Abstract
During adolescence, both rodent and human studies have revealed dynamic changes in the developmental trajectories of corticolimbic structures, which are known to contribute to the regulation of fear and anxiety-related behaviors. The endocannabinoid (eCB) system critically regulates stress responsivity and anxiety throughout the life span. Emerging evidence suggests that during adolescence, changes in eCB signaling contribute to the maturation of local and corticolimbic circuit populations of neurons, such as mediating the balance between excitatory and inhibitory neurotransmission within the prefrontal cortex. This function of the eCB system facilitates efficient communication within and between brain regions and serves a central role in establishing complex and adaptive cognitive and behavioral processing. Although these peri-adolescent changes in eCB signaling promote brain development and plasticity, they also render this period a particularly sensitive one for environmental perturbations to these normative fluctuations in eCB signaling, such as stress, potentially leading to altered developmental trajectories of neural circuits governing emotional behaviors. In this review, we focus on the role of eCB signaling on the regulation of stress and anxiety-related behaviors both during and after adolescence. Moreover, we discuss the functional implications of human genetic variation in the eCB system for the risk for anxiety and consequences of stress across development and into adulthood.
Introduction
Endocannabinoid (eCB) signaling serves an integral role in brain development and regulating stress and anxiety throughout the life span. During adolescence, dynamic changes in eCB signaling parallel normative changes in corticolimbic circuitry and fear-related behaviors. Although the exact mechanisms by which eCB signaling shapes neurodevelopmental processes remains unknown, emerging evidence suggests that the eCB system regulates the activity of local and circuit populations of neurons and is particularly important for mediating the balance between excitatory and inhibitory neurotransmission during adolescence. This function of the eCB system facilitates the efficiency of communication within and between brain regions, including corticolimbic structures critical for the regulation of fear and anxiety.
To date, the majority of research into eCB signaling during adolescence has considered the long-term consequences of disrupting the normative activity of this system during development (Lee et al, 2016; Rubino and Parolaro, 2016; Dow-Edwards and Silva, 2017). Here we focus on the role of eCB signaling for the regulation of stress and anxiety-related behaviors both during and after adolescence. Throughout this review, we also consider the importance of developmental timing and how the downstream effects of perturbations to eCB signaling after exposure to stress or exogenous cannabinoids change depending on developmental stage. This approach provides insight into how the eCB system changes across the life span as well as how it contributes to typical and atypical brain and behavioral development. Moreover, we discuss the functional implications of genetic variation in the eCB system for the consequences of stress and risk for anxiety across development and into adulthood.
The endocannabinoid system
The eCB system is largely composed of two inhibitory G-protein-coupled receptors (GPCRs), CB1 and CB2, and two major endogenous ligands, N-arachidonoylethanolamine (anandamide/AEA) and 2-arachidonoylglycerol (2-AG). In addition, eCB signaling is highly regulated by metabolic enzymes including fatty acid amide hydrolase (FAAH) and monoacylglyceride lipase (MAGL), which hydrolyze AEA and 2-AG, respectively (Figure 1). Together, dynamic interactions between these eCB system components play an important role in central nervous system development, synaptic plasticity, and the homeostatic maintenance of cognitive, behavioral, emotional, developmental, and physiological processes (Mechoulam and Parker, 2013; Lu and Mackie, 2016).
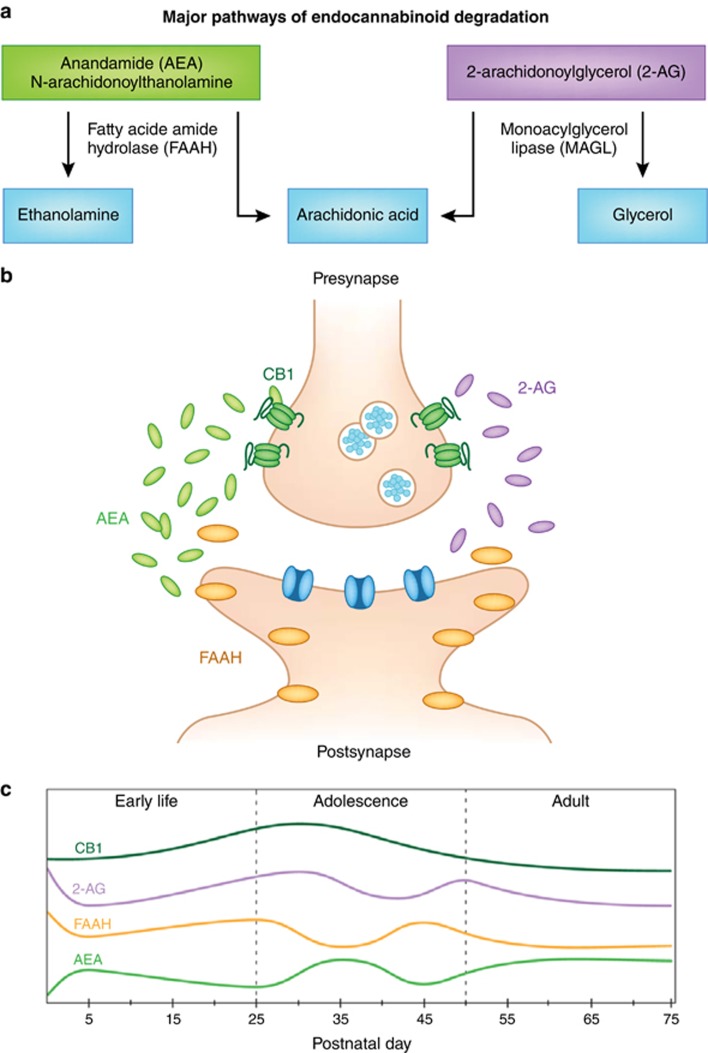
Figure 1.
Corticolimbic eCB signaling changes dynamically across rodent development. (a) Major pathways of endocannabinoid degradation. (b) Schematic of eCB signaling within a synapse. (c) Developmental trajectories of the components of the eCB system. CB1 receptor expression peaks with the onset of adolescence. 2-AG is highest around birth and may fluctuate throughout adolescence. AEA gradually increases during early life and fluctuates during adolescence. FAAH activity fluctuates in reciprocal fashion to AEA during adolescence (based on data from Berrendero et al, 1999; Ellgren et al, 2008; Fernandez-Ruiz et al, 2000; Heng et al, 2011; Lee et al, 2013; Rodríguez de Fonseca et al, 1993; Rubino et al, 2015; Wenger et al, 2002). Adapted from Lee et al (2016), Genes Brain and Behavior.
The precursors for AEA and 2-AG are present in the lipid membranes of post-synaptic neurons. Thus, AEA and 2-AG are synthesized ‘on demand’ and transferred in a retrograde manner across the synaptic cleft to bind to eCB receptors at the presynapse and regulate the release of other neurotransmitters, including glutamate, GABA, dopamine, serotonin, and acetylcholine (Figure 1; Howlett et al, 2002; Piomelli, 2003; Lovinger, 2008; Jutras-Aswad et al, 2009; Katona and Freund, 2012). AEA and 2-AG have been implicated in mediating both long- and short-term synaptic plasticity, respectively (Mackie, 2006; Lu and Mackie, 2016), evident from notable differences in the pharmacokinetic properties of the two ligands. Specifically, AEA acts as a partial agonist for the CB1 receptor, binding with high affinity, but inducing poor intracellular signal transduction (Hillard et al, 1995). AEA activity appears to represent a tonic signal that gates and maintains steady-state (homeostatic) conditions (Hill and Tasker, 2012). Conversely, 2-AG has a relatively low-binding affinity to the CB1 receptor, but produces a robust intracellular response (Hillard et al, 1995). 2-AG activity is highest in response to sustained depolarization and represents a stimulus-induced phasic signal involved in several forms of activity-dependent synaptic plasticity. Importantly, the signaling pathways mediated by AEA and 2-AG likely interact to regulate behavior (Blankman and Cravatt, 2013).
The CB1 receptors are one of the most abundant classes of GPCR, expressed on multiple neuronal populations throughout the brain, with a notable presence on GABAergic and glutamatergic neurons (Marsicano and Lutz, 1999; Hill et al, 2007). In contrast, CB2 receptors are predominantly found in peripheral tissues, with presence in the central nervous system largely localized to microglia (Cabral et al, 2008). In this review, we will focus largely on the CB1 receptor as it is responsible for most eCB signaling in the brain (Matsuda et al, 1990). However, readers are directed to a comprehensive review of the eCB system for information on additional components of the eCB system beyond the scope of this review (Lu and Mackie, 2016). CB1 receptors primarily couple to Gi and Go classes of G-protein (Howlett et al, 2002). As a result, binding induces a local reduction in cyclic adenosine monophosphate (cAMP) and an affiliated decrease in cAMP-dependent protein kinase activity (Vogel et al, 1993; Howlett et al, 2002). In turn, this leads to the activation of A-type potassium channels as well as the inhibition of voltage-gated calcium channels and disruption of the vesicle fusion process (Lovinger, 2008). Together, these downstream effects decrease the probability of neurotransmitter release from the presynaptic membrane, where the majority of CB1 receptors are localized. This mechanism contributes to the neuromodulatory capacity of the eCB system, making the system particularly well suited for the regulation of synaptic transmission in the brain and mediation of numerous forms of plasticity (Kano et al, 2009; Castillo et al, 2012).
Notably, the resultant inhibition of neurotransmitter release provides feedforward inhibition of further post-synaptic synthesis of eCB ligands. At this point, AEA and 2-AG are rapidly removed by a membrane transport process involving the enzymatic breakdown by distinct hydrolyzing enzymes. Specifically, FAAH (Cravatt et al, 1996; Di Marzo, 2011; Fu et al, 2012) breaks down AEA into arachidonic acid and ethanolamine (Ahn et al, 2008). The breakdown of 2-AG involves at least eight participating enzymes; however, the majority of 2-AG degradation in the brain occurs via the activity of MAGL (Ueda et al, 2011), resulting in arachidonic acid and glycerol (Ueda et al, 2011). The localization of these enzymes likely contributes to the nature of signaling by the eCB ligands. For example, FAAH is found post-synaptically, where its presence can contribute to feedback inhibition processes to maintain baseline synaptic regulation by eCB signaling (Häring et al, 2012). Conversely, MAGL is mostly co-localized with CB1 receptors pre-synaptically, where it contributes to the mediation of stimulus-induced 2-AG activity (Häring et al, 2012). Finally, inhibiting the enzymatic activity of FAAH and MAGL can prolong the activity of the eCB ligands (Gaetani et al, 2009). In recent years, targeting these enzymes has been used increasingly as a method of modulating eCB signaling. As will be discussed later in this review, FAAH in particular reflects an important avenue of future research, as disruptions to the normal activity of this enzyme have been linked to dramatic changes in both brain and behavioral measures (Sipe et al, 2002; Chiang et al, 2004; Hariri et al, 2009; Gunduz-Cinar et al, 2013b; Dincheva et al, 2015).
Endocannabinoid system ontogeny
The presence of the eCB system has been detected from the earliest phases of ontogenetic development, when it plays an essential role in neuronal development and circuit connectivity in a number of species, including rodents and humans (Rodríguez de Fonseca et al, 1993; Berrendero et al, 1998; Williams et al, 2003; Aguado et al, 2006; Berghuis et al, 2007; Harkany et al, 2007; Harkany et al, 2008; Mulder et al, 2008; Fride et al, 2009; Zurolo et al, 2010; MacCarrone et al, 2014). In rodents, the ability of eCBs to regulate synaptic transmission emerges around postnatal day (PND) 10 and increases throughout development and into adulthood (Rodríguez de Fonseca et al 1993; Zhu and Lovinger, 2010; Liang et al, 2014). Dynamic alterations occur in the eCB system throughout early life and well into adolescence (Ellgren et al, 2008; Heng et al, 2011; Lee and Gorzalka, 2012; Rubino et al, 2015), when overall enhanced levels of eCB signaling are observed (Figure 1).
Contributing to the heightened activity of the eCB system during adolescence are peaks in the expression of CB1 receptors as well as AEA and 2-AG (Figure 1). In rodents, the highest expression of CB1 receptors is observed at the onset of adolescence (~PND 30), particularly in prefrontal cortex (PFC) and striatum, with a subsequent decline approaching adulthood (~PND 70; Berrendero et al, 1998; Rodríguez de Fonseca et al, 1993; Ellgren et al, 2008; Schneider, 2008; Heng et al, 2011; Klugmann et al, 2011). There is currently limited evidence of how eCB signaling changes across development in humans. Although marked similarities have been observed in the developmental patterns of CB1 receptor expression between humans and rodents, expression within human PFC tissue peaks much earlier (prior to 5 years of age), after which levels gradually decrease until adulthood (Choi et al, 2012; Long et al, 2012). Overall, the available evidence indicates greater variation of the eCB system in early life relative to adulthood across a number of species.
With regard to the two major eCB ligands, AEA levels exhibit a progressive, though somewhat fluctuating, increase from early adolescence to adulthood, while 2-AG levels are high in both early and late adolescence with notably attenuated expression in mid-adolescence (Figure 1; Ellgren et al, 2008; Heng et al, 2011; Lee et al, 2013; Rubino et al, 2015). Fluctuations in AEA expression (Figure 1) have been shown to be relatively consistent across development in corticolimbic regions with prominent eCB activity, including PFC, hippocampus, amygdala, and hypothalamus (Lee et al, 2013). However, AEA and 2-AG levels in nucleus accumbens and striatum appear to exhibit unique developmental trajectories (Ellgren et al, 2008). In rodents, complementary changes in FAAH activity are observed during adolescence, which may contribute to the observed fluctuations in AEA levels (Figure 1; Lee et al, 2013). In humans, overall increases across the life span have been observed in FAAH as well as the AEA synthesizing enzyme N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD; Long et al, 2012). Peaks in FAAH expression during adolescence may indicate heightened levels of regulation over the timing of AEA availability during this period (Long et al, 2012). Less is known about the developmental trajectory of MAGL expression, but evidence in humans shows gradual decreases in MAGL from the first year of life onward, with more rapid decreases following the onset of adolescence (Long et al, 2012). A peak in expression of the 2-AG synthesizing enzyme diacylglycerol lipaseð occurs during adolescence, which when coupled with co-occurring decreases in CB1 receptor expression may reflect an increase in the spatial control of 2-AG activity (Long et al, 2012).
Given the timing of puberty and changes in the eCB system, it is possible that pubertal changes in hormone secretion relate to fluctuations in eCB signaling during adolescence. Sex differences have been observed in the timing of fluctuations in eCB system expression, with CB1 receptor expression levels peaking earlier in females than males (Rodríguez de Fonseca et al, 1993). In both sexes, peak expression occurs just before the onset of puberty, which is ~PND 35 in female rodents and ~PND 40 in male rodents. Likewise, levels of AEA in hypothalamus have also been shown to peak immediately prior to puberty in female rodents (Wenger et al, 2002). Subsequently, eCB activity fluctuates throughout the estrous and menstrual cycles as well (González et al, 2000; Bradshaw et al, 2006; El-Talatini et al, 2010). A close interaction between the endocannabinoid system and gonadal hormones (Murphy et al, 1991; Rodríguez de Fonseca et al, 1994) might contribute to the major developmental changes occurring in the eCB system during pubertal maturation. The eCB system has been shown to modulate the release and activity of gonadal hormones (androgens, estrogen, and progesterone) and gonadotrophins (follicle-stimulating hormone and luteinizing hormone) in humans and rodents alike (Kolodny et al, 1974; Dalterio et al, 1977; Dalterio et al, 1983; Kumar and Chen, 1983; Rodríguez de Fonseca et al, 1994; Wenger et al, 2001; Tsutahara et al, 2011). Overall, eCB activity appears to primarily attenuate the release of gonadal hormones in order to maintain the correct physiological levels (Gorzalka and Dang, 2012). In turn, changes to gonadal hormone functioning can influence eCB signaling through a feedback loop involving the hypothalamus, pituitary, and limbic regions (MacCarrone et al, 2000, 2001; MacCarrone et al, 2003; Nguyen and Wagner, 2006; Gorzalka and Dang, 2012). Taken together, this evidence suggests that the eCB system may contribute to the timing of pubertal onset and play a role in a number of sex-specific behaviors that are regulated by gonadal hormones.
Endocannabinoid regulation of stress, fear, and anxiety
The eCB system has a well-established role in the regulation of fear and anxiety-related behavior in both humans and rodents (Riebe et al, 2012; Ruehle et al, 2012; Higuera-Matas et al, 2015). Generally, enhanced eCB signaling is associated with reductions in conditioned fear and anxiety, whereas inhibiting the eCB system produces the opposite effect (Akirav, 2011; Gunduz-Cinar et al, 2013b; Bluett et al, 2014; Gray et al, 2015). These effects rely in large part on eCB signaling within corticolimbic structures such as PFC, amygdala, and hippocampus (Herkenham et al, 1991; Rubino and Parolaro, 2008; Hill et al, 2010; Campolongo et al, 2011; Hill and Tasker, 2012; Lee and Gorzalka, 2012; Morena et al, 2016). eCB signaling in these regions may be especially important during development, when corticolimbic regions and related emotional behaviors undergo dynamic changes and risk for anxiety disorders increases.
A Sensitive Window for Corticolimbic Circuitry and eCB Signaling
Anxiety disorders are the most common disorders during adolescence (Kessler et al, 2005; Merikangas et al, 2010; Lee et al, 2014), which is a distinctive period for fear learning and corticolimbic circuitry. Corticolimbic regions exhibit substantial changes in their reactivity and connectivity throughout development (Moriceau and Sullivan, 2006; Hare et al, 2008; Gee et al, 2013; Gabard-Durnam et al, 2016; Pattwell et al, 2016; Wu et al, 2016). Consistent with these neurodevelopmental changes, adolescents exhibit unique patterns of anxiety-related behavior and fear learning in rodents and humans alike. Specifically during adolescence, extinction learning for cued fear memory is diminished across species (McCallum et al, 2010; Pattwell et al, 2012). During this same sensitive period, expression of contextual fear is suppressed in rodents (Pattwell et al, 2011). These changes in fear learning during early adolescence correspond to a time of robust corticolimbic eCB changes (Heng et al, 2011).
The regulatory role of the eCB system has been shown to emerge early in life (Trezza et al, 2008; Higuera-Matas et al, 2015). In early development, the highest CB1 receptor expression is observed in amygdala and hippocampus (Rodríguez de Fonseca et al, 1993), which remain prominent areas for eCB signaling in adulthood along with an increased eCB presence in cortex, striatum, and cerebellum (Herkenham et al, 1991; Mackie, 2005; Jutras-Aswad et al, 2009). Though on average CB1 receptor expression peaks during adolescence and attenuates by adulthood, the most robust developmental changes occur in prefrontal and limbic regions, whereas CB1 receptor declines are not observed until later in adolescence in sensorimotor areas (Heng et al, 2011). A similar developmental pattern is observed for the functionality of CB1 receptors in prefrontal and limbic regions, as evidenced by reductions in CB1 receptor-dependent inhibition of synaptic transmission in PFC during the transition from adolescence to adulthood (Heng et al, 2011).
These variations in the regional and temporal expression of components of the eCB system across adolescent development may contribute to the emergence of a sensitive window during early adolescence when corticolimbic circuitry is particularly sensitive to perturbations of eCB signaling. Consistent with this notion, changes in the eCB system, as well as environmental modulation of the eCB system (eg, via acute or chronic stressors) during this period, have been shown to produce long-term effects on stress responses in adulthood and may alter risk for later anxiety. Indeed, CB1 receptor antagonism during adolescence results in increased mobility, an active stress coping behavior, during a forced swim test when tested in adulthood (Lee et al, 2015). Alongside changes in the behavioral stress response, adult mice who experienced CB1 receptor antagonism had reduced AEA in amygdala, increased AEA in hypothalamus, and upregulated cortical CB1 receptor expression (Lee et al, 2015). These rats also exhibited lower corticosterone levels in association with repeated restraint exposure (Lee et al, 2015), in contrast to an increase in circulating corticosterone levels following CB1 receptor antagonism in adult mice (Patel et al, 2004), suggesting that alterations to the eCB system may have different effects on HPA (hypothalamus, anterior pituitary and adrenal cortex; see below) axis reactivity in adolescence vs adulthood. In addition, exposure to CB1 receptor agonists during adolescence produces enduring increases in anxiety-related behavior measured during a number of rodent behavioral tests (Rubino and Parolaro, 2008; Schneider et al, 2005; but see Wegener and Koch, 2009; O’Shea et al, 2006). Perturbations to the maturing system may disrupt the refinement or function of corticolimbic circuits, resulting in long-term behavioral changes. Whereas disruptions in eCB signaling during adolescence may be especially influential, evidence suggests that similar manipulations of the eCB system in adulthood are not as consequential in terms of their neurobiological, cognitive, and behavioral effects (Bambico et al, 2010; Cass et al, 2014; Zamberletti et al, 2014).
Developmental Timing and Endocannabinoid Regulation of the HPA Axis
Normative maturation of the eCB system across adolescence parallels robust changes in patterns of HPA axis stress responding (Rubino et al, 2008; Lee and Gorzalka, 2012; Lee et al, 2015), indicating that eCB changes during development may contribute to developmentally specific patterns of stress reactivity and fear learning. The components of the HPA axis interact to produce and regulate the physiological response to a stressor. In the short term, activation of the neuroendocrine system in response to stressful stimuli is adaptive and necessary for an organism to respond appropriately to potential threat. However, chronically elevated levels of stress hormones may be harmful. Existing evidence implicates eCB activity in regulating basal HPA activity, as well as in the habituation of HPA activity as an organism learns that a stressor is no longer a threat (Hill and Tasker, 2012). In general, eCB signaling inhibits HPA axis activity, contributing to the maintenance of low basal levels of glucocorticoids and restricting HPA axis activity to acute stress (Gorzalka and Hill, 2009). While eCB activity suppresses the release of glucocorticoids, stress and HPA activation can also induce long-term changes in the eCB system, emphasizing the feedback-dependent regulatory role of the eCB system (Gorzalka and Hill, 2009).
HPA axis functioning changes across development, and the effects of stress on eCB signaling and corticolimbic circuitry vary depending on the timing of exposure. Although basal glucocorticoid levels tend to be comparable in adolescence and adulthood (Pignatelli et al, 2006; Romeo and McEwen, 2006; Romeo et al, 2006), habituation of the HPA axis response to a stressor differs with age. For example, adolescent animals require roughly twice as much time to return to basal levels relative to adults following the same stressor (Romeo et al, 2004; Romeo et al, 2005), indicating a greater sensitivity of the HPA axis during adolescence relative to adulthood. In line with these findings, the timing of stress exposure appears to moderate the effects of stress on eCB signaling (Lee and Hill, 2013; Reich et al, 2013). Corticolimbic structures, particularly the basolateral amygdala (BLA), are central to this close relationship between stress and eCB signaling. Indeed, CB1 receptor signaling in BLA has been shown to gate behavioral and neuroendocrine responses to stress- and fear-provoking stimuli (Hill et al, 2009; Gunduz-Cinar et al, 2013b; Bluett et al, 2014; Gray et al, 2015) with additional evidence suggesting that this regulation is mediated specifically by AEA in BLA (Hill and Tasker, 2012). Reductions in levels of AEA in the amygdala have been suggested to facilitate HPA axis responsivity and the generation of stress-related behaviors that can lead to anxiety (Gorzalka and Hill, 2009; Gray et al, 2015). Mechanistically, recent evidence suggests that corticotrophin-releasing hormone (CRH), which is upregulated in the amygdala and PFC in response to chronic corticosteroid or stress exposure, gates stress reactivity through modulation of the eCB system. Elevations in CRH evoke the rapid induction of FAAH, which in turn reduces both AEA and 2-AG levels in amygdala as well as attenuating prefrontal AEA expression (Gray et al, 2015, 2016). In this way, the data of Gray et al (2015, 2016) elucidates an important link between eCB signaling and the effects of stress on anxiety.
Endocannabinoids and the Development of Corticolimbic Circuitry
The eCB system plays a central role in the maturation of corticolimbic circuitry, supporting fundamental processes such as the balance of excitatory and inhibitory neurotransmission. Dynamic synchronized interactions between excitatory and inhibitory cortical signaling during adolescence are necessary for the selectivity of information processing, which influences how inputs from limbic regions to PFC manifests in adolescent fear responding and anxiety (O’Donnell, 2011; Uhlhaas and Singer, 2011). Information from afferents originating in limbic regions including hippocampus, amygdala, and mediodorsal thalamus is integrated into PFC, where it can be used to shape emotional and behavioral responding (Ishikawa and Nakamura, 2003; Sherwood et al, 2010). A subset of these glutamatergic afferents express CB1 receptors, providing a mechanism to enhance input selectivity to cortical neurons (Fortin and Levine, 2007). Furthermore, fine-tuning the selectivity of PFC pyramidal neuronal firing requires inhibitory control from GABAergic interneurons, which is mediated by eCB signaling (Rao et al, 2000; Trettel and Levine, 2002; Fortin et al, 2004; Bartos and Elgueta, 2012). In turn, activation of CB1 receptors expressed in glutamatergic neurons also regulates GABAergic signaling, which is critical to the developmental remodeling of local inhibitory circuits during adolescence (Fortin and Levine, 2007; Tseng et al, 2009).
Across adolescence, marked increases in synaptic pruning further contribute to the increased efficiency and selectivity of information processing (Meyer et al, 1978; Andersen et al, 2000; Nunez et al, 2002; Teicher et al, 1995; Rubia et al, 2006; Toga et al, 2006). In PFC, synaptic pruning occurs to a greater extent at excitatory than inhibitory synapses, resulting in a marked increase in prefrontal synaptic inhibition (Dow-Edwards and Silva, 2017). As a result, the balance of excitation and inhibition in individual neurons and within networks has been shown to differ greatly in adolescents compared to adults (Uhlhaas et al, 2009; Sturman and Moghaddam, 2011). Through continuous homeostatic regulation of both glutamatergic and GABAergic terminals, the eCB system makes critical contributions to the balance of excitation and inhibition as well as the strengthening and elimination of cortical excitatory synaptic connections (Marsicano et al, 2003; Katona and Freund, 2008; Bossong and Niesink, 2010; Lubman et al, 2015). By this mechanism, eCB signaling is believed to contribute to the synchronization of functional activation within and between neural networks, which facilitates the efficiency of information processing in PFC as well as the ability to encode a functional representation of behavioral and cognitive outputs (Freund et al, 2003; Gerdeman and Lovinger, 2003; Buzsáki and Draguhn, 2004; Raver et al, 2013; Sales-Carbonell et al, 2013).
The brain may be especially susceptible to disruptions in eCB signaling during adolescence. A recent study by Cass et al (2014) showed marked decreases in the inhibitory regulation of prefrontal neuronal activity in response to ventral hippocampal inputs in adult rodents that had undergone repeated pharmacological stimulation of the CB1 receptor during early adolescence (PND 35–40). The effect was age-dependent, with similar disinhibition seen in early and mid-adolescence (PND 40–45), but not late adolescence (PND 45–50) or adulthood. The authors attributed their findings in part to a downregulation of local GABAergic transmission in PFC based on the finding that delivery of the GABA-A α1 positive allosteric modulator indiplon during adulthood reversed the disinhibition caused by CB1 receptor stimulation during adolescence. While dynamic changes are occurring in prefrontal GABAergic transmission during adolescence, the brain appears to be particularly susceptible to factors that disrupt this maturational process. Interestingly, the resulting input processing patterns of adult animals exposed to CB1 receptor stimulation during early or mid-adolescence resemble those of a juvenile animal (Thomases et al, 2013), indicating that perturbations of eCB signaling during adolescence may in fact hinder maturation of PFC and its connections.
Similarly, perturbations of eCB signaling during adolescence have been suggested to impede the structural maturation of neuronal circuits in PFC (Renard et al, 2016). For example, CB1 receptor stimulation significantly alters dendritic arborization of pyramidal neurons in layer 2/3 in the medial PFC (Renard et al, 2016). Moreover, blocking eCB activity during adolescence prevented normal developmental decreases in post-synaptic density-95, GluN2A, and GluA2 (Rubino et al, 2015). Given the role of these proteins in the stabilization of excitatory synapses, it may be that eCB tone is required for the pruning of glutamatergic synapses to adult levels (Rubino et al, 2015).
Taken together, these findings highlight a critical role for eCB signaling in mediating the relative levels of excitatory and inhibitory transmission in PFC. By this mechanism, the eCB system is likely to influence the emergence of GABAergic regulation of prefrontal plasticity that has been shown to increase across adolescence (Caballero et al, 2014). The establishment of eCB-mediated regulation of synaptic transmission during adolescence sets the framework for continued cortical regulation throughout adulthood (Auclair et al, 2000). Notably, this regulatory role contributes not only to the maturation of prefrontal neurons and their output capacity, but also to the selective integration of inputs to PFC (Caballero et al, 2016). Long-lasting cognitive and behavioral effects observed in both humans and rodents after perturbation of the eCB system during adolescence have been attributed in part to disruption of normative eCB signaling that regulates PFC maturation (Realini et al, 2009; Caballero and Tseng, 2012; Rubino et al, 2015). In these ways, the eCB system mediates maturational processes in the PFC and its inputs that comprise the corticolimbic circuitry involved in fear regulation, which may be especially important in the context of increased risk for anxiety during adolescence.
Effects of FAAH Genetic Variation on Corticolimbic Circuitry and Anxiety
In recent years, cross-species translational research has provided powerful insight into the role of genetic variation in eCB signaling in both typical and atypical neurobiological and behavioral development. A common polymorphism in the human FAAH gene (C385A; rs324420) has been shown to regulate FAAH enzymatic activity. The variant FAAH A385 allele leads to reduced FAAH activity, resulting in increased levels of AEA (Chiang et al, 2004; Sipe et al, 2002). Consistent with the demonstrated role of the eCB system in anxiety, threat learning, and stress reactivity in rodents (Chhatwal et al, 2005; Gunduz-Cinar et al, 2013a; Gray et al, 2015), the FAAH C385A polymorphism in humans has been associated with variation in neural and behavioral responding to threat and stress (Hariri et al, 2009; Gunduz-Cinar et al, 2013b; Dincheva et al, 2015). Carriers of the A385 allele exhibit reduced amygdala reactivity to threat (Hariri et al, 2009) as well as quicker habituation of amygdala reactivity to threat (Gunduz-Cinar et al, 2013b), possibly due to enhanced eCB signaling. Indeed, AEA activation of the CB1 receptor in BLA is integral to the regulation of behavioral and neuroendocrine responses to stress- and fear-provoking stimuli (Hill et al, 2009; Hill and Tasker, 2012; Gunduz-Cinar et al, 2013b; Bluett et al, 2014; Gray et al, 2015). Thus, while exposure to stress typically results in the rapid mobilization of FAAH to deplete the signaling pool of AEA and increase neuronal excitability in the BLA (Gunduz-Cinar et al, 2013b), individuals expressing the variant FAAH A385 allele may sustain higher levels of AEA under stressful conditions.
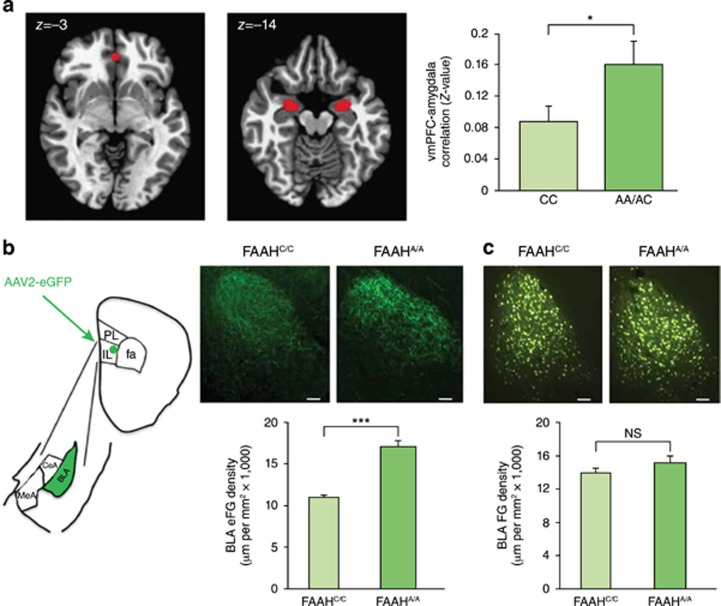
The generation of a knock-in mouse that expresses the variant A allele of the FAAH polymorphism and recapitulates the molecular and biochemical phenotypes of human A allele carriers has allowed for examination of the precise mechanisms by which genetic variation in the eCB system influences anxiety (Dincheva et al, 2015; Figure 2). In both mice and humans during adulthood, carriers of the variant A allele exhibit stronger frontoamygdala functional connectivity (Dincheva et al, 2015), a pattern of connectivity that is associated with reduced anxiety and more effective emotion regulation (Hariri et al, 2003; Urry et al, 2006; Hare et al, 2008; Kim et al, 2011; Burghy et al, 2012). Specifically, A allele carriers showed stronger resting-state functional connectivity between the amygdala and ventromedial PFC, and knock-in mice showed increased projections from infralimbic cortex (IL) to BLA. These effects were specific to this circuit, as there were no effects of genotype on connectivity between the amygdala and dorsal anterior cingulate cortex in humans or on projections from the prelimbic cortex to the BLA in mice. A allele carriers and knock-in mice demonstrated parallel effects of enhanced fear extinction and lower anxiety (Dincheva et al, 2015). The selective increase in connectivity within a circuit important for fear regulation suggests that genetic variation in the FAAH polymorphism may result in a gain of function (ie, lower anxiety) by enhancing top-down control of the amygdala.
Figure 2.
Functional and structural connectivity between ventromedial prefrontal cortex (vmPFC) and amygdala in adult humans and mice with FAAH C385A. (a) fMRI functional connectivity compared between subgenual vmPFC (x, y, z=0, 40, −3) and bilateral amygdala in A-allele carriers (n=17) relative to C homozygotes (n=18). (b) Anterograde tracer (AAV2-eGFP; eGFP), targeted to IL, labeled afferents in BLA in FAAHA/A mice (n=4) and controls (FAAHC/C; n=4). Drawing illustrates anatomical boundaries. (c) Retrograde tracer (fluorogold; FG), targeted to IL, labeled BLA cell bodies in FAAHA/A mice (n=4) and controls (FAAHC/C; n=4). (Scale bars, 100 μm.) Means±SEM. presented. *p<0.05, ***p<0.001. NS, not significant. Reprinted from Dincheva et al (2015), Nature Communications.
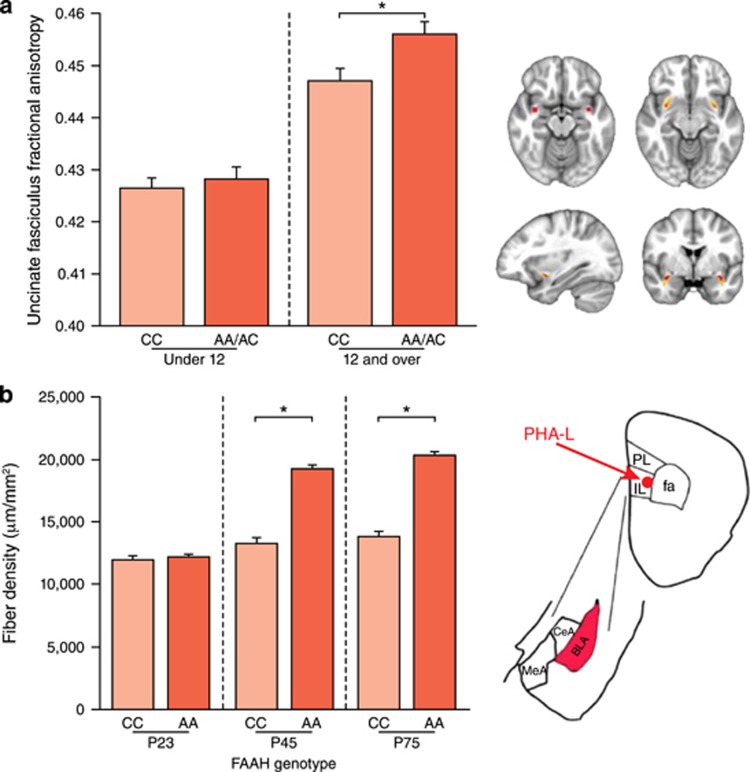
Given that anxiety disorders often emerge during adolescence, when eCB signaling is undergoing dynamic changes (Lee et al, 2016), we tested whether the effects of FAAH genetic variation differed as a function of developmental stage. Converging evidence across humans and mice demonstrated that the genotypic effects of the A allele on frontoamygdala circuitry and anxiety emerge during adolescence (Gee et al, 2016). Adolescents and adults with the A allele displayed increased structural connectivity in the uncinate fasciculus, relative to non-A allele carriers, whereas there were no effects of genotype in children. Consistent with these findings in humans, knock-in mice had increased projections from IL to BLA relative to wild-type mice during adolescence and adulthood, but not during the preadolescent period (Figure 3). Similarly, genotypic effects on anxiety emerged during adolescence across mice and humans, such that A allele carriers had lower anxiety during adolescence and adulthood, but not prior to adolescence. Phenotypic expression of the FAAH polymorphism in humans may vary as a function of developmental changes in eCB signaling, gene expression, and normative maturation of frontoamygdala circuitry. CB1 expression, FAAH activity, and AEA levels are relatively steady during childhood but fluctuate significantly across adolescence (Wenger et al, 2002; Lee and Gorzalka, 2012; Lee et al, 2013), as frontoamygdala circuitry is also undergoing dynamic changes (Hare et al, 2008; Gee et al, 2013; Gabard-Durnam et al, 2016; Wu et al, 2016). During this period of developmental change and as AEA levels begin to wane, the system may be especially sensitive to effects of the A385 allele on FAAH expression. This novel gene by development interaction observed across species provides insight into how risk for anxiety and consequences of stress vary across development (Gee and Casey, 2015) and may contribute to efforts to tailor treatments for anxiety based on genetic variation and developmental stage.
Figure 3.
Phenotypic differences in frontolimbic circuitry resulting from FAAH polymorphism emerge during adolescence in human and mouse. (a, left) Post hoc analyses revealed a significant genotypic effect on uncinate fasciculus (UF) fractional anisotropy in participants 12 years of age and older (n=509; 249 females; F(1,491)=14.02; p=0.0002) but not in those under 12 years of age (n=541; 259 females; F(1,523)=0.513; p=0.474). (a, right) Mask in Montreal Neurological Institute standard space, where UF ascends from the temporal lobe used as the seed region for probabilistic tractography in humans (upper left); UF tract mask is derived from probabilistic tractography averaged across human participants (n=1050). (b, left) Consistent with the findings in humans, a significant genotype by age group interaction (F(2,36)=58.72; p<0.0001) in IL afferent fibers to BLA emerged, such that knock-in mice (AA: n=7 per age) had higher fiber density than WT mice (CC: n=7 per age) during adolescence at age P45 (p<0.0001) and adulthood at P75 (p<0.0001). (b, right) Drawing of anatomical boundaries and anterograde tracer targeted to IL and labeling afferents in BLA. CeA, central amygdala; MeA, medial amygdala; PL, prelimbic; *p<0.05. Reprinted from Gee et al (2016), Proc Natl Acad Sci USA.
Conclusions and future directions
Despite compelling evidence for the important role of the eCB system in brain development and regulating stress and anxiety-related behaviors, the precise mechanisms by which eCB signaling shapes adolescent brain development remain unknown. Delineating the trajectory of normative eCB signaling and how this goes awry following environmental stress will be critical for understanding risk for anxiety disorders during the unique period of adolescence. Given the dynamic and interacting changes across many systems during development, and that the long-term effects of perturbations to normative eCB signaling will vary depending on the timing and duration of exposure, one of the biggest challenges for eCB research is isolating how perturbations in eCB signaling affect the brain during development. A number of recent advances are particularly promising for the rapidly evolving literature on the eCB system. For example, the development of PET ligands enabling the investigation of FAAH binding (Boileau et al, 2015; Boileau et al, 2016; Wang et al, 2016), and the assessment of circulating eCBs (Hill et al, 2009; Hill et al, 2013) are transforming the possibilities for studying eCB signaling in humans. While much of the research on genetic variation has focused on FAAH, there has also been expanded investigation into eCB-related genetic variation related to other eCB genes (eg, Carey et al, 2015). Finally, examining the effects of perturbations to eCB signaling at specific developmental stages and their association with subsequent behaviors that are particularly important during adolescence (eg, social behavior; Doenni et al, 2016) will provide critical insight into sensitive windows and their long-term effects on functioning.
The eCB system contributes to brain development and regulation of stress and anxiety across the life span, and dynamic changes in eCB signaling during adolescence may relate to changes in corticolimbic circuitry and risk for anxiety during this unique developmental period. Increases in eCB signaling specific to adolescence contribute to heightened levels of synaptic plasticity, which may allow adolescents to incorporate information from new experiences, constantly update their representations of the world, and respond in efficient and adaptive ways to salient aspects of their environment. Flexible interactions with the surrounding environment facilitate the acquisition of skills and experiences necessary for achieving independence, such as acquiring resources and engaging in social interactions necessary for survival (Spear, 2010). However, adolescence is also a particularly sensitive period for environmental influences of stress and increased risk for anxiety. Perturbations to the eCB system during this time may disrupt the refinement of cortical circuits and their interactions with limbic regions, resulting in long-term consequences for emotional behavior and stress responding. Cross-species research on developmental changes in eCB signaling and individual differences in eCB-related genetic variation will be critical for understanding the complex role of the eCB system in shaping adolescent brain development and the regulation of stress reactivity and anxiety during a time of substantial change.
Funding and disclosure
The authors declare no conflict of interest.
Acknowledgments
Drs Meyer, Lee, and Gee do not have any financial disclosures or income derived from organizations other than their employer (WCMC, Yale) or government and private funding agencies. This work was supported by a National Institutes of Health Director’s Early Independence Award to DGG (DP5 OD021370) and to FSL a National Institute of Mental Health P50 grant (MH079513), Pritzker Neuropsychiatric Disorders Research Consortium Award, the NewYork Presbyterian Youth Anxiety Center, and a generous gift by the Dr Mortimer D Sackler family.
References
- Aguado T, Palazuelos J, Monory K, Stella N, Cravatt B, Lutz B et al (2006). The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J Neurosci 26: 1551–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K, McKinney MK, Cravatt BF (2008). Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev 108: 1687–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I (2011). The role of cannabinoids in modulating emotional and non-emotional memory processes in the hippocampus. Front Behav Neurosci 5: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH (2000). Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse 37: 167–169. [DOI] [PubMed] [Google Scholar]
- Auclair N, Otani S, Soubrie P, Crepel F (2000). Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J Neurophysiol 83: 3287–3293. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Nguyen NT, Katz N, Gobbi G (2010). Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiol Dis 37: 641–655. [DOI] [PubMed] [Google Scholar]
- Bartos M, Elgueta C (2012). Functional characteristics of parvalbumin- and cholecystokinin-expressing basket cells. J Physiol 590: 669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urbán GM et al (2007). Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science 316: 1212–1216. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Garcia-Gil L, Hernandez ML, Romero JULIAN, Cebeira M, De Miguel R et al (1998). Localization of mRNA expression and activation of signal transduction mechanisms for cannabinoid receptor in rat brain during fetal development. Development 125: 3179–3188. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Sepe N, Ramos JA, Di Marzo V, Fernández-Ruiz JJ (1999). Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse 33: 181–191. [DOI] [PubMed] [Google Scholar]
- Blankman JL, Cravatt BF (2013). Chemical probes of endocannabinoid metabolism. Pharmacol Rev 65: 849–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluett RJ, Gamble-George JC, Hermanson DJ, Hartley ND, Marnett LJ, Patel S (2014). Central anandamide deficiency predicts stress-induced anxiety: behavioral reversal through endocannabinoid augmentation. Transl Psychiatry 4: e408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Tyndale RF, Williams B, Mansouri E, Westwood DJ, Foll BL et al (2015). The fatty acid amide hydrolase C385A variant affects brain binding of the positron emission tomography tracer [11C] CURB. J Cereb Blood Flow Metab 35: 1237–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Mansouri E, Williams B, Le Foll B, Rusjan P, Mizrahi R et al (2016). Fatty acid amide hydrolase binding in brain of cannabis users: imaging with the novel radiotracer [11 C] CURB. Biol Psychiatry 80: 691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossong MG, Niesink RJM (2010). Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog Neurobiol 92: 370–385. [DOI] [PubMed] [Google Scholar]
- Bradshaw HB, Rimmerman N, Krey JF, Walker JM (2006). Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. Am J Physiol 291: R349–R358. [DOI] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA et al (2012). Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci 15: 1736–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A (2004). Neuronal oscillations in cortical networks. Science 304: 1926–1929. [DOI] [PubMed] [Google Scholar]
- Caballero A, Flores-Barrera E, Cass DK, Tseng KY (2014). Differential regulation of parvalbumin and calretinin interneurons in the prefrontal cortex during adolescence. Brain Struct Funct 219: 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Tseng KY (2012). Association of cannabis use during adolescence, prefrontal CB1 receptor signaling, and schizophrenia. Front Pharmacol 3: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Granberg R, Tseng KY (2016). Mechanisms contributing to prefrontal cortex maturation during adolescence. Neurosci Biobehav Rev 70: 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral GA, Raborn ES, Griffin L, Dennis J, Marciano-Cabral F (2008). CB2 receptors in the brain: role in central immune function. Br J Pharmacol 153: 240–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolongo P, Trezza V, Ratano P, Palmery M, Cuomo V (2011). Developmental consequences of perinatal cannabis exposure: behavioral and neuroendocrine effects in adult rodents. Psychopharmacology 214: 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CE, Agrawal A, Zhang B, Conley ED, Degenhardt L, Heath AC et al (2015). Monoacylglycerol lipase (MGLL) polymorphism rs604300 interacts with childhood adversity to predict cannabis dependence symptoms and amygdala habituation: Evidence from an endocannabinoid system-level analysis. J Abnorm Psychol 124: 860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass DK, Flores-Barrera E, Thomases DR, Vital W, Caballero A, Tseng KY (2014). CB1 cannabinoid receptor stimulation during adolescence impairs the maturation of GABA function in the adult rat prefrontal cortex. Mol Psychiatry 19: 536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Younts TJ, Chávez AE, Hashimotodani Y (2012). Endocannabinoid signaling and synaptic function. Neuron 76: 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ (2005). Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology 30: 516–524. [DOI] [PubMed] [Google Scholar]
- Chiang KP, Gerber AL, Sipe JC, Cravatt BF (2004). Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum Mol Genet 13: 2113–2119. [DOI] [PubMed] [Google Scholar]
- Choi K, Le T, McGuire J, Xing G, Zhang L, Li H et al (2012). Expression pattern of the cannabinoid receptor genes in the frontal cortex of mood disorder patients and mice selectively bred for high and low fear. J Psychiatr Res 46: 882–889. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB (1996). Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384: 83–87. [DOI] [PubMed] [Google Scholar]
- Dalterio S, Bartke A, Burstein S (1977). Cannabinoids inhibit testosterone secretion by mouse testes in vitro. Science 196: 1472–1473. [DOI] [PubMed] [Google Scholar]
- Dalterio SL, Mayfield DL, Bartke A (1983). Effects of delta 9-THC on plasma hormone levels in female mice. Subst Alcohol Actions Misuse 4: 339–345. [PubMed] [Google Scholar]
- Di Marzo V (2011). Endocannabinoid signaling in the brain: biosynthetic mechanisms in the limelight. Nat Neurosci 14: 9–15. [DOI] [PubMed] [Google Scholar]
- Dincheva I, Drysdale AT, Hartley CA, Johnson DC, Jing D, King EC et al (2015). FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun 6: 6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doenni VM, Gray JM, Song CM, Patel S, Hill MN, Pittman QJ (2016). Deficient adolescent social behavior following early-life inflammation is ameliorated by augmentation of anandamide signaling. Brain Behav Immun 58: 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow-Edwards D, Silva L (2017). Endocannabinoids in brain plasticity: cortical maturation, HPA axis function and behavior. Brain Res 1654: 157–164. [DOI] [PubMed] [Google Scholar]
- Ellgren M, Artmann A, Tkalych O, Gupta A, Hansen HS, Hansen SH et al (2008). Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur Neuropsychopharmacol 18: 826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Talatini MR, Taylor AH, Konje JC (2010). The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. Fertil Steril 93: 1989–1996. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Berrendero F, Hernandez ML, Ramos JA (2000). The endogenous cannabinoid system and brain development. Trends Neurosci 23: 14–20. [DOI] [PubMed] [Google Scholar]
- Fortin DA, Levine ES (2007). Differential effects of endocannabinoids on glutamatergic and GABAergic inputs to layer 5 pyramidal neurons. Cereb Cortex 17: 163–174. [DOI] [PubMed] [Google Scholar]
- