Abstract
Salmonella is an intracellular pathogen infecting a wide range of hosts and can survive in macrophages. An essential mechanism used by macrophages to eradicate Salmonella is production of reactive oxygen species. Here, we used proteogenomics to determine the candidate genes and proteins that have a role in resistance of S. Typhimurium to H2O2. For Tn-seq, a saturated Tn5 insertion library was grown in vitro under either 2.5 (H2O2L) or 3.5 mM H2O2 (H2O2H). We identified two sets of overlapping genes required for resistance of S. Typhimurium to H2O2L and H2O2H, and the results were validated via phenotypic evaluation of 50 selected mutants. The enriched pathways for H2O2 resistance included DNA repair, aromatic amino acid biosynthesis (aroBK), Fe-S cluster biosynthesis, iron homeostasis and a putative iron transporter system (ybbKLM), and H2O2 scavenging enzymes. Proteomics revealed that the majority of essential proteins, including ribosomal proteins, were downregulated upon exposure to H2O2. On the contrary, a subset of conditionally essential proteins identified by Tn-seq were analyzed by targeted proteomics, and 70% of them were upregulated by H2O2. The identified genes will deepen our understanding on S. Typhimurium survival mechanisms in macrophages, and can be exploited to develop new antimicrobial drugs.
Introduction
Salmonella is a Gram-negative bacterium that infects humans and animals. Salmonella enterica has numerous serovars, which include typhoidal and non-typhoidal strains. In contrast to the typhoidal salmonellae which are human restricted pathogens, the non-typhoidal salmonellae (NTS), serovar Enteritidis and Typhimurium, are able to infect a wide range of hosts, causing gastroenteritis1. The NTS strains, including Salmonella enterica serovar Typhimurium, account for 11% (1.2 million cases) of the total foodborne illnesses caused by different pathogens in the United States2. It has been estimated that Salmonella is responsible for 93.8 million cases of gastroenteritis, leading to 155,000 deaths worldwide annually3. The pathogen remains a continuous threat to the food safety, and public health.
To initiate an infection and survive inside the host, Salmonella needs to overcome a myriad of host defense mechanisms. As Salmonella reaches the intestine and breaches the epithelial tissue, it enters the macrophages and activates different virulence strategies in order to survive and replicate in them4. An essential mechanism uses by the phagocytes to kill and eradicate Salmonella is production of reactive oxygen species (ROS). Hydrogen peroxide (H2O2), superoxide anion (O2 −), and the hydroxyl radical (HO) are derivatives of ROS. The short-lived O2 −, produced by the NADPH-dependent phagocytic oxidase, quickly dismutates into H2O2, which diffuses across semipermeable bacterial cell membranes. Eventually, Fe2+ reduces H2O2 to HO via the so called Fenton Reaction5–7. The ROS, including H2O2, can damage DNA, iron-sulfur cluster-containing proteins, and other biological molecules in the bacterial cells8–10.
Numerous genetic factors and proteins that are important for resistance of S. Typhimurium to H2O2 have been discovered and the underlying mechanisms have been explored11,12. Various approaches and techniques have been employed to study global response of Salmonella or related bacteria to H2O2 in vitro as a model system to simulate the bacterium’s response to ROS in phagocytic cells: (i) Two-dimensional gel electrophoresis identified H2O2-induced proteins in Salmonella 13, (ii) DNA microarray identified H2O2 induced genes in E. coli 14, and (iii) RNA-seq identified H2O2 induced genes in Salmonella 15. Yet, the factors required for fitness under the given condition cannot be identified with high confidence based on the analysis of transcriptomics or proteomics data16. Microarray-based tracking of random transposon insertions was used to identify numerous genes in Salmonella that are required for survival in mice and macrophages17,18. However, the genetic factors responsible for resistance to ROS cannot be sorted out among all of the genetic factors identified in the study that are required for fitness in the presence of multiple host stressors.
To shed more insights into the underlying mechanisms of Salmonella resistance to H2O2, more direct approach linking the gene-phenotype relationships in a genome-wide scale would be necessary. Tn-seq is a powerful approach to allow direct and accurate assessment of the fitness requirement of each gene on the entire genome of a prokaryotic organism19. In Tn-seq method, a saturated transposon insertion library (input) is exposed to a selective condition, and the mutant population altered through the selection (output) is recovered. Then, the genomic junctions of the transposon insertions are specifically amplified and sequenced from both input and output pools by high-throughput sequencing. The gene fitness can be obtained by calculating the change in relative abundance of the sequence reads corresponding to each gene in the entire genome between the two pools. Tn-seq has been employed to assign gene functions to Salmonella genomes in numerous studies: (i) Previously, our lab identified conditionally essential genes that are required for growth in the presence of bile, limited nutrients, and high temperature20, (ii) The genes required for intestinal colonization were identified in chickens, pigs, and cattle21, (iii) Candidate essential genes and genes contributing toward bile resistance were identified22, (iv) Core conserved genes for growth in rich media were identified in serovars Typhi and Typhimurium23. In addition to Tn-seq, electrospray ionization liquid chromatography tandem mass spectrometry (ESI-LC-MS/MS) is a powerful approach for identifying and quantifying proteins in a large scale. The system-wide protein regulation can be determined using mass spectrometry signal intensities of tryptic peptides obtained from two different culture conditions24. The post-translational modification in proteins can be revealed by using proteomic analysis25. Many studies took advantage of proteomic analysis of Salmonella. However, to the best of our knowledge, this study is the first to investigate proteogenomics of a bacterium by combining Tn -seq and proteome analysis simultaneously to the same stressor.
In this work, we used Tn-seq method and proteomic analysis in combination to determine system-wide responses of S. Typhimurium to two different concentrations of H2O2 (H2O2L and H2O2H). We obtained a comprehensive list of 137 genes that are putatively required for the resistance of S. Typhimurium 14028 to H2O2. The roles of 50 selected genes in resistance to H2O2 were determined by phenotypic evaluation of the individual deletion mutants. Also, we identified a set of 246 proteins that are differentially expressed in response to H2O2, using data-dependent acquisition (DDA) proteomics, which are largely overlapped with the genes identified by Tn-seq; targeted proteomics showed 70% of the proteins identified by Tn-seq were upregulated by H2O2. In addition to the genes of S. Typhimurium previously known to be important for resistance to H2O2, we identified approximately 80 genes that have not been previously associated with resistance to oxidative stress. The results of this study highlighted that the genes in aromatic amino acid biosynthesis, aroB and aroK, and iron homeostasis, ybbK, ybbL, and ybbM, are crucially important for growth fitness under H2O2 stress. The identified candidate genes will expand our understanding on the molecular mechanisms of Salmonella survival in macrophages, and serve as new antimicrobial drug targets.
Results and Discussion
The H2O2 concentrations and the selections of Tn5 library
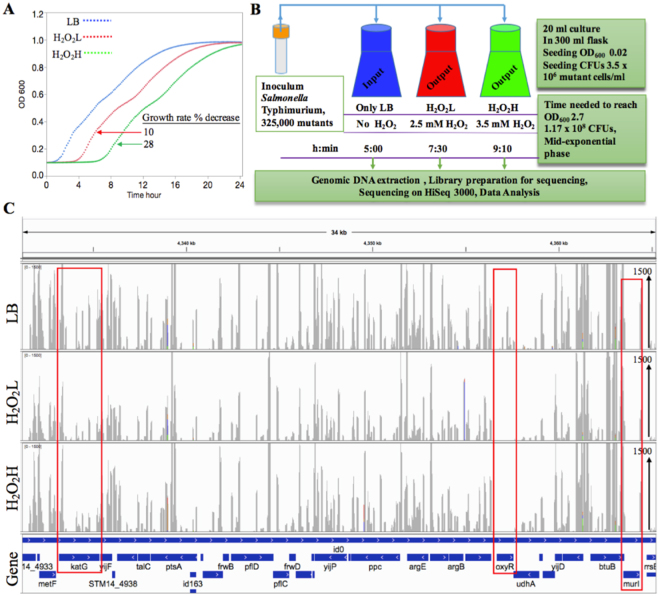
First, we sought to determine the growth response of wild type S. Typhimurium 14028 cells in LB media containing varying concentrations of H2O2. The wild type cells were grown in LB media that contain different concentrations of H2O2 in 96-well plates. After evaluating the growth rates for the cultures, 2.5 and 3.5 mM H2O2 were chosen for Tn-seq selections in our study, and termed H2O2L and H2O2H, respectively. In comparison to Salmonella grown in LB media with no H2O2, H2O2L and H2O2H reduced the growth rates by 10% and 28%, respectively (Fig. 1A). The lag time increased by a 5.7-fold (0.5 vs. 2.9 hr), and an 11-fold (0.5 vs. 5.6 hr) in H2O2L and H2O2H, respectively. The maximum OD600 decreased by only 1% for the H2O2L and 2% for the H2O2H in comparison to LB media (Fig. 1A).
Figure 1.
Study design and identification of the genes required for H2O2 resistance using Tn-seq. (A) The effect of H2O2 on the growth rate of wild type S. Typhimurium. An overnight culture of bacteria was diluted 1:200 in the LB medium containing either 0 mM (control: blue), 2.5 mM H2O2 (H2O2L: red), or 3.5 mM H2O2 (H2O2H: green). The cultures were incubated at 37 °C for 24 h in a 96-well plate. The reduced growth rates in the presence of H2O2 were in reference to LB control. (B) Schematic representation of the Tn-seq experiment design. The Salmonella transposon mutant library was inoculated into LB, H2O2L and H2O2H. The three cultures were grown until they reached mid-exponential phase. Genomic DNA was extracted from each culture and subjected to library preparation, Illumina sequencing, and data analysis. (C) The Tn-seq profiles of the three conditions for the 34 kb genomic region starting from with metF gene and ending with rrsB. The vertical axis represents number of sequencing reads with the maximum of 1500 reads. The genes highlighted in red are katG, oxyR, and murI as examples of non-essential gene, conditionally essential gene, and essential gene, respectively.
For the selection of Tn5 library, 20 ml cultures in 300 ml Erlenmeyer flasks containing LB, H2O2L, or H2O2H were inoculated with the same Tn5 library at the seeding CFUs of the library at 3.5 × 106. This seeding level provided ~10 CFUs for each Tn5 insertion mutant in the library. The cultures were grown until the mid-exponential phase, in which the CFUs reached 1.17 × 108 (SE 0.01 × 108). It required 7.5 and 9.2 h to reach the cell density as measured by optical density for H2O2L and H2O2H, respectively, in contrast to 5 h for LB medium (Fig. 1B). We observed some differences in growth responses between the cultures in a 96-well plate and in a 300-ml Erlenmeyer flask. The optical density readings by the plate reader was different in comparison to those by Bio-photometer that we used to measure optical density of the culture in the flask. As a result, the growth curve in Fig. 1A which was based on 96-well plate reader, dose not match exactly with the time required for the Tn5 library to reach the target mid-exponential phase in the flask cultures. In addition, we observed that the H2O2 is stable in LB media free of Salmonella during the window of time used for the library selection process (Fig. S1), which was also supported by Bogomolnaya et al.26.
