Abstract
GABAergic and glutamatergic dysfunction in the dorsolateral prefrontal cortex (DLPFC) are thought to be the core pathophysiological mechanisms of schizophrenia. Recently, we have established a method to index these functions from the DLPFC using the paired transcranial magnetic stimulation (TMS) paradigms of short interval intracortical inhibition (SICI) and facilitation (ICF) combined with electroencephalography (EEG). In this study, we aimed to evaluate neurophysiological indicators related to GABAA and glutamate receptor-mediated functions respectively from the DLPFC in patients with schizophrenia using these paradigms, compared to healthy controls. Given that these activities contribute to cognitive functions, the relationship between the TMS-evoked potential (TEP) modulations by SICI/ICF and cognitive/clinical measures were explored. Compared to controls, patients showed reduced inhibition in P60 (t22 = −4.961, p < 0.0001) by SICI and reduced facilitation in P60 (t22 = 5.174, p < 0.0001) and N100 (t22 = 3.273, p = 0.003) by ICF. In patients, the modulation of P60 by SICI was correlated with the longest span of the Letter-Number Span Test (r = −0.775, p = 0.003), while the modulation of N100 by ICF was correlated with the total score of the Positive and Negative. Syndrome Scale (r = 0.817, p = 0.002). These findings may represent the pathophysiology, which may be associated with prefrontal GABAA and glutamatergic dysfunctions, in the expression of symptoms of schizophrenia.
Introduction
Schizophrenia is a chronic and devastating mental disorder, which is a leading cause of years of life lost to disability1. Antipsychotics usually alleviate positive symptoms of patients with schizophrenia, however, the effects on the negative symptoms or cognitive impairments are limited2. The pathological mechanisms underlying these symptoms in schizophrenia are not fully elucidated, though, impairments in gamma-aminobutyric acid (GABA) receptor-mediated inhibitory and glutamate receptor-mediated excitatory neurotransmissions are strongly implicated3. Reduced GABA receptor-mediated inhibitory neurotransmission is a consistent finding in schizophrenia4,5, while decreased glutamate levels in the medial frontal region and glutamate receptor dysfunction are also shown in chronic schizophrenia6.
Paired pulse transcranial magnetic stimulation (TMS) is a non-invasive tool that can index neurophysiological property associated with neurotransmissions in the cortex7. Short interval intracortical inhibition (SICI) is one of the TMS neurophysiological paradigms8. The neurobiological basis of SICI has been identified through pharmacological studies combined with TMS. For example, it has been shown that GABAA agonist enhance SICI9,10, whereas GABA reuptake inhibitor tiagabine decreases SICI11. Thus, it is thought that SICI can index GABA receptor-mediated inhibitory function in the motor cortex7. In contrast, intracortical facilitation (ICF) is one of the facilitatory TMS neurophysiological paradigm8,12. Specifically, pharmacological studies with TMS have been demonstrated that NMDA antagonist decreases ICF13,14, while GABAA agonist also decreases ICF10,15. Thus, ICF is thought to be mainly associated with glutamate receptor-mediated excitatory functions in the motor cortex. Direct evidence of neurotransmitter functioning from combined TMS-EEG from the DLPFC remains relatively unexplored.
In patients with schizophrenia, a reduction of SICI in motor cortex, which is thought to be associated with GABAA receptor-mediated inhibition, has been a replicated finding5. Further, such deficits of the motor cortex in schizophrenia may be attributed to global disturbances in GABA receptor-mediated inhibitory neurotransmission, supported by findings of substantial reductions in GABA receptor-mediated inhibitory interneurons, GABA-synthesizing enzyme glutamic acid decarboxylase (GAD67), and GABA-related gene expression in post-mortem studies3. In contrast, intracortical facilitation (ICF) in motor cortex appears to be unaltered in patients with schizophrenia16,17, despite genetic and pharmacological studies suggesting potential deficits of glutamate-mediated excitatory neurotransmission through the N-methyl-D-aspartic acid (NMDA) receptor in schizophrenia18. Furthermore, postmortem studies have demonstrated decreased transcription levels and enzyme activity for glutamate carboxy peptidase in the dorsolateral prefrontal cortex (DLPFC) in patients with schizophrenia, which in turn results in reduced NMDA receptor-mediated signaling19. Taken together, dysfunction of GABA receptor-mediated inhibitory as well as glutamate receptor-mediated excitatory neurotransmissions in schizophrenia may underlie an impaired excitatory and inhibitory (E/I) balance in this disorder20.
Cognitive impairment is a core symptom of schizophrenia21 that is strongly associated with DLPFC dysfunction22,23. For example, DLPFC is involved in working memory24 and has been targeted to improve working memory deficits25 in addition to negative symptoms in schizophrenia26. We recently reported that greater working memory performance was associated with greater GABAA receptor-mediated activity indexed by SICI in motor cortex25. Recent technological advances allow us to investigate cortical integrity in a non-invasive way utilizing concurrent TMS-EEG27–29. Further, it can be possible to more directly investigate the relationship between working memory performance and neurophysiological property associated with GABAA receptor-mediated function in the DLPFC described below, which has not been determined yet.
Given that SICI and ICF deficits may represent underlying neurobiological dysfunction in schizophrenia, evaluating these TMS neurophysiology from the DLPFC and their associations with cognition may further understand the pathophysiology underlying schizophrenia. We have developed a method to measure the strength of inhibitory and excitatory neurotransmissions as indexed by SICI and ICF in the DLPFC using combined TMS–EEG30. This technique also allows us to determine the effect of SICI and ICF on individual components and frequency band activities of the TMS-evoked potential (TEP) among patients with schizophrenia compared to healthy controls. In the present study, we hypothesized that: (1) inhibitory and excitatory functions in the DLPFC as measured by the paired pulse TMS paradigms of SICI and ICF would be reduced in patients with chronic schizophrenia compared to the healthy controls; (2) in patients group, reduced SICI and ICF would correlate with cognitive and clinical measures, specifically working memory performance and clinical symptom severity based on previous research31,32. The aims of this study were to measure SICI and ICF from the DLPFC in patients with schizophrenia compared to controls and to determine if TEPs induced by these paradigms are associated with working memory performance and clinical symptom severity. In addition, we aimed to explore the modulatory effects on frequency bands by SICI and ICF between patients and controls.
Results
Demographic, clinical and cognitive measures, and medication information
Demographic of the patients and controls, and clinical and cognitive measures of the patients are detailed in Table 1. Of note, there was no correlation between the chlorpromazine equivalent dose that each patient was taking during the study and the clinical or cognitive measures. The psychotropic medications of the patients are shown in Supplementary Table.
Table 1.
