th17 (KIT, ABCB1, KLRB1) subsets. As expected, the subset-defining genes in CD26int T cells were sparse. Collectively, these findings confirm that CD26int T cells are naive and not yet committed to any subsets.
Fig. 3.
CD26 defines CD4+ T cells with naive, helper, or regulatory properties. CD4+ T cells were isolated from healthy donors and sorted by CD26 expression (Fig. 2a). Representative FACS plots (a) and phenotype data (b N = 26) from multiple healthy individuals. c Heat map of (+/−) log2-fold change in expression of CD4+ subset-associated genes (N = 3–5) prior to bead stimulation. d, e Human CD4+ T cells were sorted into bulk CD4+, CD26neg, CD26int, CD26high, Th1 (CXCR3+CCR6−), Th2 (CCR4+CCR6−), Th17 (CCR6+CCR4+), and Th1/Th17 (CXCR3+CCR6+). DNA was isolated from sorted T cells prior to expansion of TCRβ sequences using an immunoSEQ kit and subsequent analysis. Data shown is the percent TCRβ overlap between groups (d) and a Venn diagram (e) displaying the overlap frequencies of identical TCRβ sequences between CD26neg, CD26int, CD26high, and Th1/Th17 subsets (N = 4). P values for b and d were calculated using one-way ANOVA with a Kruskal-Wallis comparison. Error bars represent mean ± SEM. *P < 0.05; **P < 0.01; ****P < 0.0001
Helper T cell subsets express a specific T cell receptor β (TCRβ) profile35. Using TCRβ analysis, we found that many TCRs in CD26high T cells overlap with Th1, Th17, and Th1/Th17 cells, but not with Th2 cells (Fig. 3d). Although CD26high T cells had greater overlap with Th1 cells than CD26neg did, the overlapping TCRs between Th1 and CD26neg were more highly expressed (Supplementary Fig. 3b). This heightened TCRβ expression revealed a correlation between CD26neg and Th1 cells (r 2 = 0.297), as well as CD26high and Th1/Th17 cells (r 2 = 0.526; Supplementary Fig. 3c), thereby confirming our previous findings. Although CD26high T cells exhibited significant overlap with Th1/Th17 cells, they shared little TCRβ overlap with CD26neg or CD26int cells (Fig. 3e; Supplementary Fig. 3d, e). Likewise, CD26int cells had minimal TCRβ overlap with any of the Th subsets. Overall, these data confirm that CD26high and CD26neg T cells are differentiated cells containing multiple cell subsets while CD26int T cells are primarily uncommitted.
CD26high cells are multi-functional and enzymatically active
Given that CD26neg and CD26high T cells are differentiated, we hypothesized that they would secrete more cytokines than naive CD26int in vitro. Interestingly, CD26high T cells had heightened production of multiple cytokines, including IL-2, IFNγ, IL-17A, IL-22, and TNFα (Fig. 4a; Supplementary Fig. 4a, b). However, this heightened cytokine profile did not directly correlate with cell differentiation, as evidenced by similar cytokine production between bulk CD4+, CD26neg, and CD26int T cells. Given the numerous cytokines produced by CD26high T cells, we next investigated their capacity to secrete multiple cytokines at once (i.e., multi-functionality). Strikingly, CD26high T cells were highly multi-functional (Supplementary Fig. 4c) with roughly 25% of these cells producing 4–5 cytokines simultaneously (Fig. 4b), a phenomenon not seen in the other subsets. When we took into account the percentage of these donors not producing any cytokines, we discovered that 60–80% of CD4+, CD26neg, and CD26int T cells were incapable of producing cytokines on day 0, whereas the majority of CD26high T cells produced 3–5 cytokines simultaneously (Supplementary Fig. 4d). This vast cytokine production was maintained in CD26high T cells throughout culture (Fig. 4c). Furthermore, CD26high T cells produced more cytotoxic granules (Granzyme B, CD107A) and chemokines (MIP1β, RANTES) compared to other subsets, a property normally used to define cytotoxic CD8+ T cells (Fig. 4d; Supplementary Fig. 4e).
Fig. 4.
CD26high T cells are multi-functional and enzymatically active. CD4+ T cells from healthy individuals were isolated and sorted by CD26 expression (Fig. 2a). a, b Sorted T cells were activated with PMA/Ionomycin and Monensin for 4 h prior to intracellular staining (N = 26). In b, three independent donors were analyzed by FlowJo software and graphed to display the percentage of cells simultaneously secreting 1–5 cytokines (IL-2, IFNγ, TNFα, IL-17A, IL-22). c Supernatant was collected from cells at pre-activation (0) and 1, 6, and 12 days post-activation time points for ELISA (N = 2; data shown is representative of two individual experiments). d T cells activated in vitro were subjected to intracellular staining (N = 5–11). e 1e5 sorted cells per group from pre-activation (0) and 10 days post-activation were cultured with the CD26 ligand gly-pro-P-nitroanalide for 2 h at 37 °C and analyzed for colorimetric changes to determine enzymatic activity (N = 5). P values in a and e were calculated by One-way ANOVA with a Kruskal-Wallis comparison. Error bars represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001
As CD26 enzymatic activity has a minor role in supporting T cell function25, we next posited that enzymatic activity would be elevated in CD26high T cells compared to the other subsets. Indeed, throughout a 10-day expansion in vitro, CD26high T cells maintained the highest enzymatic activity (Fig. 4e; Supplementary Fig. 4f). Thus, CD26high T cells are uniquely multi-functional, cytotoxic, and enzymatically active.
CD26 subsets exist in peripheral blood of cancer patients
Although CD4+ T cells with distinct CD26 expression profiles exhibit unique immunological properties in healthy donors, it is unclear whether these biological assets are similar in cancer patients. To address this question, we isolated T cells with high, intermediate, or low CD26 expression from the blood of patients with malignant melanoma and examined their function, phenotype, and memory profile. CD26 was distributed similarly on CD4+ T cells from melanoma patients (Supplementary Fig. 5a) as in normal donors (Fig. 2a). Furthermore, the mean fluorescence intensity (MFI) of CD26 on all subsets was similar between cancer patients and healthy individuals, with CD26high T cells maintaining the greatest CD26 expression (Supplementary Fig. 5b). The phenotype of CD26neg, CD26int, and CD26high T cells in melanoma patients was similar to that seen in health donors. For example, CD26int T cells were naive, denoted by high CD45RA, CD62L, and CCR7 markers (Supplementary Fig. 5c). CD26high T cells expressed Th1/Th17 chemokine receptors (CCR6, CXCR3, and CD161), whereas CD26neg T cells expressed CXCR3, confirming that cell subsets within these cultures are comparable between cancer patients and healthy individuals (Supplementary Fig. 5d). Finally, CD26neg T cells from cancer patients contained Tregs, as they expressed less CD127 but greater CD39, FoxP3, and Helios (Supplementary Fig. 5e-f).