Preparation of Tn5-seq amplicon library
The Salmonella mutants were generated by using the delivery plasmid pBAM1 via conjugation. A total of 325,000 mutant colonies were recovered from 50 plates. Each mutant contained a single random insertion of Tn5 transposon in the chromosome or plasmid according to DNA sequencing of Tn5-junction sequences for a small set (n = 71) of randomly selected Tn5 mutants. We found a significant portion (~20%) of the mutants in the library that were not genuine Tn5 insertions, but the mutants generated as a result of pBAM1 integration into chromosome as determined by their ability to grow in the presence of ampicillin. To prevent the Illumina sequencing reads from being wasted on sequencing Tn5 junctions from these cointegrants, we digested genomic DNA of the input and output libraries with PvuII, which digests immediately outside the inverted repeats on both sides of Tn5. The digested DNA was then used to prepare Tn-seq amplicon library as described in Materials and Methods. Our Tn-seq data analysis indicated that our strategy of removing the DNA sequences originating from cointegrants was effective because only 0.55% of the total HiSeq reads corresponding to Tn5-junctions matched to pBAM1. It should be possible to remove them completely by ensuring complete digestion of genomic DNA with PvuII (Table S1). The method for Tn-seq amplicon library we developed and used in this study has multiple advantages over other Tn-seq protocols, because our method requires only 100 ng of the genomic DNA, and the whole process can be completed in a day27. Additionally, the average length of genomic junction sequences is much longer (Table S1; 92–94 bp) as compared to other Tn-seq methods, which allows more accurate mapping of the insertion sites. When the extension step in the protocol was performed using a conventional 20 nucleotide primer, and the final products of exponential PCR were separated on agarose gel electrophoresis, even the negative controls (the wild type genomic DNA or mutant library genomic DNA without linear extension) showed smear patterns of nonspecific background amplification. However, when dual priming oligonucleotide (DPO) primer was used in place of the conventional primer for linear extension, non-specific background amplification was completely disappeared. Therefore, we adopted the DPO primer in linear extension step for all library samples in this study. Then, the single-stranded extension products were C-tailed, and used as templates for the exponential PCR step using nested primer specific to Tn5 and poly G primer that contain Illumina adapter sequences along with sample index sequences (Fig. S2). The final PCR products were separated on an agarose gel, and the fragments within the range of 325–625 bp were gel-purified. After pooling of multiple samples, the combined library was sequenced on a HiSeq 3000 run.
Summary of Tn-seq DNA analysis
After de-multiplexing and C-tail trimming of all sequence reads, ~72 million reads of Tn5- junctions with mean read length of 94 bp were obtained. The number of the reads mapped to the complete genome of S. Typhimurium 14028 were ~25, 15, and 19 million for LB, H2O2L, and H2O2H, respectively. The number of unique insertions on the chromosome were 125,449 in the input library, excluding the plasmid (Table S1). On average, Tn5 was inserted in every 39 bp. Number of raw reads per open reading frame (ORF) for H2O2L was plotted over the corresponding number of H2O2H, which yielded an R2 of 0.91, indicating the mutants in the input library quantitatively responded in a similar way for both H2O2L and H2O2H as expected (Fig. S3). The insertions were mapped to 5,428 genes or 8,022 genes/intergenic regions. Interestingly, the ORF STM14_5121, which is 16.7 kbp long, had the highest number of insertions (~700 insertions) and reads (0.25 M).
Comparison of various bioinformatics pipelines for Tn-seq data analysis
We used 3 different Tn-seq analysis tools to identify the genes and compare the results across the methods with the goal of comprehensive identification of “all” genes required for resistance to H2O2. The first tool, ARTIST28, created small non-overlapping genomic windows of 100 bp and the reads from each window were arbitrarily assigned into the middle of the window. The default normalization script of the tool was used. Then, the relative proportions of insertion sites in the output library versus the input were tabulated. Mann-Whiney U (MWU) test was used to assess the essentiality of the locus. To consider a gene/intergenic region conditionally essential for growth in the presence of H2O2, p value had to be ≤0.05 in 90 of the 100 conducted MWU tests. Subsequently, 20 genes and 1 intergenic region were identified for H2O2L and 4 genes for H2O2H (Table S2). The fact that a smaller number of genes were identified with the higher concentration of H2O2 is counterintuitive. It is unclear if our result accurately reflected the number of the genes important for two different concentrations of H2O2 or it was due to the shortcoming in the algorithm we used for gene identification.
The second tool, Tn-seq Explorer29, counted insertions in overlapping windows of a fixed size. Using a 550 bp window size, each annotated gene was assigned an essentiality index (EI) which is determined mainly based on the insertion count in a window in this gene. The bimodal distribution of insertion counts per window divided the essential genes to the left and the non-essential genes to the right. To find conditional essential genes, the EI of the output was subtracted from the EI of the input. The genes with negative ΔEI were ranked based on the change in read fold change (Log2 (H2O2L or H2O2H/Input)). We found 114 consensus genes between H2O2L and H2O2H that had at least four-fold reduction in H2O2H read counts as compared to the input. The four-fold reduction (Log2FC = −2) threshold was chosen because some previously known genes that are important for oxidative stress such as sufS or uvrD were excluded with the more stringent cutoff such as Log2FC = −3 (Table S2).
The third tool, TRANSIT30, determined read counts of genes in the input and output library. The differences of total read counts between the input and outputs were obtained. The insertion sites were permutated for a number that is specified by the user (we used 10,000 sample). This sampling for each gene gave difference in read counts. The p value was calculated from the null distribution of the difference in read counts. We identified 8 and 21 genes for the H2O2L and H2O2H, respectively, using a p value ≤ 0.05 (Table S2).
The combined list of the genes identified by the 3 Tn-seq analysis tools for both H2O2L and H2O2H included 137 genes (Table S2). All of the genes on this list are expected to have a role in conferring resistance to H2O2 and allow Salmonella to survive and replicate in the presence of H2O2 in vitro. Utilizing DAVID and KEGG bioinformatics resources for pathway analysis31,32, the 137 genes were categorized into 15 known pathways with the details available in supplementary information. Of the 21 genes identified by TRANSIT, 19 of these genes were also identified by Tn-seq Explorer, but only 3 out of this 21 were identified by ARTIST. The 19 genes were hscA, rbsR, fepD, efp, oxyR, polA, ybaD, aroD, ruvA, xthA, dps, aroB, uvrD, tonB, uvrA, aroK, ybbM, lon, and proC. Two genes, fepD and xthA, were identified by the all 3 methods and for both conditions (Fig. S4).
The 3 Tn-seq analysis tools are very valuable for Tn-seq data analysis, but each tool has its own advantages and disadvantages. For ARTIST, (i) the user must know how to run scripts in Matlab software, (ii) the analysis is very slow on a personal computer with the HiSeq data, (iii) it has only one method for normalization, but (iv) it can search for essentiality in the intergenic regions. For Tn-seq Explorer, (i) there is no data normalization, and (ii) prediction on small genes is prone to be inaccurate, but (iii) it is very user-friendly and runs fast. For TRANSIT, (i) the user should have some knowledge on running scripts on terminal, (ii) it may need some modification in its Python script according to the way the library was prepared for sequencing, and (iii) a few software packages should be installed on the computer as TRANSIT pre-requisites, but (iv) it does have 6 different methods for data normalization and it runs very fast on a personal computer. We initially aimed at finding the most suitable analytical method to use for analysis of all Tn-seq data in this study. However, unlike our initial expectation, we found the results of the 3 methods did not overlap much (Fig. S4), making it difficult to choose one best method for our Tn-seq data. The majority of the genes uniquely identified by one method were those identified by Tn-seq Explore. However, it is not surprising considering the distinctive conceptual basis employed by Tn-seq Explorer. We argue that these differences in the 3 methods reflect that the methods are complementary to each other, and we chose to combine the results of the 3 methods to have a comprehensive gene list. Although ARTIST and Tn-seq Explorer are very useful tools for Tn-seq data analysis, we prefer using TRANSIT in our future data analysis for conditionally essential genes. In the following sections, we continued the downstream analysis mainly based on the 137 genes that include all of the genes identified by all 3 methods.
Validation of Tn-seq results using individual mutants
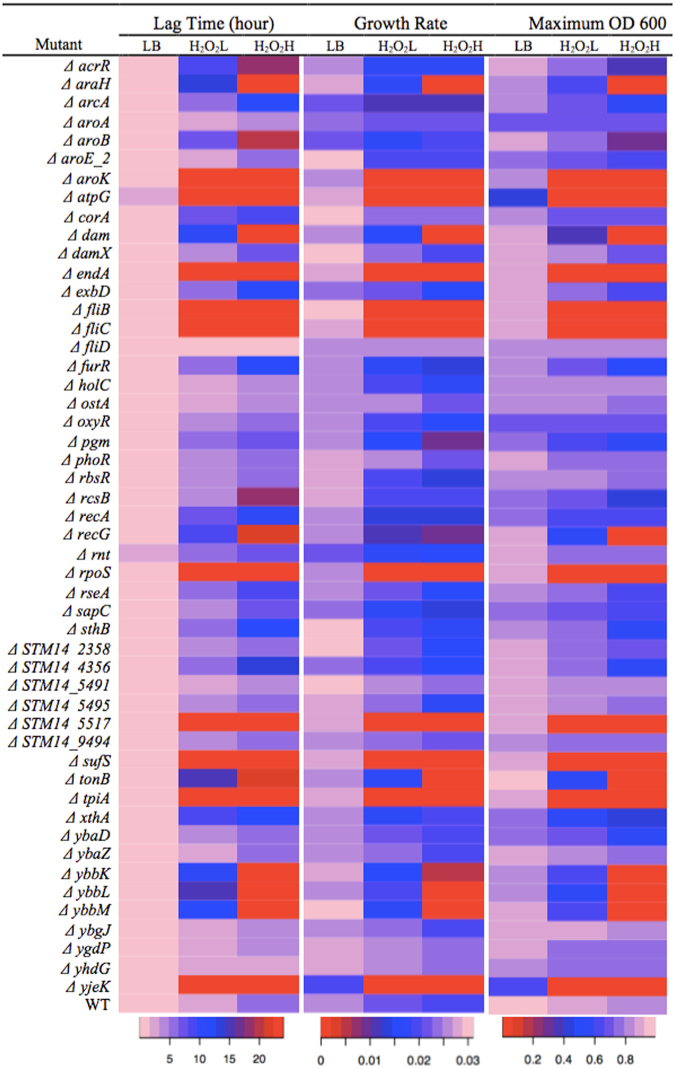
For the selected 50 genes among the 137 genes identified by Tn-seq, the growth phenotype was determined using individual single deletion mutants in LB, H2O2L, and H2O2H. The genes were considered to play a role in resistance to H2O2, if (i) lag phase time increased, (ii) growth rate reduced or (iii) maximum OD600 decreased in the presence of H2O2 in comparison to the wild type strain grown in the same conditions. Of the 50 single deletion mutants, 42 mutants were shown to have a role in resistance to H2O2 (Fig. 2 and Table S4). One gene, yhaD, was identified by all 3 analysis tools, but it did not show the expected phenotype. The fliD was also identified by ARTIST, but did not show any phenotype distinguishable from the wild type. The remaining 6 genes that did not show the phenotype was identified by Tn-seq Explorer. Based on the results of the individual mutant assay, we conclude that 84% (42/50) of the genes identified by the Tn-seq analysis and tested using single deletion mutants have a role for resistance to H2O2. These results indicate that our Tn-seq analysis identified the genes in S. Typhimurium that are required for the wild type level resistance to H2O2 with high accuracy.