Demographic, clinical, and cognitive data of the patients and controls.
| Mean (±SD) | Patients (N = 12) | Controls (N = 12) |
|---|---|---|
| Age | 41 ± 10 | 39 ± 12 |
| Male: Female | 8: 4 | 6: 6 |
| Years of Education | 15 ± 3 | 15 ± 2 |
| PANSS | ||
| Positive | 11.3 ± 3.0 | — |
| Negative | 11.7 ± 3.4 | — |
| General | 23.6 ± 2.8 | — |
| Total | 50.1 ± 6.2 | — |
| WTAR FS IQ | 106 ± 10 | — |
| Letter-Number-Span | 12 ± 3 | — |
| HVLT | — | |
| Retention (%) | 83.4 ± 14.8 | — |
| Discrimination Index | 10.5 ± 1.4 | — |
| TMT (seconds) | — | |
| A | 30.6 ± 9.7 | — |
| B | 71.7 ± 36.5 | — |
| CPZ Equivalent Doses (mg/day) | 330 ± 287 | — |
SD: standard deviation, PANSS: positive and negative symptom scale, WTAR FS IQ: Wechsler Test of Adult Reading Full Scale Intelligence Quotient, HVLT: Hopkins Verbal Learning Test, TMT: Trail Making Test, CPZ: chlorpromazine.
Modulations of the TEP amplitude by SICI and ICF paradigm from the DLPFC in patients with schizophrenia compared to controls
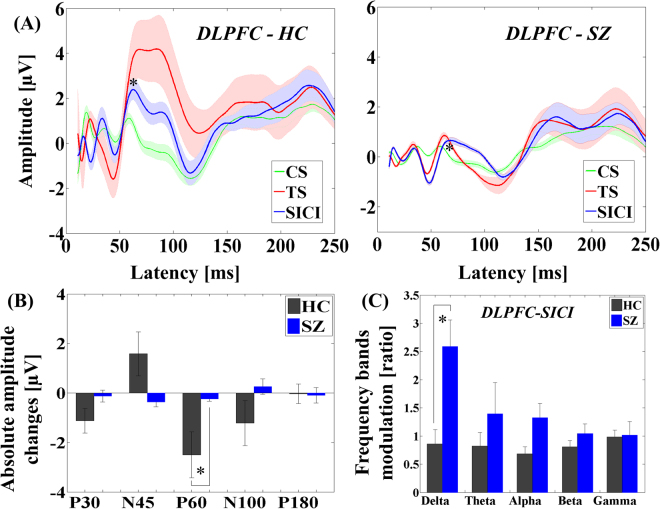
The analysis of variance (ANOVA) for TEP amplitudes in the SICI paradigm showed significant main effects of diagnosis (F1,22 = 9.231, p = 0.006) and TEP component (F4,88 = 19.603, p < 0.0001) and significant TEP component × diagnosis (F4,88 = 3.259, p = 0.015), TMS condition × TEP component (F4,88 = 3.528, p = 0.010), TMS condition × TEP component × diagnosis (F4,88 = 4.569, p = 0.002) interactions. Post-hoc analyses for TEP amplitude changes by SICI paradigm between patients and controls demonstrated a significant difference in P60 TEP (t22 = −4.961, p < 0.0001), showing that SICI induced a significantly smaller reduction in the patient group. In addition, we observed a significant TEP modulation on P60 (t11 = 2.248, p = 0.046) by SICI in patient group (see Fig. 1(A)). Of note, we observed no main effects of group between patients and controls in SICI (F1,22 = 0.661, p = 0.428) as well as ICF (F1,22 = 0.286, p = 0.598), indicating that there were no significant differences in single-pulse TEPs between the two groups.
Figure 1.
Modulation of TEPs by SICI paradigm administered TMS to DLPFC. (A) Group averaged TEPs at the region of interest (left DLPFC) in patients with schizophrenia following TS (red; delivered at a time equal to 0 ms), SICI (CS.TS) (blue) and CS alone (green line; delivered at −2 ms, i.e. 2 ms prior to TS) with standard error of mean (SEM) of each TEP trace. P60 TEP was significantly reduced in amplitude by SICI. (B) Group comparisons of TEP changes by SICI at the region of interest (left DLPFC) between healthy controls and schizophrenia patients. Patients group demonstrated significantly lower inhibitory modulation on P60 TEP compared to healthy controls. (C) Modulation of frequency bands by SICI in the DLPFC between healthy controls and patients with schizophrenia: patients with schizophrenia showed significantly less inhibitory modulation on delta frequency band with SICI at the region of interest (left DLPFC), compared to healthy controls. Error bars for each graph represent standard error.
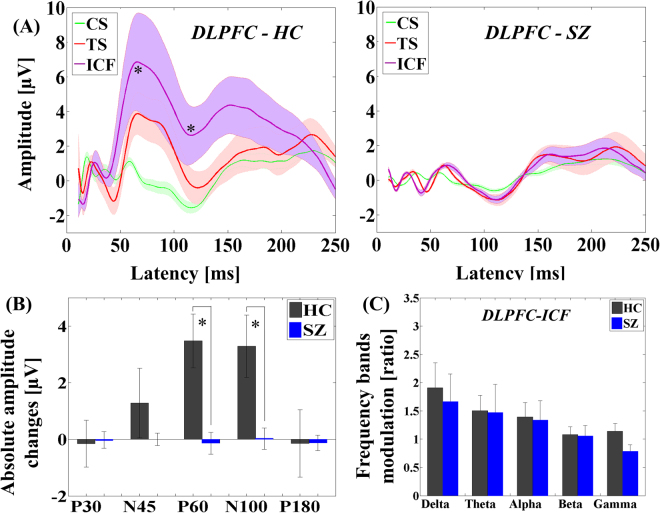
On the other hand, the ANOVA for TEP amplitude in the ICF paradigm indicated significant main effects of diagnosis (F1,22 = 18.648, p < 0.0001), TMS condition (F1,22 = 12.240, p = 0.002), and TEP component (F4,88 = 14.991, p < 0.0001) and significant TMS condition × diagnosis (F1,22 = 13.494, p = 0.001), TEP component × diagnosis (F4,88 = 5.951, p < 0.0001), TMS condition × TEP component (F4,88 = 2.760, p = 0.033), and TMS condition × TEP component × diagnosis (F4,88 = 2.857, p = 0.028) interactions. Post-hoc analyses for TEP amplitude changes by ICF paradigm between patients and controls demonstrated significant differences in P60 (t22 = 5.174, p < 0.0001) and N100 (t22 = 3.273, p = 0.003) TEPs, showing that ICF induced significantly smaller facilitations in the patient group. Additionally, there was no significant TEP modulation by ICF in patient group itself (see Fig. 2(A)).
Figure 2.
Modulation of TEPs by ICF paradigm administered TMS to DLPFC. (A) Group averaged TEPs at the region of interest (left DLPFC) in patients with schizophrenia following TS (red; delivered at a time equal to 0 ms) and ICF (CS.TS) (purple) and CS alone (green line; delivered at −10 ms, i.e. 10 ms prior to TS) with SEM of each TEP trace. No TEP amplitudes were significantly increased by ICF in patients group. (B) Group comparisons of TEP changes by ICF at the region of interest (left DLPFC) between healthy controls and schizophrenia patients. Patients group showed significantly lower facilitatory modulations on P60 and N100 TEPs compared to healthy controls. (C) Frequency band power modulations by ICF paradigms in the DLPFC between healthy controls and patients with schizophrenia: there was no significant difference of modulations on any frequency bands with ICF at the region of interest (left DLPFC) between the two groups. Error bars for each graph represent standard error.
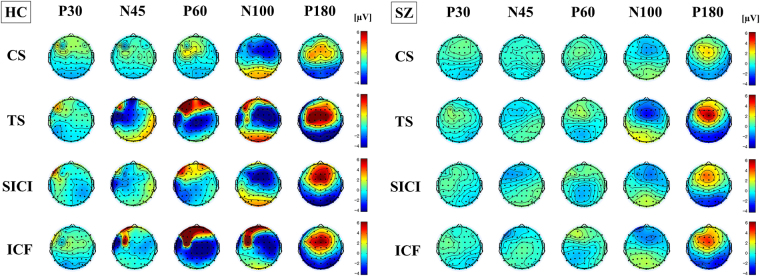
The result of TEP traces (i.e., condition stimulus, test stimulus, and SICI condition) in patients with schizophrenia with SICI paradigm is depicted in Fig. 1(A), while the result of TEP traces in the patient group with ICF paradigm is shown in Fig. 2(A). Of note, there was no correlation between the chlorpromazine equivalent dose for each patient and the modulation of TEP components induced by SICI and ICF paradigms. Further, the results of the cross-sectional comparison analyses in SICI and ICF paradigms are shown in bar graphs of Figs 1(B) and 2(B), respectively. In addition, topographical plots depicting the TEPs for each component (i.e. P30, N45, P60, N100, and P180) and condition (CS, TS, SICI and ICF paradigms) for both groups are shown in Fig. 3. The topography of healthy controls shows the TEP reduction over the prefrontal area on P60 component (t22 = −4.961, p < 0.0001) in the SICI paradigm while the TEP increases over the left prefrontal area on P60 (t22 = 5.174, p < 0.0001) (i.e. more excitatory modulation) and N100 (t22 = 3.273, p = 0.003) (i.e. less inhibitory modulation) components in the ICF paradigm. In contrast, the topographical changes in patients are poor as a whole, but in the SICI paradigm, there was a reduction of TEP on N45 component over the left prefrontal area.
Figure 3.
Topographical plots of paired pulse SICI and ICF paradigms. Left graph shows topographical distribution of healthy controls for each condition (CS, TS, SICI and ICF) and each TEP component (P30, N45, P60, N100, and P180). In healthy controls, TEPs by SICI reduced over the prefrontal area on P60 component, whereas TEPs by ICF increased over the left prefrontal area on P60 (i.e. more excitatory modulation) and N100 (i.e. less inhibitory modulation) components. In contrast, in right graph of patients group, the topographical changes are poor as a whole but there was a reduction of TEP N45 over the left prefrontal area.
Frequency band power modulations during SICI and ICF paradigms in patients with schizophrenia compared to healthy controls
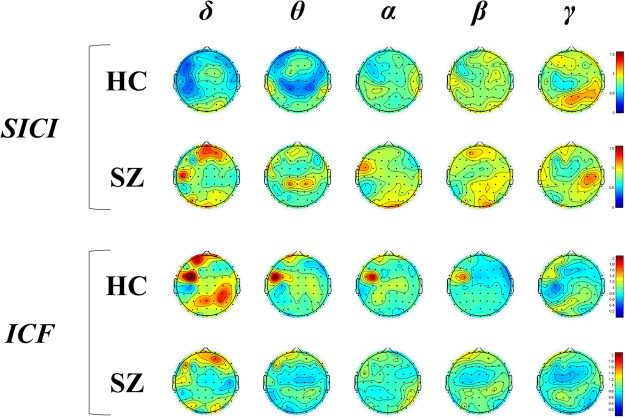
In the SICI paradigm, there was a significant frequency band power modulation difference in the delta band (t22 = −3.220, p = 0.004; α = 0.05/5; controlled by the number of frequency bands) between patients and controls. By contrast, there was no significant difference in all frequency bands power modulations between the two groups with ICF (delta: t22 = 1.261, p = 0.221; theta: t22 = 0.189, p = 0.852; alpha: t22 = 0.446, p = 0.660; beta: t22 = 0.289, p = 0.775; gamma: t22 = 1.664, p = 0.110). These modulatory effects of SICI and ICF on frequency band powers between the healthy control group and the patient group are shown in bar graphs in Figs 1c and 2c. In addition, we plotted the topographical distributions of SICI and ICF paradigms for each frequency band modulation from delta to gamma and TEP component from P30 to P180 in both groups (see Fig. 4). Specifically, when comparing healthy controls and patients with schizophrenia, the patients showed relatively higher delta power modulation (t22 = −3.220, p = 0.004) over the prefrontal area compared to healthy controls in the SICI paradigm (Fig. 4 (A)). In contrast, in the ICF paradigm, healthy controls showed relatively higher frequency band modulations from delta to beta bands (δ: t22 = 1.261, p = 0.221; θ: t22 = 0.189, p = 0.852; α: t22 = 0.446, p = 0.660; β: t22 = 0.289, p = 0.775; γ: t22 = 1.664, p = 0.110) over the left DLPFC than patients with schizophrenia, but these changes were not significant as described above (Fig. 4(B)).
Figure 4.
Topographical distributions of frequency band modulations by SICI and ICF paradigms. When comparing healthy controls and patients with schizophrenia, patients group showed relatively higher delta power modulation over the prefrontal area than healthy controls by SICI paradigm. In contrast, in ICF paradigm, healthy controls showed relatively higher frequency band modulations from delta to beta bands over the left DLPFC than patients with schizophrenia, but these changes were not significant.
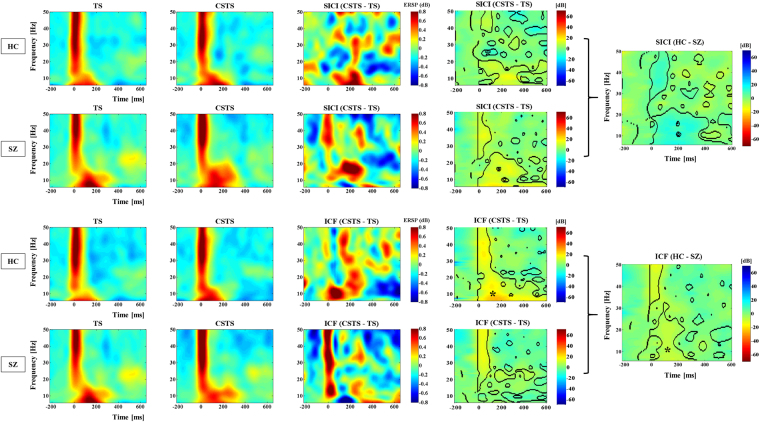
Time-frequency analysis during SICI and ICF paradigms
When comparing controls and patients, the statistical maps for the time-frequency modulation graphs during SICI as well as ICF paradigms showed significant differences in the regions inside the black dashed contours (see Fig. 5). Specifically, healthy controls showed significantly lower time-frequency modulations during SICI (blue color), compared with patients with schizophrenia, suggesting that healthy controls had a more effective suppressive effect with the SICI paradigm. In contrast, in the ICF paradigm, healthy controls showed siginificantly higher time-frequency modulations (yellow color) compared to patients, suggesting that healthy controls had a more robust excitatory response. Furthermore, the asterisks on the statistical maps in Fig. 5 indicate the significant time-frequency modulation corresponding to the significant TEP modulations as shown in Figs 1 and 2. Thus, it is suggested that patients with schizophrenia may have less cortical inhibition in the SICI as well as less cortical excitation in the ICF in comparison with healthy controls.
Figure 5.
Time-frequency analysis during SICI and ICF paradigms. When comparing controls and patients, the statistical maps for the time-frequency modulation graphs during SICI as well as ICF paradigms showed significant differences in the regions inside the black dashed contours. Specifically, healthy controls showed significantly lower time-frequency modulations during SICI (blue color), compared with patients with schizophrenia, suggesting that healthy controls had a more effective suppressive effect with the SICI paradigm. In contrast, in the ICF paradigm, healthy controls showed siginificantly higher time-frequency modulations (yellow color) compared to patients, suggesting that healthy controls had a more robust excitatory response. Furthermore, the asterisks on the statistical maps in indicate the significant time-frequency modulation corresponding to the significant TEP modulations as shown in Figs 1 and 2.
Cognitive and clinical correlations between significant TEP amplitude changes and clinical/cognitive measures in patients with schizophrenia
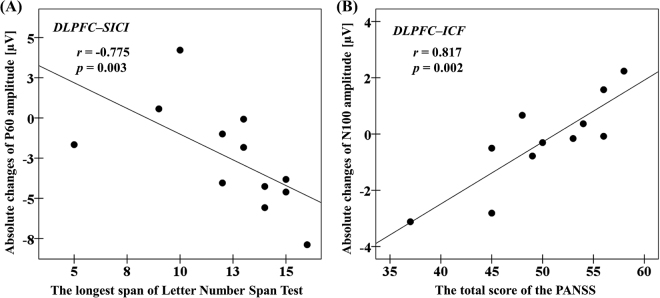
A highly significant correlation between TEP P60 amplitude change induced by SICI and the longest span of the Letter-Number Span Test (r = −0.775, p = 0.003, N = 12; α = 0.05/5; controlled by the number of cognitive/clinical measures) (see Fig. 6(A)) was observed in patients, while no other relationship was uncovered for measures of IQ or executive functioning. Furthermore, there was a highly significant correlation between TEP N100 amplitude change induced by ICF and the total score of the Positive and Negative Symptom Scale (PANSS) (r = 0.817, p = 0.002, N = 11; α = 0.05/5; see Fig. 6(B)).
Figure 6.
Cognitive and clinical correlations with modulation of TEPs at the left DLPFC by SICI and ICF in patients with schizophrenia. (A) With DLPFC-SICI paradigm, absolute changes of P60 amplitude was significantly correlated with the longest span of the Letter-Number Span Test. (B) With DLPFC-ICF paradigm, absolute changes of N100 amplitude was significantly correlated with the total score of the Positive and Negative Syndrome Scale (PANSS).
Discussion
The present study demonstrated modulatory effects of SICI and ICF paradigms on TEP components from the DLPFC in patients with schizophrenia compared to healthy controls. Patients with schizophrenia showed a significantly reduced inhibition of P60 TEP by SICI, suggesting that GABAA receptor-mediated inhibitory function may be weakening, and significantly reduced facilitations on P60 and N100 TEPs by ICF, suggesting that glutamatergic receptor-mediated function may be weakening as well, compared to healthy controls. The inhibition of delta power with SICI was selectively impaired in patients with schizophrenia compared to healthy controls. Furthermore, in patients with schizophrenia, the degree of the modulatory effect on P60 by SICI was significantly correlated with working memory performance as assessed by longest span of the Letter-Number Span Test and also the extent of modulation on N100 by ICF was significantly correlated with the severity of the symptoms of schizophrenia as evaluated with the total score of the PANSS.
Differences in the neurophysiological modulation of TEPs induced by SICI paradigm in the DLPFC between patients and controls may add to accumulating evidence of GABAAergic dysfunction in the DLPFC in schizophrenia3,33. Further, the modulation of P60 TEP with SICI was significantly correlated with performance of the Letter-Number Span Test, suggesting the relationship between working memory and GABAA activity34. This finding may support at least in part of the physiological results of previous studies that have shown a relationship between GABA levels in the DLPFC and average gamma amplitude during working memory task31. Although the relationship between glutamatergic dysfunction and cognitive impairment is well known in schizophrenia32, we did not observe any significant correlation between TEP modulations by ICF and cognitive measures in the present study. A recent meta-analysis reviewing the effects of glutamate positive modulators on cognitive deficits in patients with schizophrenia demonstrated that there is no significant effect of glutamate positive modulators on cognition35. Thus, the cortical excitatory function as indexed by ICF, which is at least in part associate with glutamatergic receptor-mediated function, may not be directly related to cognitive dysfunction in schizophrenia. In other words, the reduced cortical excitability seen in the ICF paradigm in patients with schizophrenia may be related to the chronicity of the disease rather than the cognitive dysfunction itself.
The inhibition of delta power by SICI was impaired among patients with schizophrenia compared to healthy controls. This finding is in line with previous studies that reported increased resting EEG delta power in patients with schizophrenia compared to healthy controls36, which has been associated with negative symptoms of schizophrenia37. Taken together, our findings suggest that prefrontal delta activity may be related to neurophysiological property in terms of GABAA receptor-mediated inhibitory dysfunction in patients with schizophrenia. In contrast, several studies have shown a relationship between GABAA receptor-mediated inhibition and synchronized gamma oscillations in vitro or animal model38,39 as well as in human subjects40. Thus, initially, we anticipated that the modulation of gamma band power by the SICI paradigm in patients with schizophrenia would be lower than in healthy controls. However, our result showed no significant difference in the modulation of gamma band power by SICI between the two groups. This may be partially explained by a medication effect on gamma band power in patients with schizophrenia as antipsychotic drugs are likely to have effects on SICI, which is associated with GABAA receptor-mediated inhibitory function41.
While some studies have reported decreased glutamate levels as measured by magnetic resonance spectroscopy in patients with chronic schizophrenia6,42, the evidence of glutamatergic dysfunction in schizophrenia is mixed depending on the subtype and stage of schizophrenia43,44. In the present study, there was no significant TEP modulations by ICF in patients with schizophrenia However, between patients and controls, there were significant differences of modulation of TEP P60 and N100 with ICF paradigm (controls > patients), suggesting that, at least in part, glutamatergic receptor-mediated function in the DLPFC may be reduced in patients with schizophrenia. Thus, these results may support previous findings6,42, that indicate a decrease in glutamate levels in the prefrontal cortex in patients with chronic schizophrenia.
Further, in the ICF paradigm from the DLPFC, amplitude change of the TEP N100 component was significantly correlated with the total score of PANSS in patients with schizophrenia, suggesting that patients who may have had reduced glutamatergic excitatory effect on N100 had lower clinical symptom severity. It can be speculated that patients with schizophrenia who have a lowered level of cortical excitability as indexed by ICF, may have less glutamate-mediated excitotoxicity in the prefrontal cortex45, that may be associated with a lower symptom burden.
As another important factor contributing to schizophrenia, it is well known that dopaminergic modulation plays a significant role in cognitive functions of the prefrontal cortex including functions that are impaired in patients with schizophrenia46. Specifically, on a cellular level, dopamine modulates pyramidal cell excitability through its actions on local circuits of GABAergic interneurons in the prefrontal cortex47–49. Dopamine suppresses glutamatergic excitatory transmission between pyramidal neurons through activation of D1 receptors that induce an increase in first spiking interneuron excitability, which in turn results in enhanced GABAergic inhibitory transmission to pyramidal neurons in the prefrontal cortex49. Therefore, dysfunction of tuning due to changes in dopamine and/or GABA transmission might also underlie deficits in cognitive impairments in schizophrenia3,46,50.
Furthermore, in connection with the excitatory function by ICF and the inhibitory function by SICI, previous studies have shown that there is a balanced E/I relationship between GABA receptor-mediated inhibition and glutamate receptor-mediated excitation in the healthy brain and this property is thought to be crucial for information processing20,51. However, it has been suggested that patients with schizophrenia may have impaired E/I balance in neural circuits in the prefrontal cortex52, leading to deficits in cognition and social behavior20,53,54. The present finding may support the characteristics of E/I balance in the prefrontal cortex between healthy controls and patients with schizophrenia. Moreover, in the patients group, amplitude changes of P60 component by SICI was correlated with performance of working memory, as well as amplitude changes of N100 component by ICF was correlated with severity of clinical symptoms. Therefore, taken together these clinical and cognitive correlations with the neurophysiological measures of inhibitory effect on P60 and excitatory effect on N100, as measured with SICI and ICF paradigms, respectively, may represent a pathophysiological mechanism underlying the symptoms of patients with schizophrenia.
Some limitations exist in the present study. First, concomitant medications (Supplementary Table) may have potential confounding effects on SICI and ICF in the patient group. However, no participant took benzodiazepines or glutamate modulators in the present study. In terms of potential confounding of SICI and ICF by dopamine antagonists, although a previous study has reported that haloperidol has negative impact on SICI and ICF55, there is no evidence that other dopamine antagonists have direct effects on SICI and ICF. In the present study, none of the patients were taking haloperidol. Furthermore, there was no relationship found between chlorpromazine equivalent doses and the extent of inhibition or excitation suggesting that the present results cannot readily be explained by the use of antipsychotic medication. Nonetheless, it is important to determine the effect of medication on these measures56. A second limitation to this study is that cognitive assessments were only administered in the patient group and not in the controls. The relationship between deficits in cognition and neurotransmission was of greater interest in the patient group in the present study. It was anticipated that ceiling effects in healthy controls would have limited the power to detect such relationships. Thirdly, since we did not conduct a pharmaco-TMS-EEG study, there is a limit that it cannot show direct evidence from our results. Lastly, we did not use an auditory masking noise during the experiments. Thus, there may be some potential effect of auditory input associated with click sounds of the TMS coil. However, at the stage of off-line analysis, we compared test TMS pulse and conditioned TMS pulse (i.e. SICI or ICF) that had nearly identical auditory inputs associated with the click sounds. As a result, this potential confounding may be minimal. Nonetheless, future studies may examine the relationship between SICI and ICF and cognition and whether this effect is selective to the working memory domain as we observed in the patient group.
In conclusion, the present study adds to the evidence for GABAA receptor-mediated inhibitory as well as glutamate receptor-mediated excitatory dysfunctions in schizophrenia and suggests possible pathophysiological mechanism in the emergence of clinical and cognitive symptom of the illness. Using combined TMS-EEG, it is possible to further investigate GABA receptor-mediated inhibitory and glutamate receptor-mediated excitatory neurotransmissions as possible targets or mechanisms through which treatments may exact their therapeutic effect may help to advance the treatment of this devastating disorder. The present study demonstrates the applicability of the SICI-ICF paradigms using combined TMS–EEG. Moreover, the E/I balance and its role in cognition may serve as a possible dimension to investigate across mental illness within the Research Domain Criteria (RDoC) framework57.
Materials and Methods
Participants
Twelve right-handed patients with schizophrenia (8 males, mean age: 41 ± 10 years) and 12 healthy control participants (6 males, mean age: 39 ± 12 years) were examined in the present study. SICI and ICF paradigms were administered to the DLPFC using a combined TMS–EEG in all participants. Participants were eligible for this study if they met the following criteria: (i) between ages 18 and 59; (ii) no history of neurological disorders including seizure, syncope or stroke; (iii) no current (past 6 months) of alcohol or other drug abuse or dependence; (iv) current abstinent non-smoker verified by a carbon monoxide reading (less than or equal to 4 ppm of a carbon monoxide (CO) level as measured by the breath CO monitor)58. For the healthy controls, they also met the following criteria: v) no history of neuropsychiatric disorders; or vi) no prescription medications. For patients with schizophrenia, they met the criteria as follows: vii) clinically stable determined by the PANSS score of ≤70; (viii) no anticholinergic drugs, benzodiazepines, or glutamate modulators; (ix) had not been hospitalized in the past 3 months, and were on a stable dose of antipsychotic medications for at least one month. CPZ equivalents were calculated according to established methods59. All participants were screened with the Structured Clinical Interview for DSM–IV Axis I Disorders prior to study participation. Written informed consent was obtained from each participant. The experiment was conducted in accordance with the Declaration of Helsinki and was approved by the Research Ethics Board of the Centre for Addiction and Mental Health.
Clinical and cognitive assessment for patients with schizophrenia
To assess the severity of clinical symptoms, 11 out of 12 patients were examined with the PANSS (1 patient could not complete the PANSS). For cognitive measures, the Wechsler Test of Adult Reading (WTAR), the Letter-Number Span Test, and the Trail Making Test (TMT) Parts A & B, the Hopkins Verbal Learning Test (HVLT) were performed for patients with schizophrenia.
TMS procedure and EMG measure
Monophasic TMS pulses were administered to the left primary motor cortex using a 70 mm figure–of–eight coil, and two Magstim 200 stimulators (Magstim Company Ltd., UK) connected via a Bistim module. During the TMS testing, participants sat in a chair with their eyes open and right hand relaxed throughout the study. First, the primary motor cortex hot spot for the right first dorsal interosseous muscle to evoke the largest motor evoked potential (MEP) was determined. Second, the individual intensity of resting motor threshold (RMT) to induce a minimum of 50 μV MEP amplitude from the right first dorsal interosseous muscle 5 times out of 10 trials was determined. Subsequently, the individual intensity to induce 1 mV peak–to–peak MEP amplitude of the same muscle was determined. The RMT and the intensity to induce 1 mV peak-to-peak MEP amplitude were assessed to determine the intensity parameters of SICI and ICF paradigms.
SICI and ICF measures
SICI and ICF were examined according to established methods8; specifically, the inter-stimulus interval (ISI) of SICI was set to 2 ms while the ISI of ICF was set to 10 ms. Conditioning stimulus (CS) intensity was set at 80% of RMT and test stimulus (TS) intensity was set at the intensity to evoke a 1 mV peak-to-peak MEP amplitude when TMS was delivered alone. The left DLPFC target was individually determined based on the EEG cap navigated F5 electrode site method60.
EEG recording and pre–processing
EEG was acquired through a 64–channel Neuroscan Synamps 2 EEG system with TMS-compatible EEG cap (Compumedics Neuroscan, Australia). All electrodes were referenced to an electrode placed on the vertex. Recording electrodes impedance was kept lowered to ≤5 kΩ during experiment. EEG signals were recorded at DC with a sampling rate of 20 kHz and an online lowpass filter of 200 Hz was applied. EEG data were processed offline using the MATLAB software (R2014a, The MathWorks, MA, USA). All data were down–sampled to 1000 Hz for analyses.
EEG signal processing
EEG signal processing was performed in line with published methodology30. The continuous EEG data were epoched from −1000 ms to 2000 ms relative to the TMS pulse. Baseline correction was conducted with respect to the pre-stimulus interval −500 ms to −110 ms. To avoid TMS artifacts, the epoched EEG data was re-segmented from 10 ms to 2000 ms post-TMS. Then, EEG data were visually inspected to exclude trials and channels that were highly contaminated with noise. As a result, more than 80% of trials and 95% of channels survived artifact rejection. Subsequently, independent component analysis (ICA) was applied to minimize and remove the typical TMS–related decay artifacts as well as eye–related and remaining muscle activity related components. Following the ICA cleaning, the Butterworth, zero-phase shift 1–55 Hz band pass filter (24 dB/Oct) and notch filter were applied. In each subject, the number of ICA components that were removed from original 62 ICA components was no greater than 20%. Finally, data was re-referenced to the average for further analyses.
TMS-evoked potential analyses of SICI and ICF paradigms
For the EEG analyses of SICI and ICF, TEP induced by each paradigm was analyzed individually. Specifically, the influence of each paradigm on the individual TEP components (P30, N45, P60, N100 and P180) value was computed for each condition (CS, TS, and CS.TS) at the left DLPFC region obtained from the SICI and ICF experiments from the DLPFC. We extracted the maximum/minimum amplitude values for each positive/negative deflections individually for each electrode included in the left DLPFC region. Then, values were averaged. In addition, we calculated the TEP traces over a time window of 10–250 ms after TMS pulse, but specific time windows of each TEP component were not set for the analyses because we extracted the amplitude peak and trough values manually by visual inspection for each subject. Further, we clustered the specific electrodes as regions of interest for analyses of the left DLPFC (Fp1, AF3, AF7, F1, F3, F5, F7, FC1, FC3, FC7) regions according to our previously published method30, in order to ensure consistency with previous analytical method in healthy controls and to reduce the influence of contaminated electrode channel data under the TMS coil. We created the TEP traces of each SICI and ICF paradigm for both healthy controls and patients with schizophrenia, bar graphs comparing the TEP results for both groups, and topographical plots of each paired pulse TEPs for each TEP component in both groups.
Frequency band powers analyses during SICI and ICF paradigms
Frequency band power changes induced by SICI and ICF paradigms were calculated by applying the Hilbert transform method to TEP focused on the left DLPFC and each frequency band was defined as follows: delta (1–3 Hz); theta (4–7 Hz); alpha (8–14 Hz); beta (14–30 Hz); and gamma (30–50 Hz). Then, modulations of each frequency band were calculated as a ratio of frequency band power of CS.TS over TS condition (i.e., ratio = CS.TS/TS) for both SICI and ICF paradigms in healthy controls and patients with schizophrenia groups. We created bar graphs comparing the frequency band power modulation of TEPs and topographical plots for each frequency band (i.e., delta, theta, alpha, beta, and gamma bands) and each TEP component (i.e., P30, N45, P60, N100, and P180) during the SICI and ICF paradigms in both groups.
Time-frequency analysis for SICI and ICF paradigms
Time-frequency analysis for SICI and ICF paradigms from the left DLPFC for both groups was performed to evaluate the difference of time-frequency patters qualitatively between the two groups. Specifically, the wavelet frequency analysis was applied via “Event related spectral perturbation” (ERSP) using the newtimef() function in the open source toolbox EEGLAB on the average of each trial for each condition for all subjects. The wavelet cycle was set to [30.5] and a period from 400 ms before and 1000 ms after the TMS pulse was analyzed. The baseline activity was subtracted and the ERSP was expressed in dB as 10*log10(R) where R is the ratio of power between the signal and its baseline period.
Statistical analyses
IBM SPSS Statistics 19 (Armonk, New York, USA) was used for statistical analysis. The following assessments were performed: 1) SICI or ICF effects on TEP amplitudes between patients with schizophrenia and healthy controls; 2) correlation analyses in patients with schizophrenia: i) between amplitude changes of TEP components induced with SICI and ICF and clinical and cognitive measures; and ii) between significant amplitude changes of TEPs by SICI and ICF; and 3) modulatory effects by SICI and ICF on frequency bands between patients and controls. ANOVAs were applied to TEP amplitudes to examine the significant effects of SICI and ICF on TEPs, separately. Specifically, we performed a three–way ANOVA with TEP component (P30, N45, P60, N100, and P180) and TMS condition (test pulse and SICI or ICF) as within-subject factors and diagnosis (patients vs controls) as a between-subject factor for each SICI and ICF paradigm. Cross-sectional comparison analyses were performed for TEP amplitude changes by SICI or ICF by using post–hoc independent t–tests. We performed correlation analyses with Pearson’s correlation coefficient for the significant results obtained from above ANOVA. Pearson’s correlation was applied based on the assumption of normal distribution calculated by the Shapiro-Wilk test. A significant level of α = 0.05 was applied. Further, modulatory effects on the frequency band powers from delta to gamma over the left DLPFC during the SICI and ICF paradigms were exploratory examined with independent t–tests compared to the healthy control group with Bonferroni correction (α = 0.05/5). In addition, with respect to the time-frequency analysis for SICI and ICF paradigms over the left DLPFC, we applied the EEGLAB bootstrapping statistics (N = 1000) with an alpha value set at 0.01. Further the false discovery rate (FDR)-controlling procedures was used to correct multiple comparisons.
Electronic supplementary material
Acknowledgements
This research was supported by the Temerty Centre for Therapeutic Brain Intervention, the Campbell Family Research Institute through the CAMH Foundation, and Canada Foundation for Innovation.
Author Contributions
Y.N., R.F.H.C., R.C., M.S.B., Z.J.D., and D.M.B. conception and design of research; Y.N., M.S.B., and R.F.H.C. performed experiments; Y.N. and R.Z. analyzed data; Y.N., R.F.H.C., M.S.B., F.F., R.C., Z.J.D., and D.M.B. interpreted results of experiments; Y.N. prepared figures; Y.N. and M.S.B. drafted manuscript; Y.N., M.S.B., R.F.H.C., F.F., T.K.R., R.C., Z.J.D., and D.M.B. edited and revised manuscript; Y.N., M.S.B., R.Z., R.F.H.C., F.F., T.K.R., R.C., Z.J.D., and D.M.B. approved final version of manuscript.
Competing Interests
Y.N. receives postdoctoral fellowship from the Centre for Addiction and Mental Health (CAMH) Foundation. M.S.B. receives research support from the Brain and Behavior Research Foundation (Formerly NARSAD) Young Investigator Grant and Schizophrenia Junior Faculty Grant from the CAMH Foundation. R.F.H.C. was supported by a Canadian Institutes of Health Research (CIHR) – Dystonia Medical Research Foundation Fellowship award. F.F. receives funding from NARSAD, Slaight Family Centre for Youth in Transition at the CAMH, Natural Sciences and Engineering Research Council of Canada (NSERC), the Ontario Brain Institute (OBI). T.K.R. received research support from Brain Canada, Brain and Behavior Research Foundation, Canada Foundation for Innovation, the CIHR, Ontario Ministry of Health and Long–Term Care, Ontario Ministry of Research and Innovation, the US National Institute of Health (NIH), and the W. Garfield Weston Foundation. R.C. received research support from the CIHR, the Catherine Manson Chair in Movement Disorders, Medtronic Inc and Merz Pharma. Z.J.D. has received research support from the Ontario Mental Health (OMH) Foundation, the CIHR, the Brain and Behaviour Research Foundation (Formerly NARSAD), and the Temerty family and Grant family through the CAMH Foundation and the Campbell Research Institute. Z.J.D. received research and equipment in kind support for an investigator–initiated study through Brainsway Inc., and a travel allowance through Merck. Z.J.D. has also received speaker funding through Sepracor Inc., and AstraZeneca, served on advisory boards for Hoffmann–La Roche Limited and Merck, and received speaker support from Eli Lilly. D.M.B. has received research support from the CIHR, NIH, Brain Canada and the Temerty Family through the CAMH Foundation and the Campbell Research Institute. He receives research support and in-kind equipment support for an investigator-initiated study from Brainsway Ltd. and he is the site principal investigator for three sponsor-initiated studies for Brainsway Ltd. He receives in-kind equipment support from Magventure for an investigator-initiated study. He receives medication supplies for an investigator-initiated trial from Indivior.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-17052-3.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Whiteford HA, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 2.Hyman SE, Fenton WS. Medicine. What are the right targets for psychopharmacology? Science. 2003;299:350–351. doi: 10.1126/science.1077141. [DOI] [PubMed] [Google Scholar]
- 3.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 4.Frankle WG, et al. In vivo measurement of GABA transmission in healthy subjects and schizophrenia patients. The American journal of psychiatry. 2015;172:1148–1159. doi: 10.1176/appi.ajp.2015.14081031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogasch NC, Daskalakis ZJ, Fitzgerald PB. Cortical inhibition, excitation, and connectivity in schizophrenia: a review of insights from transcranial magnetic stimulation. Schizophrenia bulletin. 2014;40:685–696. doi: 10.1093/schbul/sbt078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marsman A, et al. Glutamate in schizophrenia: a focused review and meta-analysis of (1)H-MRS studies. Schizophrenia bulletin. 2013;39:120–129. doi: 10.1093/schbul/sbr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziemann U. TMS and drugs. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2004;115:1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Kujirai T, et al. Corticocortical inhibition in human motor cortex. The Journal of physiology. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Lazzaro V, et al. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2000;111:794–799. doi: 10.1016/S1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- 10.Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Experimental brain research. 1996;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- 11.Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. The Journal of physiology. 1999;517(Pt 2):591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. The Journal of physiology. 1996;496(Pt 3):873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziemann U, Chen R, Cohen LG, Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology. 1998;51:1320–1324. doi: 10.1212/WNL.51.5.1320. [DOI] [PubMed] [Google Scholar]
- 14.Schwenkreis P, et al. Influence of the N-methyl-D-aspartate antagonist memantine on human motor cortex excitability. Neuroscience letters. 1999;270:137–140. doi: 10.1016/S0304-3940(99)00492-9. [DOI] [PubMed] [Google Scholar]
- 15.Ziemann U, Lonnecker S, Paulus W. Inhibition of human motor cortex by ethanol. A transcranial magnetic stimulation study. Brain: a journal of neurology. 1995;118(Pt 6):1437–1446. doi: 10.1093/brain/118.6.1437. [DOI] [PubMed] [Google Scholar]
- 16.Radhu N, et al. A meta-analysis of cortical inhibition and excitability using transcranial magnetic stimulation in psychiatric disorders. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2013;124:1309–1320. doi: 10.1016/j.clinph.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Strube W, et al. Impairments in motor-cortical inhibitory networks across recent-onset and chronic schizophrenia: a cross-sectional TMS Study. Behav Brain Res. 2014;264:17–25. doi: 10.1016/j.bbr.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 18.Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Archives of general psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 19.Hakak Y, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yizhar O, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer BW, Dawes SE, Heaton RK. What do we know about neuropsychological aspects of schizophrenia? Neuropsychology review. 2009;19:365–384. doi: 10.1007/s11065-009-9109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cannon TD, et al. Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Archives of general psychiatry. 2005;62:1071–1080. doi: 10.1001/archpsyc.62.10.1071. [DOI] [PubMed] [Google Scholar]
- 23.Potkin SG, et al. Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophrenia bulletin. 2009;35:19–31. doi: 10.1093/schbul/sbn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eldreth DA, et al. Evidence for multiple manipulation processes in prefrontal cortex. Brain research. 2006;1123:145–156. doi: 10.1016/j.brainres.2006.07.129. [DOI] [PubMed] [Google Scholar]
- 25.Bridgman AC, et al. Deficits in GABAA receptor function and working memory in non-smokers with schizophrenia. Schizophrenia research. 2016;171:125–130. doi: 10.1016/j.schres.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Barr MS, et al. Can repetitive magnetic stimulation improve cognition in schizophrenia? Pilot data from a randomized controlled trial. Biological psychiatry. 2013;73:510–517. doi: 10.1016/j.biopsych.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 27.Guller Y, et al. Probing thalamic integrity in schizophrenia using concurrent transcranial magnetic stimulation and functional magnetic resonance imaging. Archives of general psychiatry. 2012;69:662–671. doi: 10.1001/archgenpsychiatry.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrarelli F, et al. Reduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: a TMS/EEG study. The American journal of psychiatry. 2008;165:996–1005. doi: 10.1176/appi.ajp.2008.07111733. [DOI] [PubMed] [Google Scholar]
- 29.Hasan A, et al. Deficient inhibitory cortical networks in antipsychotic-naive subjects at risk of developing first-episode psychosis and first-episode schizophrenia patients: a cross-sectional study. Biological psychiatry. 2012;72:744–751. doi: 10.1016/j.biopsych.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Cash, R. F. et al. Characterisation of Glutamatergic and GABAA Mediated Neurotransmission in Motor and Dorsolateral Prefrontal Cortex using Paired-Pulse TMS-EEG. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology, 10.1038/npp.2016.133 (2016). [DOI] [PMC free article] [PubMed]
- 31.Chen CM, et al. GABA level, gamma oscillation, and working memory performance in schizophrenia. Neuroimage Clin. 2014;4:531–539. doi: 10.1016/j.nicl.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anticevic A, et al. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16720–16725. doi: 10.1073/pnas.1208494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duncan CE, et al. Prefrontal GABA(A) receptor alpha-subunit expression in normal postnatal human development and schizophrenia. J Psychiatr Res. 2010;44:673–681. doi: 10.1016/j.jpsychires.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Egeland JMW. Memory With Digit Span and the Letter-Number Sequencing Subtests From the WAIS-IV: Too Low Manipulation Load and Risk for Underestimating Modality Effects. Appl Neuropsychol Adult. 2015;22:445–451. doi: 10.1080/23279095.2014.992069. [DOI] [PubMed] [Google Scholar]
- 35.Iwata Y, et al. Effects of glutamate positive modulators on cognitive deficits in schizophrenia: a systematic review and meta-analysis of double-blind randomized controlled trials. Molecular psychiatry. 2015;20:1151–1160. doi: 10.1038/mp.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JW, et al. Diagnostic utility of quantitative EEG in un-medicated schizophrenia. Neuroscience letters. 2015;589:126–131. doi: 10.1016/j.neulet.2014.12.064. [DOI] [PubMed] [Google Scholar]
- 37.Chen YH, et al. Frontal slow-wave activity as a predictor of negative symptoms, cognition and functional capacity in schizophrenia. Br J Psychiatry. 2016;208:160–167. doi: 10.1192/bjp.bp.114.156075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 39.Christian EP, et al. EEG-beta/gamma spectral power elevation in rat: a translatable biomarker elicited by GABA(Aalpha2/3)-positive allosteric modulators at nonsedating anxiolytic doses. Journal of neurophysiology. 2015;113:116–131. doi: 10.1152/jn.00539.2013. [DOI] [PubMed] [Google Scholar]
- 40.Saxena N, et al. Enhanced stimulus-induced gamma activity in humans during propofol-induced sedation. PLoS One. 2013;8:e57685. doi: 10.1371/journal.pone.0057685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamura S, et al. Effects of quetiapine on monoamine, GABA, and glutamate release in rat prefrontal cortex. Psychopharmacology. 2009;206:243–258. doi: 10.1007/s00213-009-1601-9. [DOI] [PubMed] [Google Scholar]
- 42.Thakkar, K. N. et al. 7 T Proton Magnetic Resonance Spectroscopy of Gamma-Aminobutyric Acid, Glutamate, and Glutamine Reveals Altered Concentrations in Patients With Schizophrenia and Healthy Siblings. Biological psychiatry, 10.1016/j.biopsych.2016.04.007 (2016). [DOI] [PubMed]
- 43.Liemburg E, et al. Prefrontal NAA and Glx Levels in Different Stages of Psychotic Disorders: a 3 T 1 H-MRS Study. Sci Rep. 2016;6:21873. doi: 10.1038/srep21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Natsubori T, et al. Reduced frontal glutamate+ glutamine and N-acetylaspartate levels in patients with chronic schizophrenia but not in those at clinical high risk for psychosis or with first-episode schizophrenia. Schizophrenia bulletin. 2014;40:1128–1139. doi: 10.1093/schbul/sbt124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plitman E, et al. Glutamate-mediated excitotoxicity in schizophrenia: a review. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2014;24:1591–1605. doi: 10.1016/j.euroneuro.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends in neurosciences. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Zhou FM, Hablitz JJ. Dopamine modulation of membrane and synaptic properties of interneurons in rat cerebral cortex. Journal of neurophysiology. 1999;81:967–976. doi: 10.1152/jn.1999.81.3.967. [DOI] [PubMed] [Google Scholar]
- 48.Gorelova N, Seamans JK, Yang CR. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. Journal of neurophysiology. 2002;88:3150–3166. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- 49.Gao WJ, Goldman-Rakic PS. Selective modulation of excitatory and inhibitory microcircuits by dopamine. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2836–2841. doi: 10.1073/pnas.262796399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spencer KM, et al. Abnormal neural synchrony in schizophrenia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carcea I, Froemke RC. Cortical plasticity, excitatory-inhibitory balance, and sensory perception. Prog Brain Res. 2013;207:65–90. doi: 10.1016/B978-0-444-63327-9.00003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lewis DA, Hashimoto T. Deciphering the disease process of schizophrenia: the contribution of cortical GABA neurons. Int Rev Neurobiol. 2007;78:109–131. doi: 10.1016/S0074-7742(06)78004-7. [DOI] [PubMed] [Google Scholar]
- 53.Ma L, Skoblenick K, Seamans JK, Everling S. Ketamine-Induced Changes in the Signal and Noise of Rule Representation in Working Memory by Lateral Prefrontal Neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35:11612–11622. doi: 10.1523/JNEUROSCI.1839-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rowland LM, et al. Frontal Glutamate and gamma-Aminobutyric Acid Levels and Their Associations With Mismatch Negativity and Digit Sequencing Task Performance in Schizophrenia. JAMA Psychiatry. 2016;73:166–174. doi: 10.1001/jamapsychiatry.2015.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ziemann U, Tergau F, Bruns D, Baudewig J, Paulus W. Changes in human motor cortex excitability induced by dopaminergic and anti-dopaminergic drugs. Electroencephalography and clinical neurophysiology. 1997;105:430–437. doi: 10.1016/S0924-980X(97)00050-7. [DOI] [PubMed] [Google Scholar]
- 56.Liu SK, Fitzgerald PB, Daigle M, Chen R, Daskalakis ZJ. The relationship between cortical inhibition, antipsychotic treatment, and the symptoms of schizophrenia. Biological psychiatry. 2009;65:503–509. doi: 10.1016/j.biopsych.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 57.Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13:28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cropsey KL, et al. How low should you go? Determining the optimal cutoff for exhaled carbon monoxide to confirm smoking abstinence when using cotinine as reference. Nicotine Tob Res. 2014;16:1348–1355. doi: 10.1093/ntr/ntu085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. The Journal of clinical psychiatry. 2003;64:663–667. doi: 10.4088/JCP.v64n0607. [DOI] [PubMed] [Google Scholar]
- 60.Rusjan PM, et al. Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation. Human brain mapping. 2010;31:1643–1652. doi: 10.1002/hbm.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.