CD26high T cells also secreted more IL-2, TNFα, IL-17A, IFNγ, and MIP-1β than other subsets following stimulation with PMA and Ionomycin (Supplementary Fig. 5g, h). CD26neg T cells from melanoma patients secreted elevated IL-4 (Supplementary Fig. 5i), correlating with our previous finding that Th2 cells are prevalent in this culture (Fig. 3). Finally, CD26high T cells expressed more CCR2 and CCR8 than other subsets (Supplementary Fig. 5j). As CCR8 promotes T cell trafficking to the skin, it is intriguing that this chemokine receptor is abundant on CD26high T cells from melanoma patients. Collectively, we found that CD26neg, CD26int, and CD26high T cells from melanoma patients possess a similar biological profile as those identified in healthy donors.
CD26int and CD26high T cells regress/inhibit tumor growth
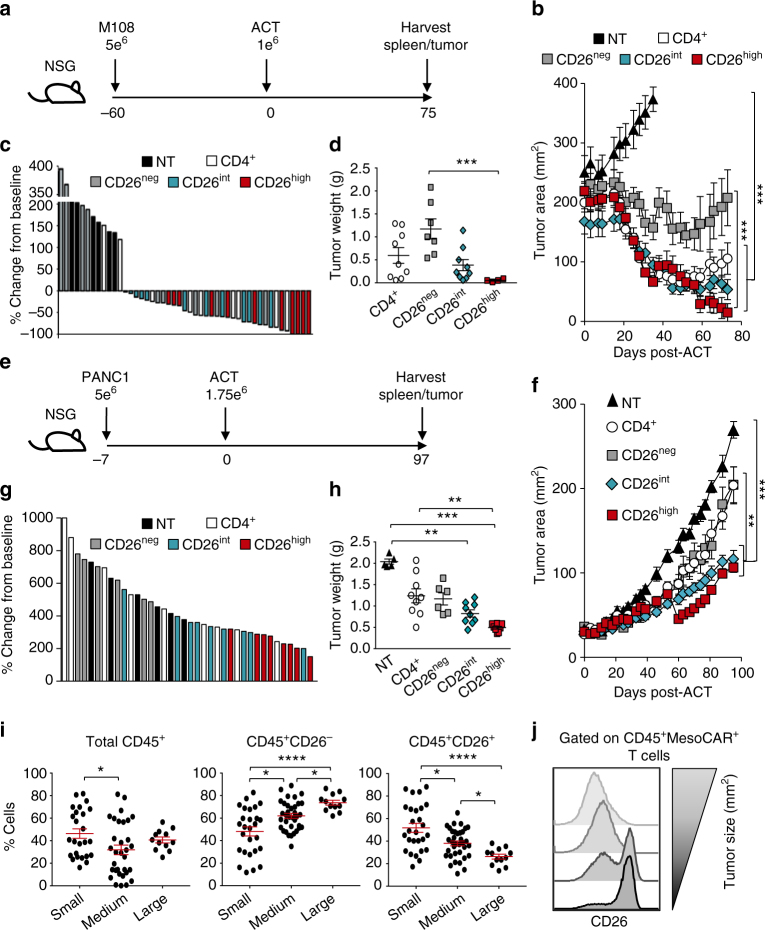
As enzymatically active CD26high T cells are more differentiated than CD26int T cells, we hypothesized that CAR+CD26int T cells would clear tumor and persist better than CAR+CD26high T cells. To address this hypothesis, we redirected human CD4+, CD26neg, CD26int, and CD26high T cells to recognize mesothelin via a MesoCAR (Supplementary Fig. 6a). Ten days post-expansion, CAR-T cells were infused into NSG mice bearing large M108 mesothelioma (Fig. 5a). Surprisingly, despite the seemingly exhausted phenotype of CD26high T cells, they regressed tumor slightly, but not significantly, better than CD26int T cells (Fig. 5b; Supplementary Fig. 6b). CD4+ T cells were only slightly less effective than CD26int or CD26high, whereas 26neg T cells proved to be incapable of regressing mesothelioma. Our tumor curve data in Fig. 5b directly correlated with both percent tumor change from baseline (Fig. 5c) and tumor weights (Fig. 5d). Collectively, we found that CAR+ T cells that express CD26 are more effective at clearing tumors.
Fig. 5.
Human CD26int and CD26high T cells regress/slow established tumors. CD4+ T cells were isolated from healthy individuals, sorted by CD26 expression, transduced to express MesoCAR, and expanded for 10 days. a, b NSG mice bearing large M108 mesothelioma tumor (established for 60 days) were infused with 1e6 human CD4+, CD26neg, CD26int, or CD26high T cells. Post-ACT, tumors were measured bi-weekly until mice were killed and organs harvested at 75 days post-ACT (N = 7–9 mice per group). P values for the tumor curve were calculated by one-way ANOVA with a Kruskal-Wallis comparison on the final day when mice from all comparison groups were still alive (NT vs. all groups = day 38; CD26neg vs. CD26high = day 59). c Percent change in tumor size from baseline (day 0) to endpoint (day 75) was calculated and graphed as a Waterfall plot. d Graphical representation of tumor weights (g) harvested from treated mice 75 days post-ACT. e, f NSG mice bearing established pancreatic tumors (PANC1) were infused with 1.75e6 human CD4+, CD26neg, CD26int, and CD26high T cells and tumors were measured bi-weekly for more than 3 months (N = 6–9 mice per group). P values for the tumor curve were calculated by one-way ANOVA with a Kruskal-Wallis comparison on the final day when mice from all comparison groups were still alive (All groups = day 84). g Graphical representation of tumor weight (g) harvested from mice 97 days post-ACT. h–j Tumors from all treated mice were harvested, digested, and run through a strainer. Resulting cell suspension was stained for flow cytometry and graphed relative to tumor size (small <100 mm2, medium = 100–200 mm2, large >200 mm2). P values for d, h and i were calculated by one-way ANOVA with a Kruskal-Wallis component. Data with error bars represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001
In contrast to human mesothelioma mouse models, CAR-T therapy does not inhibit the growth of pancreatic tumors (Supplementary Fig. 6c). Thus, we next sought to determine if CAR-T cells that express CD26 (CD26high and CD26int) could better control pancreatic cancer (PANC1) in mice to a greater extent than CD26neg T cells. To test this, we used a similar treatment strategy (Fig. 5e) as performed in our M108 model. Following CAR transduction (Supplementary Fig. 6d), both CD26int and CD26high T cells significantly slowed the progression of pancreatic tumors, whereas bulk CD4+ and CD26neg T cells yielded little-to-no antitumor response (Fig. 5f; Supplementary Fig. 6e). These findings were confirmed by decreased tumor growth and weight in mice treated with CD26int or CD26high T cells compared to mice treated with CD4+ or CD26neg T cells (Fig. 5g, h). Overall, our data demonstrate that differentiated CD26high T cells exhibit a similar antitumor response as naive CD26int T cells.
Given that CD26int and CD26high T cells yielded the best antitumor response, we next posited that CD26 expression on T cells in the tumor itself might correlate with overall response. To test this, we isolated tumor-infiltrating lymphocytes from all mice at experimental endpoint and assessed donor cell persistence and phenotype by flow cytometry. When graphed by tumor size (small <100 mm2, medium = 100–200 mm2, large >200 mm2), we unexpectedly discovered that the percentage of CD45+ T cells was similar between the groups and had no correlation with antitumor response (Fig. 5i, left). On the contrary, we found that CD26 expression on TIL tightly correlated with response as smaller tumors exhibited heightened percentages of CD26+ TIL (Fig. 5i, middle and right; Fig. 5j).
We next sought to determine whether CD26 expression on the endogenous TIL of mice that had not received therapy also correlated with tumor size. To do this, mice were subcutaneously injected with B16F10 melanoma and CD26 expression on TIL was assessed by flow cytometry. Similar to our ACT model, we found that the expression of CD26 on endogenous TIL decreased as tumor size increased (Supplementary Fig. 6f). These observations not only exhibit that distinct CD26−expressing CD4+ T cell subsets exist in both endogenous and adoptively transferred TIL, but also identify a direct correlation between CD26 and their subsequent ability to slow/regress tumors.
Although we found that CD4+CD26high T cells exhibit greater antitumor activity than other CD4+ subsets, how they compare to the commonly used cytotoxic CD8+ T cells is unknown. To test this concept, human CD4+CD26high and CD8+ T cells were isolated and engineered to be MesoCAR+. An in vitro cytotoxic assay using mesothelin-expressing K562 cells revealed that CD4+CD26high T cells have a similar, if not enhanced, capacity to kill cancer compared to CD8+ cells (Supplementary Fig. 6g). Furthermore, adoptive transfer into mesothelioma-bearing mice showed that CD4+CD26high T cells clear tumors significantly better than traditionally used CD8+ T cells (Supplementary Fig. 6h, i). This finding, in addition to our previously discussed NSG mouse models in which no inherent immune system exists, reveals that CD4+CD26high T cells are directly cytotoxic and capable of clearing tumors in the absence of other immune cells, including CD8+ T cells.
CD26high T cells have enhanced migration and stemness
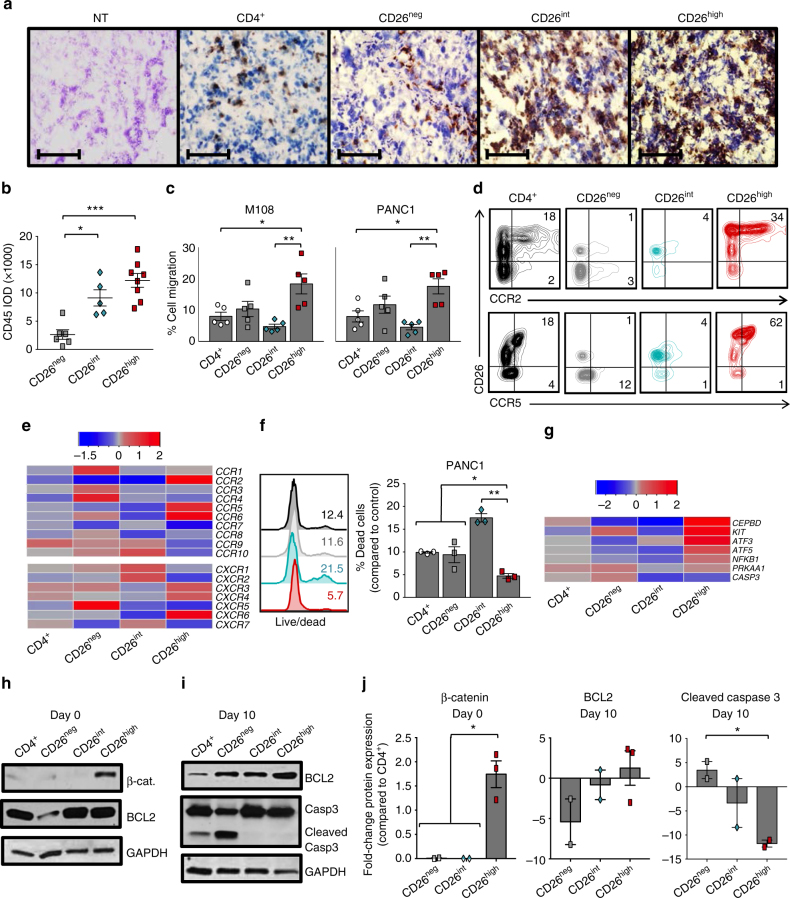
We next sought to determine the mechanisms underlying the effectiveness of CD26int and CD26high T cells. Owing to the multi-functional and cytotoxic nature of CD26high T cells (Fig. 4; Supplementary Fig. 4), we hypothesized that they would cause immediate tumor regression in mice, but would not persist like CD26int lymphocytes. Surprisingly, CD26high T cells persisted as well as CD26int cells in the tumor (Supplementary Fig. 7a). Conversely, few CD26neg T cells were detected. These findings were confirmed by immunohistochemistry, which revealed a direct correlation between CD26 and enhanced CD45+ donor cell persistence in the tumor (Fig. 6a, b). Collectively, these findings reveal that CD26int and CD26high T cells persist in the tumor to a greater extent than bulk CD4+ and CD26neg T cells.
Fig. 6.
CD26high T cells have stemness and increased migratory capacity. a, b Tumors from treated PANC1-bearing mice (from Fig. 5) were harvested and then frozen in cryomatrix. Tumor samples were then sliced and used for immunohistochemistry analysis (purple = H&E, brown = CD45; N = 5–9 tumors per group). Magnification = ×10. In b, the integrated optical density (IOD) of CD45 in CD26-sorted groups was quantified with ImageJ software and graphed (N = 5–8 per group). c Sorted T cells were activated with CD3/ICOS beads and expanded in 100 IU/ml IL-2 for 10 days prior to testing cell migration via a transwell assay. 0.75e6 sorted cells were re-suspended and assessed for percent cell migration towards M108 or PANC1 supernatant in a 2 h time period (N = 5). d, e Sorted T cells were analyzed for chemokine receptor expression by flow cytometry (d N = 12–15) and gene array (e N = 3–5) prior to bead stimulation. In e, data shown in heat map as (+/−) log2-fold change in chemokine receptor expression compared to bulk CD4+. f Viability of T cells that migrated towards PANC1 was determined by live/dead staining (N = 3). g Anti-apoptotic and stemness genes were analyzed and displayed as a (+/−) log2-fold change compared to bulk CD4+ (N = 3–5). h–j Protein from pre-activation (day 0) and post-activation (day 10) cells was isolated and used for western blot analysis (N = 2–3). In j, the fold change of β-catenin, BCL2 and cleaved Caspase-3 for each subset compared to bulk CD4+ T cells was graphed (N = 2–3). P values were calculated using a One-way ANOVA with a Kruskal-Wallis comparison. Data with error bars represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001
Given the ample amount of donor T cells persisting in the tumors of mice treated with CD26int and CD26high T cells, we next sought to determine if they have an enhanced capacity to migrate. To test this, sorted human CD4+ T cells were activated and expanded in culture for 10 days as previously described. On day 10, cells from each group were placed in the top well of a transwell plate and assayed for their migration towards tumor-secreted supernatant (or controls) following a 2 h incubation period. CD26neg and CD26high T cells migrated slightly better in control conditions than bulk CD4+ and CD26int T cells (Supplementary Fig. 7b), perhaps due to the elevated expression of genes that aid in migration, including RGS1, RHOA, ROCK1, and DIAPH1 (Supplementary Fig. 7c). Furthermore, we discovered that CD26high T cells migrated to M108 and PANC1 in vitro to a greater extent than bulk CD4+, CD26neg, or CD26int T cells (Fig. 6c). Given that the chemokine receptors CCR2 and CCR5 have been shown to be important in migration towards mesothelioma tumors, we next observed the expression of these receptors on CD26high T cells. Indeed, we found that CD26high T cells not only expressed elevated levels of CCR2 and CCR5, but also expressed more CCR6, CXCR3, CXCR4, and CXCR6 (Fig. 6d, e; Supplementary Fig. 7d). Interestingly, the ligands for these chemokine receptors (CCL2, RANTES, CCL20, CXCL9-10, CXCL12, and CXCL16, respectively) are produced by pancreatic tumors36. In addition, CD26high T cells were more viable following migration (Fig. 6f).
Given this finding, we next tested if CD26high T cells were resistant to apoptosis. We found that CD26neg T cells were more apoptotic than bulk CD4+, CD26int, and CD26high T cells (Supplementary Fig. 7e). Conversely, CD26high T cells expressed many anti-apoptotic genes, such as KIT, CEBPD, and ATF3 (Fig. 6g). As Th17 cells have stemness and durable memory despite their differentiated appearance22, we next assessed if CD26high T cells expressed proteins in the Wnt/β-catenin pathway. Compared to naive CD26int or regulatory CD26neg T cells, CD26high T cells had greater β-catenin expression (Fig. 6h) and maintained heightened BCL2 with minimal caspase-3 cleavage (Fig. 6i). These findings repeated among several healthy donors (Fig. 6j) and were confirmed by the upregulation of Lef1 concurrently with CD26 expression (Supplementary Fig. 7f). Collectively, our findings reveal that CD26high T cells have remarkable self-renewal potential and a profound ability to migrate, survive, and persist in the tumor long-term.
As visualized in Fig. 7, CD26 identifies three human CD4+ T cell subsets with distinct immunological properties. First, CD26neg T cells exhibit poor persistence and antitumor activity due to increased Treg expression and lack of stemness properties. CD26int T cells are mainly naive, persistent, and effectively slow/regress human tumors. Finally, CD26high T cells possess qualities of stemness and migration factors that support their persistence and antitumor activity in solid tumors.
Fig. 7.
CD26 identifies three CD4+ T cell subsets with distinct properties. Depiction of our observations on CD26 expression and cellular therapy described herein. CD26neg T cells, despite their enhanced capacity to migrate, fail to regress tumors due to regulatory properties, decreased persistence, and increased sensitivity to cell death. CD26int and CD26high T cells exhibit similar antitumor activity, but have vastly different immunological properties. Despite their decreased migration, CD26int T cells are naive and capable of persisting long-term. CD26high T cells, despite their differentiated phenotype, exhibit several anti-apoptotic and stemness features, persist long-term, co-secrete multiple cytokines, and cytotoxic molecules and have a natural capacity to migrate towards various established solid tumors
Discussion
The T cell subsets that optimally elicit robust memory responses to tumors have been hotly debated. Recent reports underscore the need for naive or less differentiated memory T cells to maintain a long-term antitumor response in vivo37–39. Unfortunately, T cells become unresponsive quickly during tumorigenesis40. Moreover, exhausted and terminally differentiated T cells are epigenetically stable, regardless of PD-1 blockade therapy, and thus fail to become memory T cells41. One advantage to ACT therapy is the ability to infuse selected T cells with long-lived immunity into cancer patients. Several markers, such as CD45RA, CCR7, CD62L, and CD95, identify naive or stem memory T cells42. Our data suggest that this long list of markers can be consolidated to one: CD26. Collectively, we demonstrate that CD26 expression identifies three distinct human T helper subsets—regulatory, naive, and stem memory—with varying levels of antitumor activity.
We discovered that CD26int T cells were naive, CD26neg cultures contained Treg, Th1 and Th2 cells, and CD26high T cells displayed a Th1/Th17 profile and were highly multi-functional/cytotoxic. Although cells with heightened cytotoxicity lyse tumors in vitro, they often have diminished replicative and antitumor capacity in vivo39. Surprisingly, CD26high T cells controlled pancreatic tumors and regressed mesothelioma slightly, but not significantly, better than CD26int. In addition to regressing tumors in multiple cancer models, CD26high T cells also exhibited long-term persistence. Similar to Th17 cells that persist in vivo via the Wnt/β-catenin signaling pathway despite their effector memory profile22, we discovered that CD26high T cells display markers of stemness, such as increased β-catenin, BCL2 and Lef1. Studies similar to that by Graef et al.21, which showed that a single antigen-specific mouse CD8+CD62L+ T cell could reconstitute and protect mice from infection post-serial transfer, would be interesting to investigate in order to confirm the stemness of CD26high T cells. On the basis of our work herein, we posit that CD26+ T cells would persist and protect the host from malignancy, whereas CD26− T cells would be short-lived and unable to prevent tumor growth. Also, CD26high T cells naturally expressed the chemokine receptors CCR2 and CCR5, which likely helped CD26high T cells migrate to the tumor site; a notion supported by data showing that transduction of CCR2 into transferred T cells improved their trafficking to tumors43, 44. Thus, CD26high T cells employ key mechanisms, including enhanced stemness and migration, to persist and lyse tumor.
In addition, we found it intriguing that CD26high T cells express multiple co-stimulatory and co-inhibitory markers. Activating T cells via OX40/CD40L agonists45, 46 or PD-1/LAG3 antagonists1, 47, 48 enhances antitumor immunity. GITR, a co-stimulatory receptor known for it’s expression on Tregs, has recently been shown to enhance effector T cell function and bestow protection against Treg-mediated immunosuppression49, 50. The elevated expression of these markers on CD26high T cells begs the question of whether checkpoint modulators regulate the frequency and function of these cells in cancer patients, thereby dictating treatment outcome. Therefore, the simple addition of a CD26 detection antibody in immune-monitoring cores in cancer centers could easily shed light on this key question.
Finally, we discovered that mice bearing melanoma, mesothelioma or pancreatic cancer experienced a better treatment outcome when a heightened percentage of CD26+ donor T cells infiltrated the tumor. These data suggest a potential role of CD26 in tumor immunity, which could entail several mechanisms. First, our finding that human MesoCAR+ T cells and endogenous mouse TIL expressing CD26 correlate with reduced tumor size suggests that CD26 augments antitumor immunity in an MHC-independent manner. Given the polyfunctionality of CD4+CD26high T cells (indicated by their ability to co-express IL-17A, IFNγ, IL-2, TNFα, and Granzyme B), our findings could be the result of enhanced inflammation at the tumor site (i.e., induction of a “hot” tumor), which has been shown to associate with tumor regression51, 52. It is also worth noting that human regulatory T cells express little-to-no CD2628, a fact that could implicate that the enhanced regression seen in mice expressing a greater percentage of CD26+ TIL is simply due to a greater Teff to Treg ratio at the tumor site. Finally, as CD26 increases on T cells following stimulation53, its expression could also correlate with TIL activation in vivo.
Although the majority of our work herein utilized CAR-engineered CD4+ T cells, we also identified a similar phenotypic and functional correlation between CD26 expression on both TCR-specific and endogenous TIL. Given that previous reports identify CD26 as a marker of memory T cells with an enhanced response to recall antigens33, it is possible that the CD26+ compartment of T helper cells express TCRs that recognize tumor antigens. In addition, recent reports have shown that mutation-specific CD4+ T cells in a patient with metastatic cholangiocarcinoma have clinical benefit54. Intriguingly, the phenotype described by Tran et al., specifically the functional nature and increased OX40 and 4-1BB on erbb2 mutation-specific T helper cells, is similar to our CD26high T cells described herein. Therefore, our data might also imply that a small cohort of CD26-expressing CD4+ T cells recognize mutated tumor antigens displayed on MHC II-positive tumors, which has been shown to mediate antitumor efficacy in patients treated with cell therapy and checkpoint modulators54–59. If this were true, CD26 could be an ideal marker to isolate CD4+ TIL with responses against mutated antigens that can be exploited to regress established tumors. Collectively, our findings indicate that CD4+CD26+ T cells could be used for TCR-based immunotherapy, as they may potentially mediate enhanced responses to mutated tumor antigen in an MHC II-restricted fashion. Thus, it will be worthwhile to deduce the TCR specificity on human CD4+ TILs expressing low, intermediate, and high levels of CD26. Regardless of the putative mechanism by which CD26+ T cells mount immunity to tumors, our data suggest that CD26 could have a role—be it functional or as a biomarker—in T cell-based therapies, including neoantigen-specific vaccines, ICB, and ACT.
One prevalent role of CD26 in autoimmune diseases such as diabetes is via the enzymatic activity, which inactivates peptides (GLP-1 and GIP) critical for stabilizing blood glucose levels26. Contrary to the known role of CD26 enzymatic activity in diabetes, its effect on antitumor activity has been controversial. One study found that CD26 inhibition following ACT or checkpoint therapy enhanced immunity by renewing the recruitment of CXCR3+ T cells to the tumor60. On the contrary, inhibiting CD26 has also been shown to accelerate tumor metastasis via NRF2 activation61. Herein, we discovered that CD26high T cells maintain elevated enzymatic activity in vitro. Further exploration on the biological role of CD26 in antitumor immunity will be insightful. Genetic or pharmaceutical regulation of the enzymatic and multi-functional properties of CD26 will determine its role in T cell therapy and uncover if targeting CD26 can enhance immunotherapy in cancer patients.
To our knowledge, this is the first report that defines three human CD4+ subsets by CD26 expression and examines their unique impact in tumor immunity. Data herein suggest that although CD26int and CD26high T cells are promising for cancer immunotherapy, CD26neg T cells are deleterious to this field but may have an important role in suppressing autoimmune diseases, such as GVHD or rheumatoid arthritis. Specifically, our major findings are threefold. First, we comprehensively characterized CD26int T cells and confirmed their naive phenotype. Given the extensive sorting or bead strategies required to isolate naive cells from patients, this finding could revolutionize ACT by simplifying the strategy for enriching ideal lymphocytes. Second, our findings reveal that CD26high T cells are not terminally differentiated, but rather have durable memory, enabling them to persist and regress multiple solid tumors. These discoveries appear to be, in part, due to their enhanced stemness properties and migration towards the tumor. Finally, our finding that mice with better responses had more CD26+ T cells in both mesothelioma and pancreatic tumors reveals a possible significance of this marker in cancer immunotherapy.
Methods
Mice and tumor lines
C57BL/6 (B6), TRP-1 TCR transgenic, and NOD scid gamma (NSG) mice were purchased from The Jackson Laboratory and housed in the comparative medicine department at the Medical University of South Carolina Hollings Cancer Center (MUSC, Charleston, SC). NSG mice were housed in microisolator cages to ensure specific pathogen-free conditions and given ad libitum access to autoclaved food and acidified water. All housing and experiments were conducted in accordance with MUSC’s Institutional Animal Care and Use Committee’s (IACUC) procedures and with the aid of the Division of Lab Animal Resources (DLAR). B16F10 (H-2b) melanoma (gift, N.P. Restifo), M108 mesothelioma (gift, C.H. June), and PANC1 pancreatic cancer (gift, M.R. Rubinstein) cells were utilized for tumor experiments.
T cell subset isolation
Peripheral blood cells from healthy, de-identified individuals were purchased as a buffy coat (Plasma Consultants) or a leukophoresis (Research Blood Components). Lymphocytes were enriched via centrifugation with Lymphocyte Separation Media (Mediatech). Untouched CD4+ T cells were isolated by magnetic bead separation (Dynabeads, Invitrogen) and cultured overnight in CM and rhIL-2 (100 IU/ml; NIH repository). The next morning, CD4+ T cells were stained with PE-CD26 (C5A5b; BioLegend) and v500-CD4 (RPAT4) or APCCy7-CD4 (OKT4; BDPharmingen) and sorted on a BD FACSAria Ilu Cell Sorter into bulk CD4+, CD26neg, CD26int, and CD26high. Following sort, cells were cultured overnight in CM, 100 IU/ml rhIL-2, 10 μg/ml kanamycin/ampicillin, and 20 μg/ml anti-mycotic.
T cell expansion
TRP-1: Splenocytes from transgenic TRP-1 mice were isolated and cultured with 1 μl/ml TRP-1106–130 peptide (SGHNCGTCRPGWRGAACNQKILTVR) and feeder T cells at a ratio of 1 feeder: 5 TRP-1 CD4+ T cells. Cells were programmed to a Th17 phenotype with polarizing cytokines (10 ng/ml hIL-1β, 100 ng/ml hIL-21, 100 ng/ml hIL-6, 30 ng/ml hTGFβ, 10 μg/ml αm-IFNγ, 10 μg/ml αm-IL-4), and 100 IU/ml IL-2 for 6 days.
Human: Cells were cultured in CM supplemented with 100 IU/ml rhIL-2 at a 1:5 bead to T cell ratio using magnetic beads (Dynabeads, Life Technologies) decorated with antibodies to CD3 (OKT3) and ICOS (ISA-3, eBioscience), which were produced in the lab according to manufacturer’s protocol. Cells were de-beaded on day four and culture media/100 IU/ml rhIL-2 was replaced as needed.
Flow cytometry
Antibodies for extracellular stains were incubated with cells for 20 min in FACS buffer (PBS + 2%FBS). For intracellular staining, cells were activated with PMA/Ionomycin for 1 h, combined with Monensin (BioLegend) and incubated another 3 h prior to staining in Fix and Perm buffers (BioLegend). For transcription factors, the FOXP3 kit (BioLegend) was used according to manufacturer’s protocol. Live/dead staining was performed using the Zombie Aqua Fixable Viability Kit (BioLegend). Data were acquired on a BD FACSVerse (BD Biosciences) and analyzed using FlowJo software (Tree Star). A complete list of antibodies can be found in Supplementary Table 1.
MicroArray
The Qiagen RNeasy Mini kit was used to isolate RNA from sorted CD4+ T cells. Frozen RNA samples were sent to Phalanx Biotech Group for processing using their OneArray platform (San Diego, CA). NanoDrop ND-1000 was utilized to assess the quality and purity of the RNA. Absorbance ratios had a pass criteria of 260/280 ≥ 1.8 and A260/230 ≥ 1.5, indicating acceptable RNA purity. Agilent RNA 6000 Nano assay was used to ascertain RIN values, with a pass criteria of RIN value established at >6 indicating acceptable RNA integrity. Gel electrophoresis was used to evaluate gDNA contamination. Target preparation was performed using an Eberwine-based amplification method with Amino Allyl MessageAmp II aRNA Amplification Kit (AM1753, Ambion) to generate amino-allyl antisense RNA (aa-RNA). Prior to hybridization, labeled aRNA coupled with NHS-CyDye was prepared and purified. Purified coupled aRNA was quantified using NanoDrop ND-1000 with a pass criteria for CyDye incorporation efficiency at >15 dye molecular/1000 nt.
For data analysis, GPR files were loaded into Rosetta Resolver System. The Rosetta error model calculation was used to estimate random factors and systematic biases. Duplicate probes were averaged and median scaling was performed for normalization. Differentially expressed genes were defined as having a (+/−) log2-fold change ≥1 and P < 0.05. Where log2 ratios = “NA”, the differences in intensity between the two samples had to be ≥1000.
Heatmaps were constructed in R (version 3.1.2) using gplots (version 2.16.0) log2 values for bulk CD4+ T cells were averaged and used as a baseline for the genes of interest. For each sample, the fold change relative to baseline was calculated and the median value for the triplicates was used for generating figures.
T cell receptor β sequencing
CD4+ T cells were isolated from four individual healthy individuals and sorted into the following groups: bulk CD4+, CD26neg, CD26int, CD26high, Th1 (CXCR3+CCR6−), Th2 (CCR4+CCR6−), Th17 (CCR4+CCR6+), and Th1/Th17 (CCR6+CXCR3+). Sorted subsets were then centrifuged and washed in PBS prior to extracting genomic DNA via Wizard Genomic DNA purification kit (Promega). Spectrophotometric analysis using NanoDrop (ThermoScientific) was used to assess the quantity and purity of genomic DNA. The ImmunoSEQ hsTCRβ kit (Adaptive Biotechnologies Corp, Seattle, WA) was used according to manufacturer’s protocol to amplify the TCR genes. TCRβ sequencing was performed using the Illumina MiSeq platform at the Hollings Cancer Center Genomics Core. Analysis and graphing was performed using ImmunoSEQ software.
ELISA
T cells utilized for ELISA assays were plated at 2e5 cells/200 μl CM for 12–15 h prior to supernatant collection. Supernatant was then used to detect IL-17A, IL-17F, IL-22, GMCSF, IFNγ, and TNFα by DuoSet ELISA kits (R&D) per manufacturer’s instructions.
CD26 enzymatic assay
To determine CD26 enzymatic activity, a microplate-based fluorescence assay was performed. Briefly, 1e5 T cells from days 0, 5, and 10 days post-activation were washed and re-suspended in 100 μl 4% gly-pro-P-nitroanalide in PBS. After 2 h at 37 °C, the release of pNA from the substrate was assessed using a Multiskan FC plate reader (ThermoScientific) at 405 nm.
Metastatic melanoma patients
Lymphocytes were enriched from peripheral blood drawn from de-identified metastatic melanoma patients prior to treatment with Nivolumab or Pembrolizumab. Cells were stained immediately for flow cytometry analysis or cryopreserved for future use. CD4+ T cells from melanoma patients were sorted or gated by CD26 expression and compared to healthy donors in parallel experiments. All patients gave written, informed consent in accordance with the Declaration of Helsinki. The Medical University of South Carolina Institutional Review Board approved this study.
Adoptive cell transfer
B16F10: B6 mice were subcutaneously injected with 4e5 B16F10 melanoma 10 days prior to adoptive cell transfer of 5e4 CD4+Vβ14+ CD26neg or CD26high T cells. Mice received non-myeloablative 5 Gy total body irradiation 1 day pre-ACT (N = 6 mice/group, two independent experiments).
M108: Fig. 1: NSG mice were subcutaneously injected with 5e6 M108 mesothelioma (50% M108 in PBS, 50% Matrigel) 40 days prior to intravenous ACT of 1e5 redirected MesoCAR+ human CD26neg or CD26 high T cells (N = 10 mice per group). Fig. 5: 1e6 MesoCAR+ CD4+, CD26neg, CD26int, or CD26high T cells were infused into NSG mice bearing M108 tumors that were established for 60 days (N = 7–9 mice per group). Supplementary Fig. 6h: 5e6 MesoCAR+ CD4+CD26high or CD8+ T cells expanded in IL-2 were infused into M108-bearing mice and tumor regression assessed at 60 days post-ACT (N = 6 mice per group).
PANC1: NSG mice were subcutaneously injected with 5e6 PANC1 cells (50% PANC1, 50% Matrigel) 7 days prior to intravenous ACT of 1.75e6 CD4+, CD26neg, CD26int, or CD26high T cells (N = 6–9 mice per group). All mice were measured and equally distributed among treatment groups based on tumor size. Tumors were measured bi-weekly by caliper in a blinded fashion until tumor endpoint (>400 mm2).
Immunohistochemistry
Cryosections of xenograft tumor tissues (5 μm thick) were incubated in −20 °C acetone for 10 min, rinsed and exposed to 0.3% hydrogen peroxide for 10 min before blocking and subsequent incubation with primary antibody (CD45, 2B11_PD7/26, 1:100; DAKO) overnight at 4 °C. Slides were washed and incubated with secondary antibody (Polymer-HRP) and developed with DAB substrate kit (DAKO). Slides were counterstained with hematoxylin before visualization on an Olympus BX60 microscope. IHC-stained antigen spots were counted using a computer-assisted image analyzer (Olympus Microimage Image Analysis V4.0 software for Windows). The intensities of color related to CD45 antigen spot were quantified using ImageJ and expressed as mean pixel IOD.
Transwell migration assay
Sorted human T cells (7.5e4) were re-suspended in 75 μl RPMI + 0.1% FBS and placed in the top well of a transwell plate. Chemoattractants (235 μl) were placed in the bottom well as follows: control media (RPMI + 0.1% FBS), RPMI + 10% FBS, M108 supernatant, or PANC1 supernatant. Supernatant from cancer cells was collected 15 h after plating. The ability of subsets to migrate at 37 °C for 2 h was assayed by flow cytometry.
Western blot
Protein was isolated and concentration quantified using a BSA Protein Assay Kit (ThermoScientific). Ten-20 μg of total protein was separated on a Mini-PROTEAN TGX, Any kDTM gel followed by transfer onto PVDF membranes (Bio-Rad). The membranes were blocked (5% non-fat dry milk in TBS + 0.5% Tween20) prior to overnight incubation at 4 °C with primary antibodies to β-catenin (BD), Bcl-2 (D17C4), Caspase-3 (8G10), or GAPDH (D16HH, Cell Signaling). Following washes, membranes were incubated for 1 h at room temperature with secondary HRP-conjugated goat antibodies to mouse or rabbit IgG (Cell Signaling). Chemiluminescence was performed using Western ECL Blotting Substrate (Bio-Rad) followed by X-ray film-based imaging. Films were scanned and quantified for integrated optical density (IOD) using ImageJ software. To remove antibodies, membranes were incubated for 15 min at room temperature in Restore Western Blot Stripping Buffer (ThermoScientific).
Statistical analysis
Experiments comparing two groups were analyzed using a Mann–Whitney U test. For multi-group comparison, a one-way analysis of variance (ANOVA) was performed with a post comparison of group using Kruskal–Wallis. Graphs utilizing error bars display the center values as the mean and error bars indicate SEM. TCRβ sequencing analysis was based on the log-linear model and the relative risks were calculated with a 95% confidence interval. For tumor curves, Mann–Whitney or Kruskal–Wallis tests were performed at the final dates where all mice from compared groups were still alive. As these experiments were exploratory, there was no estimation to base the effective sample size; therefore, we based our animal studies using traditional sample sizes ≥6.
Data availability
All data are available from authors upon reasonable request. OneArray data can be found at GEO accession number GSE106726.
Electronic supplementary material
Acknowledgements
We would like to thank Hannah Knochelmann, Logan Huff and Kristina Schwartz from the Department of Microbiology & Immunology for their assistance. We also thank Adam Soloff, Zachary McPherson, Corinne Livingston and Kirsten McDanel at the MUSC Flow Cytometry and Cell Sorting Core and Chris Fuchs at Regenerative Medicine Department Flow Core for sorting cells. We thank Kent Armeson for his guidance on statistics and acknowledge Carmine Carpenito for his assistance in comparing the M108 and PANC1 tumor models (Supplementary Fig. 6c). We thank Mark Rubinstein (PANC1), Nicholas Restifo (B16F10) and Carl June (M108, MesoCAR) for their reagents and support. Finally, we acknowledge Zihai Li, Shikhar Mehrotra, Laura Kasman, and Ramsay Camp for their constant feedback and encouragement. Finally, we acknowledge our funding sources as follows: F31 CA192787 (NCI; S.R.B.), 122709-PF-13-084-01-L1B (ACS; M.H.N.), F30 CA200272 (NCI; J.S.B.), R01 CA175061 (NCI; C.M.P.), R01 CA208514 (NCI; C.M.P.), P01 CA154778(NCI; C.M.P, M.J.Z, M.I. Nishimura) and MUSC start-up funds.
Author contributions
S.R.B. designed and executed experiments, analyzed data, created the figures, and wrote/edited the manuscript; M.H.N., K.M., M.M.W., A.S.S. and C.C. performed experiments; M.J.Z. analyzed the data; K.S. consented and obtained blood samples from melanoma patients; C.H.J. provided reagents and intellectual feedback and C.M.P. directed the project, designed experiments, and edited the manuscript. All authors critically read and approved the manuscript.
Competing interests
S.R.B, M.H.N. and C.M.P. have a provisional patent for the use of CD26high T cells for adoptive cell transfer therapy. The remaining authors declare no competing financial interests.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at doi:10.1038/s41467-017-01867-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Stefanie R. Bailey, Email: flemins@musc.edu
Chrystal M. Paulos, Email: paulos@musc.edu
References
- 1.Topalian SL, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedicord VA, Montalvo W, Leiner IM, Allison JP. Single dose of anti-CTLA-4 enhances CD8+ T cell memory formation, function, and maintenance. Proc. Natl Acad. Sci. USA. 2011;108:266–271. doi: 10.1073/pnas.1016791108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.June CH. Adoptive T cell therapy for cancer in the clinic. J. Clin. Invest. 2007;117:1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan RA, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 2013;36:133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robbins PF, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frey NV, Porter DL. The promise of chimeric antigen receptor T cell therapy. Oncology. 2016;30:880–888. [PubMed] [Google Scholar]
- 7.Frigault MJ, Maus MV. Chimeric antigen receptor-modified T cells strike back. Int. Immunol. 2016;28:355–363. doi: 10.1093/intimm/dxw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beatty GL, O’Hara M. Chimeric antigen receptor-modified T cells for the treatment of solid tumors: Defining the challenges and next steps. Pharmacol. Ther. 2016;166:30–39. doi: 10.1016/j.pharmthera.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newick K, Moon E, Albelda SM. Chimeric antigen receptor T cell therapy for solid tumors. Mol. Ther. Oncol. 2016;3:16006. doi: 10.1038/mto.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudley ME, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan RA, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizvi NA, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brahmer JR, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawaoka T, et al. Adoptive immunotherapy for pancreatic cancer: cytotoxic T lymphocytes stimulated by the MUC1-expressing human pancreatic cancer cell line YPK-1. Oncol. Rep. 2008;20:155–163. [PubMed] [Google Scholar]
- 15.Kunk PR, Bauer TW, Slingluff CL, Rahma OE. From bench to bedside a comprehensive review of pancreatic cancer immunotherapy. J. Immunother. Cancer. 2016;4:14. doi: 10.1186/s40425-016-0119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melero I, Rouzaut A, Motz GT, Coukos G. T cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov. 2014;4:522–526. doi: 10.1158/2159-8290.CD-13-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim WA, June CH. The principles of engineering immune cells to treat. Cancer Cell. 2017;168:724–740. doi: 10.1016/j.cell.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pule MA, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg SA, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T cell transfer immunotherapy. Clin. Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yee C, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl Acad. Sci. USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graef P, et al. Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8(+) central memory T cells. Immunity. 2014;41:116–126. doi: 10.1016/j.immuni.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Muranski P, et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972–985. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paulos CM, et al. The inducible costimulator (ICOS) is critical for the development of human T(H)17 cells. Sci. Transl. Med. 2010;2:55ra78. doi: 10.1126/scitranslmed.3000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bengsch B, et al. Human Th17 cells express high levels of enzymatically active dipeptidylpeptidase IV (CD26) J. Immunol. 2012;188:5438–5447. doi: 10.4049/jimmunol.1103801. [DOI] [PubMed] [Google Scholar]
- 25.Fan H, et al. Dipeptidyl peptidase IV/CD26 in T cell activation, cytokine secretion and immunoglobulin production. Adv. Exp. Med. Biol. 2003;524:165–174. doi: 10.1007/0-306-47920-6_20. [DOI] [PubMed] [Google Scholar]
- 26.Bae EJ. DPP-4 inhibitors in diabetic complications: role of DPP-4 beyond glucose control. Arch. Pharm. Res. 2016;39:1114–1128. doi: 10.1007/s12272-016-0813-x. [DOI] [PubMed] [Google Scholar]
- 27.Ohnuma K, et al. Role of CD26/dipeptidyl peptidase IV in human T cell activation and function. Front. Biosci. 2008;13:2299–2310. doi: 10.2741/2844. [DOI] [PubMed] [Google Scholar]
- 28.Salgado FJ, et al. CD26: a negative selection marker for human Treg cells. Cytometry A. 2012;81:843–855. doi: 10.1002/cyto.a.22117. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur. J. Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 30.Yu N, et al. CD4(+)CD25 (+)CD127 (low/−) T cells: a more specific Treg population in human peripheral blood. Inflammation. 2012;35:1773–1780. doi: 10.1007/s10753-012-9496-8. [DOI] [PubMed] [Google Scholar]
- 31.Liu W, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weng NP, Araki Y, Subedi K. The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. Nat. Rev. Immunol. 2012;12:306–315. doi: 10.1038/nri3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krakauer M, Sorensen PS, Sellebjerg F. CD4(+) memory T cells with high CD26 surface expression are enriched for Th1 markers and correlate with clinical severity of multiple sclerosis. J. Neuroimmunol. 2006;181:157–164. doi: 10.1016/j.jneuroim.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Alsuliman A, et al. A subset of virus-specific CD161+ T cells selectively express the multidrug transporter MDR1 and are resistant to chemotherapy in AML. Blood. 2017;129:740–758. doi: 10.1182/blood-2016-05-713347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becattini S, et al. T cell immunity. Functional heterogeneity of human memory CD4(+) T cell clones primed by pathogens or vaccines. Science. 2015;347:400–406. doi: 10.1126/science.1260668. [DOI] [PubMed] [Google Scholar]
- 36.Hedin KE. Chemokines: new, key players in the pathobiology of pancreatic cancer. Int. J. Gastrointest. Cancer. 2002;31:23–29. doi: 10.1385/IJGC:31:1-3:23. [DOI] [PubMed] [Google Scholar]
- 37.Klebanoff CA, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl Acad. Sci. USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hinrichs CS, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc. Natl Acad. Sci. USA. 2009;106:17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gattinoni L, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J. Clin. Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schietinger A, et al. Tumor-specific T cell dysfunction is a dynamic antigen-driven differentiation program initiated early during tumorigenesis. Immunity. 2016;45:389–401. doi: 10.1016/j.immuni.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pauken KE, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. 2016;354:1160–1165. doi: 10.1126/science.aaf2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gattinoni L, et al. A human memory T cell subset with stem cell-like properties. Nat. Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moon EK, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin. Cancer Res. 2011;17:4719–4730. doi: 10.1158/1078-0432.CCR-11-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Craddock JA, et al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J. Immunother. 2010;33:780–788. doi: 10.1097/CJI.0b013e3181ee6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linch SN, et al. Combination OX40 agonism/CTLA-4 blockade with HER2 vaccination reverses T cell anergy and promotes survival in tumor-bearing mice. Proc. Natl Acad. Sci. USA. 2016;113:E319–E327. doi: 10.1073/pnas.1510518113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khalil M, Vonderheide RH. Anti-CD40 agonist antibodies: preclinical and clinical experience. Update Cancer Ther. 2007;2:61–65. doi: 10.1016/j.uct.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang RY, et al. LAG3 and PD1 co-inhibitory molecules collaborate to limit CD8+ T cell signaling and dampen antitumor immunity in a murine ovarian cancer model. Oncotarget. 2015;6:27359–27377. doi: 10.18632/oncotarget.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ribas A, et al. PD-1 blockade expands intratumoral memory T cells. Cancer Immunol. Res. 2016;4:194–203. doi: 10.1158/2326-6066.CIR-15-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suvas S, et al. In vivo kinetics of GITR and GITR ligand expression and their functional significance in regulating viral immunopathology. J. Virol. 2005;79:11935–11942. doi: 10.1128/JVI.79.18.11935-11942.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stephens GL, et al. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+ CD25+ T cells. J. Immunol. 2004;173:5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 51.Ding ZC, et al. Polyfunctional CD4(+) T cells are essential for eradicating advanced B-cell lymphoma after chemotherapy. Blood. 2012;120:2229–2239. doi: 10.1182/blood-2011-12-398321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan J, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc. Natl Acad. Sci. USA. 2008;105:20410–20415. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fleischer B. A novel pathway of human T cell activation via a 103 kD T cell activation antigen. J. Immunol. 1987;138:1346–1350. [PubMed] [Google Scholar]
- 54.Tran E, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsuzaki J, et al. Direct tumor recognition by a human CD4(+) T cell subset potently mediates tumor growth inhibition and orchestrates anti-tumor immune responses. Sci. Rep. 2015;5:14896. doi: 10.1038/srep14896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malandro N, et al. Clonal abundance of tumor-specific CD4(+) T cells potentiates efficacy and alters susceptibility to exhaustion. Immunity. 2016;44:179–193. doi: 10.1016/j.immuni.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lal N, Beggs AD, Willcox BE, Middleton GW. An immunogenomic stratification of colorectal cancer: implications for development of targeted immunotherapy. Oncoimmunology. 2015;4:e976052. doi: 10.4161/2162402X.2014.976052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alexandrov LB, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwek SS, et al. Diversity of antigen-specific responses induced in vivo with CTLA-4 blockade in prostate cancer patients. J. Immunol. 2012;189:3759–3766. doi: 10.4049/jimmunol.1201529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barreira da Silva R, et al. Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat. Immunol. 2015;16:850–858. doi: 10.1038/ni.3201. [DOI] [PubMed] [Google Scholar]
- 61.Wang H, et al. NRF2 activation by antioxidant antidiabetic agents accelerates tumor metastasis. Sci. Transl. Med. 2016;8:334ra351. doi: 10.1126/scitranslmed.aad6095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available from authors upon reasonable request. OneArray data can be found at GEO accession number GSE106726.