Figure 2.
Growth curve of 50 mutants and a wild type S. Typhimurium in LB, H2O2L and H2O2H. The lag phase time, growth rate, and maximum OD600 of the individual S. Typhimurium mutants and the wild type were measured in LB media supplemented with no H2O2 (LB), 2.5 mM H2O2 (H2O2L) or 3.5 mM H2O2 (H2O2H). Overnight culture of each of the mutants and wild type was diluted 1:200 in LB, H2O2L and H2O2H. The cultures were incubated at 37 °C for 24 h in a 96-well plate and the OD600 was recorded every 10 min. The lag phase time, growth rate, and maximum OD600 were calculated and shown here as a graphical representation. The pale pink color indicates a short lag phase time, a high growth rate, and a high OD600. The red color indicates that the bacteria stayed in a lag phase, growth rate was close to a zero, and OD600 of the culture did not increase during the 24 h time of the assays. The data of this figure can be found in Table S4.
Proteomics of H2O2 response
With ESI-LC-MS/MS in data-dependent acquisition (DDA) mode, the protein regulation was determined using MS1 filtering technique that skyline software offers33. It uses signal intensities of tryptic peptides derived from the proteins of wild type strain grown in the presence of H2O2 in comparison to the control (LB). As described in Materials and Methods section, trypsin digestion of the protein extracts under different conditions generates tryptic peptides that are uniquely related to individual proteins. Tryptic peptides separated by liquid chromatography from the complex samples were first subjected to simple mass measurement (MS1) followed by intensity dependent fragmentation of these peptide ions to produce sequence specific fragment ions by collision-induced dissociation (MS/MS). Tryptic peptides were then identified using these sequence specific fragment ions via MASCOT database search software34, where the sequence specific fragment ions were matched to the proteins in S. Typhimurium 14028 reference proteome database24,35. This method of protein analysis is normally referred to as data dependent analysis (DDA). At the beginning of data analysis, the H2O2L and H2O2H data were compared to LB separately, however it turned out that comparison was not sensitive enough to differentiate between H2O2L and H2O2H conditions. Hence, the data of H2O2L and H2O2H were combined for analysis in comparison to LB. We identified 1,104 proteins of Salmonella for the 3 conditions (Table S5); of these, 246 proteins were differentially expressed in response to H2O2 with p values ≤ 0.05 and 90% CI. Proteomics analysis showed that 121 and 125 proteins were upregulated and downregulated in response to stress by H2O2, respectively. Since Tn-seq revealed genetic requirements for fitness under the selection conditions, the identified genes are expected to express corresponding proteins under the conditions to perform their cellular functions. Often the proteins required for fitness under a given condition are overexpressed under the condition, but it may not be the case for some proteins. In this study, we had a unique opportunity to comparatively analyze both Tn-seq and the MS data to understand the relationship between genetic requirements and changes in protein expression level under the condition of interest, which was H2O2 in this study. We also obtained the list of essential genes based on our Tn-seq data, which could not tolerate insertions by definition, and if we were not certain about essentiality of a gene from our Tn-seq data, the gene was searched for essentiality in the previously reported list of Salmonella essential genes22. The comprehensive list of essential genes allowed us to study any correlation between the essentiality and the changes in protein expression. Among the 246 proteins, there were 78 essential and 168 non-essential proteins. Among the 78 essential proteins, 25 were upregulated whereas 53 were downregulated. On the contrary, the majority (n = 96) of the detected non-essential proteins were upregulated, while 72 non-essential proteins were downregulated. To further examine the quantitative relationships closely, 64 genes/proteins identified by both methods (Table S5) were focused on. Among the 64 genes/proteins, 57 genes showed negative Log2FC based on Tn-seq data, and 41 proteins among the 57 were upregulated at protein level. However, only 12 upregulated proteins (AhpC, ArcA, Crr, DksA, FliC, IcdA, OxyR, Pgm, RecA, RpoS, SlpA, and WecE) had p values of ≤0.05. The Venn diagram in Fig. S5A shows the common genes/proteins that were identified by both approaches.
Using KEGG pathway analysis31,32, 150 proteins among the 246 were enriched in 21 pathways (Table S6). Interestingly, of the all 59 30 S and 50 S ribosomal proteins in S. Typhimurium, 37 of these proteins (63%) were downregulated in response to H2O2. Moreover, of the 8 identified proteins in TCA cycle, 6 proteins were downregulated, including 2 essential proteins.
Although DDA method can be used to search for all proteins in a complex sample, it is prone to miss identification of important proteins due to the fact that fragmentation of tryptic peptides from these proteins may not be triggered as a result of lower peptide ion intensities compared to the threshold set. To quantify proteins expressed for the genes identified by Tn-seq more accurately, we used targeted-proteomic approach by employing liquid chromatography coupled with triple quadrupole mass spectrometry (LC-QQQ-ESI-MS). Here, tryptic peptides of the protein were targeted for fragmentation (MS/MS) independent of their intensities, as described in Materials and Methods, and the observed sequence specific fragment ion intensities from three unique tryptic peptides were utilized for protein quantitation. Of the 137 Tn-seq identified genes, we selected 33 genes to quantify their proteins in response to H2O2 by using targeted-proteomics based on one of the multiple criteria (Table S5): (1) the protein expression was not supported by DDA, (2) the protein is a part of the pathways encoded by aroABK or ybbKLM, (3) the protein whose role was validated by mutant assay, or (4) the protein was identified by both Tn-seq and DDA (Fig. S5B). Interestingly, 23 (70%) of the 33 tested proteins were upregulated in response to H2O2. This shows a good agreement between the results of the Tn-seq and the targeted-proteomics. These genes that are conditionally required for fitness in the presence of H2O2 are usually expected to show upregulated protein expression in response to the same stimuli. However, it is also possible a protein is conditionally required while the expression is constitutive, and not regulated by H2O2.
Aromatic amino acid biosynthesis genes are required for H2O2 resistance
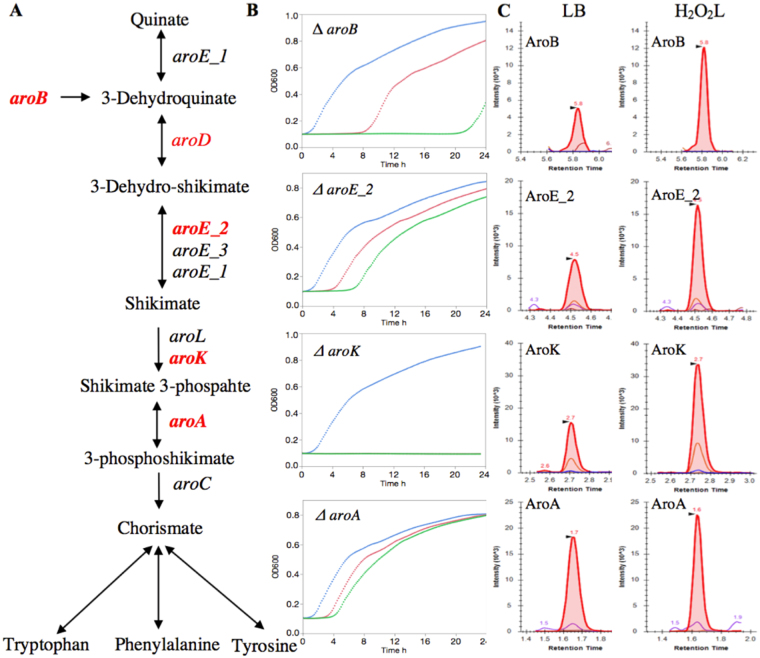
Interestingly, our Tn-seq data revealed that the aromatic amino acid biosynthesis and metabolism pathway play a role in conferring resistance in Salmonella to H2O2 (Fig. 3A and B). Five genes, aroB, aroD, aroE_2, aroK, and aroA in the aromatic amino acid biosynthesis pathway were identified by Tn-seq, and the fitness of the mutants were significantly reduced in the presence of H2O2. To confirm this, 4 of these genes were evaluated using individual mutant assays. The Salmonella aroK mutant showed the strongest phenotype, because it failed to grow in the presence of H2O2L or H2O2H during 24 h incubation time. Also, the aroB mutant exhibited a strong phenotype, significantly extending lag phase for both H2O2 conditions. The aroE_2 mutant also exhibited an extended lag time, but the aroA mutant did not show any difference in growth phenotype in the presence of H2O2. In addition, targeted-proteomics also showed that all these 5 proteins were upregulated in response to H2O2 (Fig. 3C and Table S5).
Figure 3.
The role of aromatic amino acid biosynthesis genes in resistance to H2O2. (A) Schematic representation of aromatic amino acid biosynthesis pathway, adapted from the KEGG pathway database. The genes in red color were identified by the Tn-seq analysis as conditionally essential for H2O2 resistance in Salmonella. The genes in bold type indicated that the mutant phenotype was also validated by individual mutant assay. (B) The overnight cultures of the individual mutants were diluted 1:200 in LB (no H2O2) and LB containing either 2.5 mM H2O2 (H2O2L) or 3.5 mM H2O2 (H2O2H). The cultures were incubated at 37 °C for 24 h in a 96-well plate. The growth curves are shown LB (blue), H2O2L (red), and H2O2H (green). In the growth curve of ΔaroK, the red color is overlapped with the green color. (C) Upregulation of Salmonella proteins in response to H2O2L in comparison to LB. Wild type S. Typhimurium strain was grown in LB, H2O2L, and H2O2H until mid-exponential phase. Targeted-proteomics was used to quantify AroB, AroE_2, AroK, and AroA protein expressions in response to H2O2L.
ROS damages a variety of biomolecules via Fenton reaction, which consequently lead to metabolic defects, specifically auxotrophy for some aromatic amino acids10. E. coli mutants that lack superoxide dismutase enzymes are unable to grow in vitro unless the medium are supplemented with aromatic (Phe, Trp, Tyr), branched-chain (Ile, Leu, Val), and sulfur-containing (Cys, Met) amino acids36. We identified the genes in the aromatic amino acid biosynthesis pathway that are critically important for resistance to H2O2. In this pathway, aroK catalyzes the production of shikimate 3-phosphate from shikimate, which consequently leads to the production of tryptophan, phenylalanine, tyrosine and some metabolites from the chorismate precursor in E. coli. Further, aroK mutant in E. coli displays increased susceptibility to protamine, a model cationic antimicrobial peptide. It has been suggested that resistance to protamine is probably due to the aromatic metabolites and product of aroK gene, which act as a signal molecule to simulate the CpxR/CpxA system and Mar regulators37. In our Tn-seq data, cpxR/cpxA and marBCRT were in the list of non-required genes, but the proteomics data indicated that CpxR was upregulated. Also, aroK mutant in E. coli is resistance to mecillinam, a beta-lactam antibiotic specific to penicillin-binding protein 2. It has been concluded that the AroK has a secondary activity in addition to the aromatic amino acid biosynthesis, probably related to cell division38. In addition, aroK gene presents a promising target to develop a non-toxic drug in Mycobacterium tuberculosis because aroK is the only in vitro essential gene among the aromatic amino acid pathway genes and blocking aroK kills the bacterium in vivo 39. Moreover, aroK gene plays a general role in S. Typhimurium persistence in pigs40. The aroB is another gene in the pathway that was identified by Tn-seq, which encodes 3-dehydroquinate synthase in the Shikimate pathway, aromatic amino acid biosynthesis pathway. Salmonella lacking aroB showed a strong growth defect in the presence of H2O2. When this mutant was grown in the presence of H2O2, it increased the lag phase time by a 114-fold for the H2O2L and a 347-fold for the H2O2H as compared to the mutant grown in the absence of H2O2. S. Typhimurium mutant lacking the aroB gene is attenuated in BALB/c mice41. In addition to aroK and aroB, aroE_2 was also shown to be important for resistance to H2O2, because deletion of the aroE_2 reduced the growth rate by 35% in the presence of H2O2 and increased the lag phase time, too. All these 3 genes in this pathway are required for systemic infection of Salmonella in BALB/c mice in a more recent study18. We observed that there was a strong correlation between the fitness based on Tn-seq data, growth rates measured by individual mutant assays, and upregulation of their proteins quantified via targeted proteomics. This demonstrates the power of proteogenomic approach in discovering and characterizing the genes that are required for growth under a specific condition.
The ybbM, ybbK, and ybbL have a role in H2O2 resistance
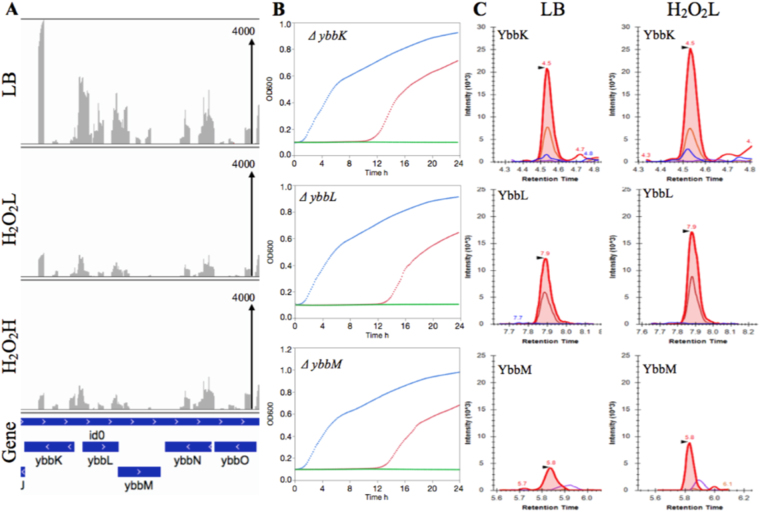
The mutants with single deletion in each of ybbK, ybbL, and ybbM genes on the same pathway showed a strong phenotype against the activity of H2O2 in a dose-dependent manner. Based on Tn-seq data, the fitness of ybbM was −1.16 and −1.79 for H2O2L and H2O2H, respectively (Fig. 4A). The ybbM mutant demonstrated decreased growth rate by 38% for H2O2L and 100% for the H2O2H as compared to the mutant grown in the absence of H2O2. This mutant also increased the lag time by a 126-fold and a 267-fold for H2O2L and H2O2H, respectively (Fig. 4B). Also, the fitness score of ybbK was −0.92 for H2O2L and −1.81 for H2O2H. The ybbK mutant showed decrease of growth rate for H2O2L and H2O2H by 85% and 95%, respectively. The deletion increased the lag phase by a 46-fold and a 114-fold in the presence of H2O2L and H2O2H, respectively (Fig. 4B). Moreover, the fitness of ybbL mutant was −1.05 and −1.73 for H2O2L and H2O2H, respectively. Deleting the ybbL in Salmonella led to decrease in growth rate by 27% for H2O2L and 92% for H2O2H. The lag phase time for this mutant increased by a 22-fold and a 33-fold for H2O2L and H2O2H, respectively. In addition, YbbM, YbbL, and YbbK proteins were upregulated in response to H2O2; YbbM was the most upregulated protein among the 3 proteins (1.46-fold), followed by YbbL (1.29-fold), and YbbK (1.25-fold) (Fig. 4C and Table S5). The fitness scores of the Tn-seq of these 3 genes are correlated strongly with the growth rate, lag time of their respective mutants, and upregulation of their proteins. As the number of reads depletes after the selection for a mutant, (i) there was more reduction in growth rate, (ii) the mutant stays longer in the lag phase, and (iii) the protein expression elevates. These observations clearly point to their role in conferring resistance to the H2O2-mediated stress. These genes were described in the Salmonella reference genome as follows: ybbM, putative YbbM family transport protein, metal resistance protein; ybbK, putative inner membrane proteins; ybbL, putative ABC transporter, ATP-binding protein YbbL. To the best of our knowledge, there is only one published study on the ybbM and ybbL 42. Based on their findings, YbbL and YbbM have a role in iron homeostasis in E. coli and are important for survival when the bacterium was challenged with 10 mM H2O2 for 30 min; this putative ABC transporter transports iron and lessens ROS species formation that generates via H2O2. In this study, we identified an additional gene, ybbK, in the same pathway as the gene required for resistance to H2O2, strongly establishing the role of these 3 genes in resistance to H2O2.
Figure 4.
The ybbK, ybbL, and ybbM have a role in resistance to H2O2. (A) The Tn-seq profiles of LB (no H2O2), H2O2L (2.5 mM), and H2O2H (3.5 mM) corresponding to the ~6 kb genomic region ranging from ybbK and ybbO. The read scale on vertical axis is 4000. (B) The growth curve of ΔyybK, ΔyybL, and ΔyybM. The overnight cultures of these three mutants were diluted 1:200 in LB, H2O2L, and H2O2H. The cultures were incubated at 37 °C for 24 h in a 96-well plate. The growth curves are shown for LB (blue), H2O2L (red) and H2O2H (green). (C) The wild type S. Typhimurium strain was grown in LB, H2O2L, and H2O2H until mid-exponential phase. Targeted-proteomics was used to quantify YbbK, YbbL, and YbbM protein expressions in response to H2O2L.
The H2O2 scavenging and degrading genes
Salmonella employs redundant enzymes to degrade or scavenge ROS. The katE, katG, and katN genes encode catalases, which are involved in H2O2 degradation. The ahpCF, tsaA, and tpx genes encode peroxidases, which scavenge H2O2. The sodA, sodB, sodCI, and sodCII genes encode superoxide dismutases and these enzymes specifically scavenge O2 11,12,43–45. However, none of these were present in the list of genes identified by Tn-Seq. Even though katE, katG, ahpC, sodA, sodCI, and sodCII showed reduced fitness, they did not meet the statistical threshold. However, the proteomics data indicated that AhpC (1.48-fold), SodB (1.46-fold), and TpX (1.39-fold) were upregulated in the presence of H2O2 (Table S5) and KatG was also upregulated, but its p value was 0.054. This reveals that these 4 proteins were important enzymes for H2O2 resistance under our experimental conditions. Salmonella containing an ahpC promoter-gfp fusion shows that expression of the ahpC is regulated by ROS that is generated from macrophages or exogenous H2O2 and the response to H2O2 is in a dose-dependent manner46. Salmonella mutant that lacks katE, katG, or ahpCF can degrade micromolar concentrations of H2O2. However, Salmonella mutant that has deletions in the all 5 genes, katE, katG, katN, ahpCF and tsaA (HpxF), cannot degrade H2O2, is unable to proliferate in macrophages, and show reduced virulence in mice11. This emphasizes that ahpC, sodB, and tpx may be the primary players in scavenging and degrading H2O2 in our experiment. Why Tn-seq did not detect any of these genes, while proteomics detected only these 3 proteins among others? It may reflect the functional redundancy in the genetic network that prevented single deletions in one of these genes from exhibiting fitness defect. Alternatively, when these mutants were grown together with all other mutants in the library, the functional protein lacking in one mutant due to Tn5 insertion could have been compensated by the other mutants in the library.
In addition to these genes, oxyR was detected by Tn-seq (Fig. 1C) and DDA proteomics. The oxyR was identified by all 3 analysis methods of Tn-seq data and it was on the top of the list, indicating a severe fitness defect of the mutant. The oxyR gene encodes H2O2 sensor and transcription factor, which mediates protection against ROS. The katG and ahpCF are regulated by OxyR, peroxide response regulator13,14. Although Salmonella OxyR regulon is induced in the Salmonella-containing vacuole in macrophage, the oxyR mutant was virulent in a BALB/c mouse and can grow well in human neutrophils in vitro 47,48. The fitness of oxyR mutant was reduced in both H2O2L and H2O2H with the respective fitness score of −4.96 and −5.94. Salmonella oxyR mutant exhibited a growth rate reduction by 24% and 40% for H2O2L and H2O2H, respectively. Comparing this reduction in growth rate to the other mutants such as rpoS or aroK, we observed that the oxyR mutant did not show severe phenotype and the mutant escaped from the lag phase easily. Moreover, our targeted proteomics indicated that the OxyR was not upregulated significantly. Further studies are needed to uncover the exact role of OxyR in response to ROS. However, previous studies implied that OxyR plays an essential role in resistance to H2O2 by regulating other proteins. OxyR induces Dps in E. coli, a ferritin-like protein that sequesters iron49. Sequestering of iron impairs the Fenton reaction, which consequently provides protection against ROS and reduces the damage of biomolecules. The dps gene was identified by the Tn-seq and its fitness score was −2.48. However, the Dps protein was downregulated based on the DDA proteomic analysis. To confirm this unexpected finding, we conducted the proteomic assay twice and each time with at least 4 technical replicates, but the Dps protein was significantly downregulated with p = 0.001. Further, the targeted-proteomics demonstrated the same result, pointing to the downregulation of Dps in response to H2O2. This is contrary to the previously reported works on Dps in Salmonella and the reason for the discrepancy is unclear.
DNA repair system is important for H2O2 resistance
The imposed exogenous H2O2 activates DNA repair system in Salmonella in order to repair or eliminate the damage that occurred on the nucleotides. The E. coli RecA protein repairs double-strand DNA lesions through recombination50. In our Tn-seq analysis, the fitness score of this mutant was −5.36 for both concentrations, and in proteomics, the RecA was upregulated (1.79-fold). Salmonella recA mutant decreased the maximum OD600 by 16% for H2O2L and 22% for H2O2H as compared to the same mutant grown in LB. Salmonella recA mutant was also sensitive to exogenous H2O2 in aerated rich medium26. Moreover, recG, recombination and DNA repair gene51, showed a stronger phenotype than recA mutant. The regG deletion in Salmonella caused the growth rate reduction by 52% for H2O2L and 60% for H2O2H. This disruption in recG also caused the cells to stay in lag phase for a longer time in the presence of H2O2 as compared to LB; the lag time increased by a 62-fold and a 159-fold for H2O2L and H2O2H, respectively. In the blood of patients with Salmonella Typhi bacteremia, the proteins encoded by recA, recG, and xthA genes were detected, suggesting these proteins are actively expressed in the blood environment52. The XthA protein is another enzyme that participates in DNA repair mechanism induced by H2O2 and iron-mediated Fenton reaction. The xthA encodes exonuclease III, which repairs the damaged DNA. We found that the xthA gene was required based on the Tn-seq assay and its mutant had a reduced fitness score of −3.06 for H2O2L and a −4.38 for the H2O2H. Further, Salmonella lacking the xthA increased the lag time by 8-fold and a 12-fold for H2O2L and H2O2H, respectively. Targeted-proteomics showed upregulation of XthA (1.64-fold) in response to H2O2. Salmonella enterica serovar Enteritidis defective in xthA is susceptible to egg albumin53. E. coli xthA mutant is hypersensitive to H2O2 54. The xthA is also required for Mycobacterium tuberculosis to infect C57BL/6 J mice55. In addition to the aforementioned genes involved in DNA repair system, uvrA encoding Holliday junction DNA helicase motor protein, uvrC encoding exonuclease ABC subunit A, uvrD encoding DNA-dependent helicase II, and polA encoding DNA polymerase I were among the top of the list of the genes identified by Tn-seq as required for resistance to H2O2. Collectively, DNA repair system is crucial for the survival of the Salmonella in a niche that contains H2O2.
Flagellar genes have a role for H2O2 resistance
Some flagellar genes, fliC and fliB, were shown to be important for resistance to H2O2. These two genes were identified by Tn-seq and their proteins were shown to be upregulated. Salmonella lacking either of these genes exhibited a strong phenotype in the presence of H2O2. During 24 h of incubation, fliC and fliB mutants could not grow in both H2O2 conditions. However, growth of fliD mutant was not affected by H2O2. The fliC was shown to have a role in Salmonella Typhi interaction with human macrophages and Salmonella Typhimurium fliB mutant was defective in swarming motility56,57. Currently it remains unclear how flagella genes can be involved in the resistance of Salmonella to oxidative stress, which warrants future study into this direction.
Fe-S cluster biogenesis system is required for H2O2 resistance
Salmonella requires the genes from Fe-S cluster biogenesis system in order to resist H2O2. Our Tn-seq analysis identified 5 genes in this system that are required for the resistance. In isc operon (Fe-S cluster), iscA, hscB, and hscA were among the genes required to resist H2O2. Particularly, the hscA is on the top of the gene list identified by Tn-seq. In E. coli, this operon is regulated by IscR, iron sulfur cluster regulator58; in Salmonella the gene iscR encoding this transcription regulator is named yfhP. The HscB and HscA chaperones are believed to be involved in the maturation of Fe–S proteins59,60. The second operon that is involved in Fe-S protein biogenesis is the suf, sulfur mobilization operon. Tn-seq found that two genes in this operon were required for Salmonella to resist H2O2; sufS and sufC. Salmonella mutant lacking sufS exhibited a strong phenotype in the presence of H2O2 and could not grow during the 24 h of incubation. The SufS with SufE in E. coli form a heterodimeric cysteine desulphurase and SufB, SufC, and SufD form a pseudo-ABC-transporter that could act as a scaffold60; this operon is regulated by OxyR14. The other known genes in these two operons that are present in Salmonella are iscA, sufA, sufB, and sufD; they showed a reduced fitness, while their p values were >0.05. The damage of Fe-S clusters is not only problem for the defective proteins, but also it fuels the Fenton reaction via the released iron and H2O2 10. Thus, Salmonella uses Fe-S cluster repair system as an arsenal to overcome the damage imposed by H2O2.
DNA adenine methylase is important for H2O2 resistance
DNA adenine methylase genes, dam and damX, are involved in Salmonella resistance against H2O2. Our Tn-seq data showed that fitness of dam and damX mutants were reduced in the presence of H2O2. To confirm this, Salmonella dam mutant was grown in both conditions. Under H2O2L, the growth rate was reduced by 42% as compared to the mutant in LB and the mutant could not grow under H2O2H during the 24 h of incubation. In addition, the lag time of the Salmonella dam mutant was extended by a 19-fold for H2O2L. While the Salmonella damX mutant displayed a moderate phenotype as compared to the dam mutant, the damX mutant also showed that the growth rate decreased by 23% and 33% for H2O2L and H2O2H, respectively. The lag time was extended for this mutant by a 25-fold and a 49-fold for H2O2L and H2O2H, respectively. The dam regulates virulence gene expression in S. Typhimurium61. The different levels of Dam affects virulence gene expression, motility, flagellar synthesis, and bile resistance in the pathogenic S. Typhimurium 1402862. Dam methylation activates the gene that are involved in lipopolysaccharide synthesis63. Moreover, Salmonella defective in damX is very sensitive to bile64. Collectively, our study demonstrates the critical role of DNA adenine methylase in Salmonella resistance against H2O2.
Other genes for H2O2 resistance
Beside the important pathways described above, there were many additional genes also important for resistance to H2O2. Among those, the 3 unrelated genes, rpoS, pgm, and tonB, are important ones that deserve more attention. The rpoS mutant showed reduced fitness and its protein was upregulated in the presence of H2O2. Salmonella mutant defective in rpoS showed a strong phenotype when grown in the presence of H2O2. The rpoS encodes the alternative sigma factor σS, a subunit of RNA polymerase; it is the master regulator of stress response65. This implies that rpoS is an important component of the genetic regulatory network that Salmonella employs in order to resist H2O2. Furthermore, the fitness of pgm mutant was reduced and its protein was upregulated in the presence of H2O2. Knock out of pgm in Salmonella caused a decrease in growth rate, increase the lag phase time, and reduce the maximum OD600 in the presence of H2O2. The pgm encodes phosphoglucomutase which are required for catalysis of the interconversion of glucose 1-phosphate and glucose 6-phosphate66. This gene contributes to resistance against antimicrobial peptides, is required for in vivo fitness in the mouse model, and participates in LPS biosynthesis67. Lastly, the fitness of tonB mutant was also reduced. Salmonella lacking tonB exhibited a strong phenotype in the presence of H2O2 as compared to the mutant grown in LB. The gene mediates iron uptake in the Salmonella 45. In addition, seven of the genes identified in our study (proC, arcA, barA, exbD, flhD, fliC, and fliD) were previously shown to be important for interaction of Salmonella Typhi with human macrophages56.
In summary, we applied Tn-seq and proteomic analysis to find the genes and proteins that are required in S. Typhimurium to resist H2O2 in vitro. As the concentration of H2O2 increased, the growth rate reduced, the lag time extended, the fitness of mutants decreased, and some proteins were differentially expressed. Validation of Tn-seq results with individual mutant assays indicated the accuracy of the identified genes in response to the two H2O2 concentrations. The targeted-proteomics had a good agreement with Tn-seq. We found many genes that have not been associated to resistance to H2O2 previously and these genes will be focus of our future research. Salmonella employs multiple pathways to resist H2O2 and the most important ones are ROS detoxifying enzymes, amino acid biosynthesis (aroK and aroB), putative iron transporters (ybbK, ybbL, ybbM), iron homeostasis, Fe-S cluster repair, DNA repair, flagellar and DNA adenine methylase genes. Our study is focused on single gene knockout mutants and their phenotypes, and therefore was not designed nor expected to reveal any synthetically essential genes in the presence of H2O2. However, Tn-seq can be also used for genetic interaction mapping as previously demonstrated19. We expect that genetic interaction mapping study in the future based on Tn-seq would reveal more complete picture of H2O2 resistance. The genes identified in this study will broaden our understanding on the mechanisms used by Salmonella to survive and persist against ROS in macrophages.
Our unbiased system-wide approach, Tn-seq, was successful in identifying novel genetic determinants that have not been implicated previously in Salmonella resistance to oxidative stress. Based on our literature review, the number of genes that are involved in resistance to oxidative stress is over 60 across different bacterial species, and our findings have doubled this number (n = 137) of conditionally required genes for H2O2 resistance. Furthermore, the combined use of quantitative proteomic approach has provided additional insights on the function or mode of action of the identified genetic determinants in resisting oxidative stress. As expected, the majority of the proteins important for resistance to H2O2 were upregulated in response to the same stressor. However, the expression level did not increase for some proteins, in spite of their known roles in resistance to H2O2. Interestingly, the downregulation of Dps and other proteins was counterintuitive to the common mode of protein regulation and function, yet it may point to some unknown aspects of how Salmonella regulates the expression of those proteins to better cope with the oxidative stress during infection in macrophage. The genes identified in this study will broaden our understanding on the mechanisms used by Salmonella to survive and persist against ROS in macrophages.
Methods
Bacterial strain and construction of Tn5 mutant library
Salmonella enterica subsp. enterica serovar Typhimurium ATCC 14028 s (with spontaneous mutation conferring resistance to nalidixic acid (NA) was used in this study. All procedures involving this pathogen (Biosafety level 2) were conducted according to the protocol approved by Institutional Biosafety Committee (IBC). We mutagenized S. Typhimurium ATCC 14028s by biparental mating using Escherichia coli SM10 λpir carrying a transposon-delivery plasmid vector pBAM1 (Ampicillin (Amp) resistance) as a donor strain68. The plasmid pBAM1 was generously provided by Victor de Lorenzo (Systems and Synthetic Biology Program, Centro Nacional de Biotecnología, Madrid, Spain). The donor strain, E. coli Sm10 λpir (pBAM1), was grown overnight in LB with 50 µg/ml Amp and recipient strain was grown in LB with 50 µg/ml NA at 37 °C. Equal volumes (1 ml) of donor and recipient were mixed, centrifuged, washed in 10 mM MgSO4, and re-suspended in 2 ml PBS (pH 7.4). Then, the mating mixture was concentrated and laid on a 0.45-µm nitrocellulose filters (Millipore). The filter was incubated at 37 °C on the surface of LB agar plate. After 5 h of conjugation, the cells on the filter was washed in 10 mM MgSO4, and resuspended in 1 ml MgSO4. The conjugation mixture was plated on LB agar containing 50 µg/ml NA and 50 µg/ml kanamycin (Km). After approximately 24 h at 37 °C, we scraped the colonies into LB broth containing 50 µg/ml Km and 7% DMSO. The yield was approximately 68,000 individual colonies from each conjugation. Five independent conjugations were conducted to yield approximately 325,000 mutants. The library was stored at −80 °C in aliquots. To determine the frequency of the mutants that have been produced by integration of the entire delivery plasmid, the colonies were picked randomly and streaked on LB plates (Km) and LB plates (Km and Amp). It was shown that ~20% of the cells in the library were resistant to Amp, indicating a significant portion of the Km-resistant colonies was not from authentic transposition events.
Measuring growth responses of S. Typhimurium to H2O2
To determine the effect of H2O2 concentrations on growth parameters, overnight culture of the wild type S. Typhimurium 14028 s was inoculated into fresh LB broth media (1:200 dilution) to give a seeding concentration corresponding to OD600 of ~0.1. The LB broth contained freshly prepared H2O2 to give the final concentrations ranging from 0.05 to 10 mM. The cultures were directly added into 96-well microplate (200 μl/well). The microplate was incubated in a Tecan Infinite 200 microplate reader at 37 °C, with shaking duration 5 s, shaking amplitude 1.5 mm, and reading OD600 every 10 min. The number of replicates were at least three. The lag time, growth rate, and maximum OD600 were calculated using GrowthRates script69. Growth Rate % decrease was calculated as follows70: Growth Rate % decrease = ((μPC − μS)/μPC) × 100; where μ = the maximum slope (growth rate), μPC = growth rate of positive control (without H2O2), μS = growth rate in the presence of H2O2.
Selection of the mutant library for Tn-seq analysis
The transposon library was thawed at room temperature and diluted 10−1 in fresh LB broth. To activate the library, the diluted library was incubated at 37 °C with shaking at 225 rpm for an hour. Then, the culture was washed twice with PBS and resuspended in LB broth medium. The library was inoculated to 20 ml LB broth and LB broth supplemented with either 2.5 or 3.5 mM H2O2 (H2O2L and H2O2H, respectively), seeding CFU was 3.5 × 106 per ml. Then, when the cultures reached mid-exponential phase, OD600 of 2.7 (~1.17 × 108 CFU/ml), the incubation was stopped, and the culture was immediately harvested by centrifugation, and stored at −20 °C.
Preparation of Tn-seq amplicon libraries
Genomic DNA was extracted from the harvested cells using DNeasy Blood & Tissue kit (Qiagen), and quantified using Qubit dsDNA RB Assay kit (Invitrogen). As described above, 20% of the mutants in the library were the result of the integration of pBAM1 into chromosome. To remove the Tn5-junction sequences originated from the plasmid in the Tn-seq amplicon libraries, genomic DNA was digested with PvuII-HF (New England Biolabs), which digests immediately outside the inverted repeats on both sides of Tn5 in pBAM1, and purified with DNA Clean & Concentrator-5 kit (Zymo Reaerch). Then, a linear PCR extension was performed using a Tn5-specific primer in order to produce single stranded DNA corresponding to Tn5-junction sequences. To increase the specificity in extending into Tn5-junction sequences, the linear PCR was conducted with a dual priming oligonucleotide Tn5-DPO (5′-AAGCTTGCATGCCTGCAGGTIIIIICTAGAGGATC-3′) that is specific to Tn5 end71. The PCR reaction contained 25 μl Go Taq Colorless Master Mix (Promega), 20 μM Tn5-DPO primer, 100 ng gDNA, and 50 μl MQ-H2O. The PCR cycle consisted of the initial denaturation at 95 °C for 2 min, followed by 50 cycles at 95 °C for 30 sec, 62 °C for 45 sec, and 72 °C for 10 sec. The PCR product was purified with DNA Clean & Concentrator-5 kit and eluted in 13 μl TE buffer. After that, C-tail was added to the 3′ end of the single-stranded DNA. The C-tailing reaction was consisted of 2 μl terminal transferase (TdT) buffer (New England Biolabs), 2 μl CoCl2, 2.4 μl 10 mM dCTP, 1 μl 1 mM ddCTP, 0.5 μl TdT and 13 μl purified linear PCR product. The reaction was performed at 37 °C for 1 h and the enzyme was inactivated by incubation at 70 °C for 10 min. The C-tailed product was purified with DNA Clean & Concentrator-5 kit and eluted in 12 μl TE. Next, the exponential PCR was performed with forward primer, P5-BRX-TN5-MEO, 5′-AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCTNNNNAG-6 nt barcode-CCTAGGCGGCCTTAATTAAAGATGTGTATAAGAG-3′ and reverse primer, P7–16G, 5′-CAAGCAGAAGACGGCATACGAGCTCTTCCGATCTGGGGGGGGGGGGGGGG-3′ to attach Illumina adapter sequences along with the sample barcodes. The PCR reaction contained 25 μl Go Taq Green Master Mix, 10 μM P5-BRX-TN5-MEO primer, 10 μM P7–16G primer, 1 μl purified C-tailed genomic junctions, and MQ-H2O to 50 μl; the PCR condition started with initial denaturation at 95 °C for 2 min, followed by 36 cycles of 95 °C for 30 sec, 60 °C for 30 sec, and 72 °C for 20 sec, with the final extension at 72 °C for 5 min. Then, the size selection of the DNA was performed using agarose gel electrophoresis. The 50 μl PCR products were incubated at 60 °C for 15 min and incubated on ice for 5 min, and immediately loaded on the 1% agarose gel in 0.5% TAE buffer. After running the gel, the DNA fragment of size 325–625 bp was cut and put in a microtube for each sample. The DNA was extracted from the gel using Zymoclean Gel DNA Recovery kit (Zymo Reaerch). The prepared DNA libraries were quantified using Qubit dsDNA RB Assay kit. Since each library has its own barcode, the libraries were combined and sequenced on a flow cell of HiSeq. 3000 using single end read and 151 cycles (Illumina) at the Center for Genome Research & Biocomputing in Oregon State University.
Tn-seq data analysis
The preliminary data analysis was conducted by using a super computer in the High Performance Computing Center (AHPCC) at the University of Arkansas. The libraries that were multiplexed for sequencing were de-multiplexed using a custom Python script. The script searched for the six-nucleotide barcode for each library for perfect matches. In order to extract the transposon genomic junctions, we used Tn-Seq Pre-Processor (TPP) tool30 with some modifications in the script. The TPP searched for the 19 nucleotide inverted repeat (IR) sequence and identified five nucleotides (GACAG) at the end of the IR sequence, allowing one nucleotide mismatch. The Tn5-junctions that start immediately after GACAG were extracted and the C-tails at the end of junctions were removed. Tn5-junction sequences less than 20 nucleotides were discarded and remaining Tn5-junctions were mapped to the Salmonella enterica serovar Typhimurium 14028 s genome and plasmid using BWA-0.7.1272. To identify genes that are required for H2O2 resistance, the following three Tn-seq analysis tools were used for comparative analysis: (i) ARTIST28: the genomic junctions were mapped to the reference genome using Bowtie 2.2.773. The number of insertions and reads were determined for genes and intergenic regions. The data were normalized with default script in the ARTIST. Then, the relative abundance of Tn5 insertions in the output library versus the input were calculated. Later, the p values were calculated from a 100 independent Mann–Whitney U test (MWU) analysis that were carried out on input and output data for each gene. Finally, the genes were considered conditionally essential if the p values were ≤0.05 in the 90 of the 100 MWU tests. (ii) Tn-seq Explorer29: The output SAM files from the TTP were used as input to the Tn-seq Explorer. The unique insertions with less than 20 reads were removed from the input and outputs. Using the window size of 550 and excluding 5% of beginning of genes and 20% of the end of genes, Essentiality Index (EI), number of unique insertions, and total number of reads per gene were counted. The EI of more than 10 were removed from the input. Genes with less than 300 nucleotides were removed. Deferential EI were calculated from input and outputs (∆IE = output EI–input EI) and genes with ∆IE more than −1 were removed. Log2 fold change of reads were calculated from input and output (Log2FC = log2 (output reads/input reads)) and the genes were ranked based on the Log2FC value from least value to highest. The genes with Log2FC value of less than −2 from the H2O2H and present in H2O2L were considered conditionally essential. (iii) TRANSIT30: The output wig files from the TTP was used as input data file for TRANSIT. The comparative analysis was conducted with Tn5 resampling option. The reads were normalized with trimmed total reads (TTR). Insertions outside the 5% and 10% sequences from 5′- and 3′- ends were removed, respectively. The genes were considered conditionally essential if p values ≤ 0.05.
Phenotypic evaluation of individual deletion mutants
The mutants were obtained from Salmonella enterica subsp. enterica, 14028 s (Serovar Typhimurium) Single-Gene Deletion Mutant Library through BEI Resources (www.beiresources.org). The overnight cultures of S. Typhimurium mutants were added into fresh LB broth media containing freshly prepared H2O2 (2.5, or 3.5 mM/ml) (1:200 dilution) to give seeding OD600 of 0.1. The cultures were directly added into 96-well microplates and incubated in Tecan Infinite 200 at 37 °C for 24 h. The lag time, growth rate, and maximum OD600 were calculated using GrowthRates69.
Sample preparation for proteomics and mass spectrometry analysis
The overnight culture of the wild type S. Typhimurium 14028 was diluted 1:200 in 50 ml LB medium, and LB containing either 2.5 or 3.5 mM H2O2 in a 300-ml flask. The cultures were grown until mid-exponential phase (OD600 of 2.7), and the 50 ml volume of cultures were used for a total protein extraction by using Qproteome Bacterial Protein Prep kit (Qiagen). Disulfide bonds within proteins were reduced with 2-Mercaptoethanol and separated by SDS-PAGE gel electrophoresis. For each condition, there were three lanes with approximately 300 μg of proteins. The gel then was stained via coomassie blue dye. The gel portions of 3 lanes for each condition were cut out and chopped into small pieces, pooled together, washed twice with 50 mM NH4HCO3, destained with NH4HCO3/ 50% Acetonitrile (ACN), and dried with pure ACN. Then, the proteins were reduced using 10 mM Dithiothreitol in 50 mM NH4CO3 and the alkylation was conducted with 10 mg/ml Iodoacetamide Acid in 50 mM NH4CO3. After that, the proteins were washed with NH4HCO3, and dried with pure ACN. Mass spectrometry grade Trypsin gold from Promega (~20 ng/μl in 50 mM NH4HCO3) was added to dried gels, and left it overnight for efficient in-gel digestion of the proteins at 37 °C. During the digestion, tryptic peptides diffused out into the solution. Gel pieces then were extracted three times by 50% CAN/0.1% TFA solution and incubated at 37 °C for 15 minutes. Later, these digests were analyzed by ESI-LC-MS/MS at State Wide Mass Spectrometry Facility, University of Arkansas at Fayetteville. Data dependent analysis (DDA) for the in-gel trypsin digested samples from each condition were performed by using an Agilent 1200 series micro flow HPLC in line with Bruker Amazon-SL quadrupole ion trap ESI mass spectrometer (QIT-ESI-MS). All the ESI-MS analyses were performed in a positive ion mode using Bruker captive electrospray source with a dry nitrogen gas temperature of 200 °C, with nitrogen flow rate of 3 L/minute. LC-MS/MS data were acquired in the Auto MS(n) mode with optimized trapping condition for the ions at m/z 1000. MS scans were performed in the enhanced scanning mode (8100 m/z/second), while the collision-induced dissociation or the MS/MS fragmentation scans were performed automatically for top ten precursor ions with a set threshold for one minute in the UltraScan mode (32,500 m/z/second). Tryptic peptides were separated by reverse-phase high-performance liquid chromatography (RP-HPLC) using a Zorbax SB C18 column, (150 × 0.3 mm, 3.5 µm particle size, 300 Å pore size, Agilent Technologies), with a solvent flow rate of 4 µL/minute, and a gradient of 5%–38% consisting of 0.1% FA (solvent A) and ACN (solvent B) over a time period of 320 minutes. Tryptic peptides were then identified by searching MS/MS data in S. Typhimurium 14028 s reference proteome database24,35 by using MASCOT database search software34. MS1 intensities of the integrated areas of these identified tryptic peptides were compiled and grouped in skyline software according to the replicates/conditions to perform statistical analysis, there were 4 technical replicates. Targeted protein work were performed using Shimadzu UPLC-20A coupled to 8050 triple quadrupole ESI-MS with heated probe. Sequence specific fragment ion intensities from at least three unique tryptic peptides from the protein of interest were used in the protein quantitation. Multiple reaction monitoring (MRM) events corresponding to sequence specific fragment ions derived from the precursor tryptic peptides were targeted to operate at a certain specific retention time intervals predicted by in house retention time library. This library was generated using the correlation of relative hydrophobicity of the tryptic peptides with their retention times (RT) from highly common housekeeping proteins, for the UPLC method used in this analysis as described below. While the RT were correlated well within 99% confidence, sufficient number of sequence specific fragment ions were used as basis for identification of the tryptic peptide by MS/MS alone. Specificity and the confidence was achieved by incorporating RT prediction. In addition to the application of skyline in quantitation, skyline software was also used in predicting RT and optimizing parameters such as collision energies and voltages with the help of Shimadzu Labsolution software. Tryptic peptides were separated by reverse-phase ultra-high-performance liquid chromatography (RP-UPLC) compatible Shimadzu C18, 1.9-micron particle size, 50 × 2.1 mm column (SN # 16041880 T), with a solvent flow rate of 0.3 mL/minute, and a gradient of 5%–90% consisting of 0.1% FA (solvent A) and 0.1% FA in ACN (solvent B) over a time period of 10 minutes.
Data availability
Tn-seq sequencing data are available on NCBI Sequence Read Archive. LB: PRJNA352537; H2O2L: PRJNA352862; H2O2H: PRJNA352865. Proteomics data are available on https://panoramaweb.org/labkey/cMOPwI.url.
Electronic supplementary material
Acknowledgements
We thank Jack Jiang, a high school student, for writing the demultiplexing Python script. We are thankful for Dr. Thomas R. Ioerger and Dr. Michael A. DeJesus at Texas A&M University for the help on the use of TRNASIT. We extend our special thanks to Dr. Jeff F. Pummill and Dr. Pawel Wolinski at Arkansas High Performance Computing Center for their help to use the facility. This work was partially supported by Arkansas Biosciences Institute. This research was also supported by the Arkansas High Performance Computing Center which is funded through multiple National Science Foundation grants and the Arkansas Economic Development Commission. The first author was supported by his parents, Cell and Molecular Biology (CEMB) program at the University of Arkansas, and Human Capacity Development Program-Kurdistan Regional Government (HCDP-KRG).
Author Contributions
Conceived and designed the experiments: Y.K., S.K. Performed the experiments, analyzed the data, wrote the manuscript: S.K. Proteomics work: R.L., S.K., A.Q., J.L. Revised the manuscript: Y.K., S.K. All authors read the final version of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-17149-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.LaRock DL, Chaudhary A, Miller SI. Salmonellae interactions with host processes. Nat. Rev. Microbiol. 2015;13:191–205. doi: 10.1038/nrmicro3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scallan, E. et al. Foodborne illness acquired in the United States – major pathogens. Emerg. Infect. Dis.17 (2011). [DOI] [PMC free article] [PubMed]
- 3.Majowicz SE, et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010;50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 4.Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 2008;6:53–66. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- 5.Nauseef W. Assembly of the phagocyte NADPH oxidase. Histochem. Cell Biol. 2004;122:277–291. doi: 10.1007/s00418-004-0679-8. [DOI] [PubMed] [Google Scholar]
- 6.Seaver LC, Imlay JA. Hydrogen peroxide fluxes and compartmentalization inside growing. Escherichia coli. J. Bacteriol. 2001;183:7182–7189. doi: 10.1128/JB.183.24.7182-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park S, You X, Imlay JA. Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx- mutants of Escherichia coli. Proc. Natl. Acad. Sci. USA. 2005;102:9317–9322. doi: 10.1073/pnas.0502051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imlay JA, Linn S. DNA damage and oxygen radical toxicity. Science. 1988;240:1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- 9.Imlay JA. Iron-sulphur clusters and the problem with oxygen. Mol. Microbiol. 2006;59:1073–1082. doi: 10.1111/j.1365-2958.2006.05028.x. [DOI] [PubMed] [Google Scholar]
- 10.Slauch JM. How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol. Microbiol. 2011;80:580–583. doi: 10.1111/j.1365-2958.2011.07612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hébrard M, Julie PMV, Méresse S, Barras F, Aussel L. Redundant Hydrogen Peroxide Scavengers Contribute to Salmonella Virulence and Oxidative Stress Resistance. J. Bacteriol. 2009;191:4605–4614. doi: 10.1128/JB.00144-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horst SA, et al. Thiol Peroxidase Protects Salmonella enterica from Hydrogen Peroxide Stress In Vitro and Facilitates Intracellular Growth. J. Bacteriol. 2010;192:2929–2932. doi: 10.1128/JB.01652-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan RW, Christman MF, Jacobson FS, Storz G, Ames BN. Hydrogen Peroxide-Inducible Proteins in Salmonella Typhimurium Overlap with Heat Shock and Other Stress Proteins. Proc. Natl. Acad. Sci. USA. 1986;83:8059–8063. doi: 10.1073/pnas.83.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng M, et al. DNA Microarray-Mediated Transcriptional Profiling of the Escherichia coli Response to Hydrogen Peroxide. J. Bacteriol. 2001;183:4562–4570. doi: 10.1128/JB.183.15.4562-4570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroger C, et al. An Infection-Relevant Transcriptomic Compendium for Salmonella enterica Serovar Typhimurium. Cell Host Microbe. 2013;14:683–695. doi: 10.1016/j.chom.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Turner KH, Wessel AK, Palmer GC, Murray JL, Whiteley M. Essential genome of Pseudomonas aeruginosa in cystic fibrosis sputum. Proc. Natl. Acad. Sci. USA. 2015;112:4110. doi: 10.1073/pnas.1419677112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan K, Kim CC, Falkow S. Microarray-Based Detection of Salmonella enterica Serovar Typhimurium Transposon Mutants That Cannot Survive in Macrophages and Mice. Infect. Immun. 2005;73:5438–5449. doi: 10.1128/IAI.73.9.5438-5449.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva-Valenzuela, C. et al. Analysis of Two Complementary Single-Gene Deletion Mutant Libraries of Salmonella Typhimurium in Intraperitoneal Infection of BALB/c Mice. Front. Microbiol. 6 (2016). [DOI] [PMC free article] [PubMed]
- 19.van Opijnen T, Bodi KL, Camilli A. Tn-seq: high-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nat. Methods. 2009;6:767–772. doi: 10.1038/nmeth.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khatiwara A, et al. Genome Scanning for Conditionally Essential Genes in Salmonella enterica Serotype Typhimurium. Appl. Environ. Microbiol. 2012;78:3098–3107. doi: 10.1128/AEM.06865-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhuri R, et al. Comprehensive Assignment of Roles for Salmonella Typhimurium Genes in Intestinal Colonization of Food-Producing Animals. PLoS Genet. 2013;9:e1003456. doi: 10.1371/journal.pgen.1003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langridge GC, et al. Simultaneous assay of every Salmonella Typhi gene using one million transposon mutants. Genome Res. 2009;19:2308–2316. doi: 10.1101/gr.097097.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barquist L, et al. A comparison of dense transposon insertion libraries in the Salmonella serovars Typhi and Typhimurium. Nucleic Acids Res. 2013;41:4549–4564. doi: 10.1093/nar/gkt148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Domon B, Aebersold R. Options and considerations when selecting a quantitative proteomics strategy. Nat. Biotechnol. 2010;28:710–721. doi: 10.1038/nbt.1661. [DOI] [PubMed] [Google Scholar]
- 25.Jensen ON, Mann M. Proteomic analysis of post-translational modifications. Nat. Biotechnol. 2003;21:255–261. doi: 10.1038/nbt0303-255. [DOI] [PubMed] [Google Scholar]
- 26.Bogomolnaya L, et al. The ABC-Type Efflux Pump MacAB Protects Salmonella enterica serovar Typhimurium from Oxidative Stress. Mbio. 2013;4:e00630. doi: 10.1128/mBio.00630-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon YM, Ricke SC, Mandal RK. Transposon sequencing: methods and expanding applications. Appl. Microbiol. Biotechnol. 2016;100:31–43. doi: 10.1007/s00253-015-7037-8. [DOI] [PubMed] [Google Scholar]
- 28.Pritchard JR, et al. ARTIST: high-resolution genome-wide assessment of fitness using transposon-insertion sequencing. PLoS Genet. 2014;10:e1004782–e1004782. doi: 10.1371/journal.pgen.1004782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solaimanpour S, Sarmiento F, Mrazek J. Tn-Seq Explorer: A Tool for Analysis of High-Throughput Sequencing Data of Transposon Mutant Libraries. PLoS One. 2015;10:e0126070. doi: 10.1371/journal.pone.0126070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeJesus MA, Ambadipudi C, Baker R, Sassetti C, Ioerger TR. TRANSIT–A Software Tool for Himar1 TnSeq Analysis. PLoS Comput. Biol. 2015;11:e1004401–e1004401. doi: 10.1371/journal.pcbi.1004401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacLean B, et al. Bioinformatics. 2010. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments; pp. 966–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perkins D, Pappin D, Creasy D, Cottrell J. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 35.Cravatt BF, Yates JR, III, Simon GM. The biological impact of mass-spectrometry-based proteomics. Nature. 2007;450:991–1000. doi: 10.1038/nature06525. [DOI] [PubMed] [Google Scholar]
- 36.Carlioz A, Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986;5:623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weatherspoon-Griffin N, Yang D, Kong W, Hua Z, Shi Y. The CpxR/CpxA Two-component Regulatory System Up-regulates the Multidrug Resistance Cascade to Facilitate Escherichia coli Resistance to a Model Antimicrobial Peptide. J. Biol. Chem. 2014;289:32571–32582. doi: 10.1074/jbc.M114.565762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vinella D, Gagny B, Joseleau-Petit D, D’Ari R, Cashel M. Mecillinam resistance in Escherichia coli is conferred by loss of a second activity of the AroK protein. J. Bacteriol. 1996;178:3818–3828. doi: 10.1128/jb.178.13.3818-3828.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaur P, et al. Unravelling the Secrets of Mycobacterial Cidality through the Lens of Antisense. PLoS One. 2016;11:e0154513. doi: 10.1371/journal.pone.0154513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Parys A, et al. Tissue-Specific Salmonella Typhimurium Gene Expression during Persistence in Pigs. PLoS One. 2011;6:e24120. doi: 10.1371/journal.pone.0024120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Günel-Özcan A, Brown KA, Allen AG, Maskell DJ. Salmonella Typhimurium aroB mutants are attentuated in BALB/ c mice. Microb. Pathog. 1997;23:311–316. doi: 10.1006/mpat.1997.0157. [DOI] [PubMed] [Google Scholar]
- 42.Nicolaou SA, Fast AG, Nakamaru-Ogiso E, Papoutsakis ET. Overexpression of fetA (ybbL) and fetB (ybbM), Encoding an Iron Exporter, Enhances Resistance to Oxidative Stress in Escherichia coli. Appl. Environ. Microbiol. 2013;79:7210–7219. doi: 10.1128/AEM.02322-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robbe‐Saule V, Coynault C, Ibanez‐Ruiz M, Hermant D, Norel F. Identification of a non‐haem catalase in Salmonella and its regulation by RpoS (σS) Mol. Microbiol. 2001;39:1533–1545. doi: 10.1046/j.1365-2958.2001.02340.x. [DOI] [PubMed] [Google Scholar]
- 44.Craig M, Slauch J. Phagocytic Superoxide Specifically Damages an Extracytoplasmic Target to Inhibit or Kill Salmonella. PLoS One. 2009;4:e4975. doi: 10.1371/journal.pone.0004975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsolis RM, Bäumler AJ, Heffron F, Stojiljkovic I. Contribution of TonB- and Feo-mediated iron uptake to growth of Salmonella Typhimurium in the mouse. Infect. Immun. 1996;64:4549–4556. doi: 10.1128/iai.64.11.4549-4556.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aussel L, et al. Salmonella detoxifying enzymes are sufficient to cope with the host oxidative burst. Mol. Microbiol. 2011;80:628–640. doi: 10.1111/j.1365-2958.2011.07611.x. [DOI] [PubMed] [Google Scholar]
- 47.Taylor PD, Inchley CJ, Gallagher MP. The Salmonella Typhimurium AhpC Polypeptide Is Not Essential for Virulence in BALB/c Mice but Is Recognized as an Antigen during Infection. Infect. Immun. 1998;66:3208–3217. doi: 10.1128/iai.66.7.3208-3217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papp-Szabò E, Firtel M, Josephy PD. Comparison of the sensitivities of Salmonella Typhimurium oxyR and katG mutants to killing by human neutrophils. Infect. Immun. 1994;62:2662–2668. doi: 10.1128/iai.62.7.2662-2668.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Imlay J. Diagnosing oxidative stress in bacteria: not as easy as you might think. Curr. Opin. Microbiol. 2015;24:124–131. doi: 10.1016/j.mib.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carlsson J, Carpenter VS. The recA+ gene product is more important than catalase and superoxide dismutase in protecting Escherichia coli against hydrogen peroxide toxicity. J. Bacteriol. 1980;142:319–321. doi: 10.1128/jb.142.1.319-321.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishioka K, Iwasaki H, Shinagawa H. Roles of the recG gene product of Escherichia coli in recombination repair: Effects of the Delta recG mutation on cell division and chromosome partition. Genes Genet. Syst. 1997;72:91–99. doi: 10.1266/ggs.72.91. [DOI] [PubMed] [Google Scholar]
- 52.Sheikh A, et al. In Vivo Expression of Salmonella enterica Serotype Typhi Genes in the Blood of Patients with Typhoid Fever in Bangladesh. PLoS Negl. Trop. Dis. 2011;5:e1419. doi: 10.1371/journal.pntd.0001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu S, Killoran PB, Riley LW. Association of Salmonella enterica Serovar Enteritidis YafD with Resistance to Chicken Egg Albumen. Infect. Immun. 2003;71:6734–6741. doi: 10.1128/IAI.71.12.6734-6741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Demple B, Halbrook J, Linn S. Escherichia coli xth mutants are hypersensitive to hydrogen peroxide. J. Bacteriol. 1983;153:1079–1082. doi: 10.1128/jb.153.2.1079-1082.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sassetti CM, Rubin EJ. Genetic Requirements for Mycobacterial Survival during Infection. Proc. Natl. Acad. Sci. USA. 2003;100:12989–12994. doi: 10.1073/pnas.2134250100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sabbagh S, Lepage C, McClelland M, Daigle F. Selection of Salmonella enterica Serovar Typhi Genes Involved during Interaction with Human Macrophages by Screening of a Transposon Mutant Library. PLoS One. 2012;7:e36643. doi: 10.1371/journal.pone.0036643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bogomolnaya L, et al. Identification of Novel Factors Involved in Modulating Motility of Salmonella enterica Serotype Typhimurium. PLoS One. 2014;9:e111513. doi: 10.1371/journal.pone.0111513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwartz CJ, et al. IscR, An Fe-S Cluster-Containing Transcription Factor, Represses Expression of Escherichia coli Genes Encoding Fe-S Cluster Assembly Proteins. Proc. Natl. Acad. Sci. USA. 2001;98:14895–14900. doi: 10.1073/pnas.251550898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Unciuleac M, et al. Vitro activation of apo-aconitase using a [4Fe-4S] cluster-loaded form of the IscU [Fe-S] cluster scaffolding protein. Biochemistry (N. Y.) 2007;46:6812–6821. doi: 10.1021/bi6026665. [DOI] [PubMed] [Google Scholar]
- 60.Py B, Barras F. Building Fe–S proteins: bacterial strategies. Nat. Rev. Microbiol. 2010;8:436–446. doi: 10.1038/nrmicro2356. [DOI] [PubMed] [Google Scholar]
- 61.Balbontín R, et al. DNA Adenine Methylation Regulates Virulence Gene Expression in Salmonella enterica Serovar Typhimurium. J. Bacteriol. 2006;188:8160–8168. doi: 10.1128/JB.00847-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Badie G, Heithoff DM, Sinsheimer RL, Mahan MJ. Altered Levels of Salmonella DNA Adenine Methylase Are Associated with Defects in Gene Expression, Motility, Flagellar Synthesis, and Bile Resistance in the Pathogenic Strain 14028 but Not in the Laboratory Strain LT2. J. Bacteriol. 2007;189:1556–1564. doi: 10.1128/JB.01580-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sarnacki SH, et al. Dam Methylation Controls O-Antigen Chain Length in Salmonella enterica Serovar Enteritidis by Regulating the Expression of Wzz Protein. J. Bacteriol. 2009;191:6694–6700. doi: 10.1128/JB.00839-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lopez-Garrido J, Cheng N, Garcia-Quintanilla F. Garcia-del Portillo, F. & Casadesus, J. Identification of the Salmonella enterica damX Gene Product, an Inner Membrane Protein Involved in Bile Resistance. J. Bacteriol. 2010;192:893–895. doi: 10.1128/JB.01220-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hengge-Aronis R. Signal Transduction and Regulatory Mechanisms Involved in Control of the σS (RpoS) Subunit of RNA Polymerase. Microbiol. Mol. Biol. Rev. 2002;66:373–395. doi: 10.1128/MMBR.66.3.373-395.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu M, Kleckner N. Molecular cloning and characterization of the pgm gene encoding phosphoglucomutase of Escherichia coli. J. Bacteriol. 1994;176:5847–5851. doi: 10.1128/jb.176.18.5847-5851.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paterson GK, Cone DB, Peters SE, Maskell DJ. The enzyme phosphoglucomutase (Pgm) is required by Salmonella enterica serovar Typhimurium for O-antigen production, resistance to antimicrobial peptides and in vivo fitness. Microbiology. 2009;155:3403–3410. doi: 10.1099/mic.0.029553-0. [DOI] [PubMed] [Google Scholar]
- 68.Martínez-García E, Calles B, Arévalo-Rodríguez M, de Lorenzo V. pBAM1: an all-synthetic genetic tool for analysis and construction of complex bacterial phenotypes. BMC Microbiol. 2011;11:38–38. doi: 10.1186/1471-2180-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hall B, Acar H, Nandipati A, Barlow M. Growth Rates Made Easy. Mol. Biol. Evol. 2014;31:232–238. doi: 10.1093/molbev/mst187. [DOI] [PubMed] [Google Scholar]
- 70.Yao Q, et al. Assembly of nanoions via electrostatic interactions: ion-like behavior of charged noble metal nanoclusters. Sci. Rep. 2014;4:3848–3848. doi: 10.1038/srep03848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chun J, et al. Dual priming oligonucleotide system for the multiplex detection of respiratory viruses and SNP genotyping of CYP2C19 gene. Nucleic Acids Res. 2007;35:e40–e40. doi: 10.1093/nar/gkm051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Langmead B, Trapnell C, Pop M, Salzberg S. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25–R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Tn-seq sequencing data are available on NCBI Sequence Read Archive. LB: PRJNA352537; H2O2L: PRJNA352862; H2O2H: PRJNA352865. Proteomics data are available on https://panoramaweb.org/labkey/cMOPwI.url.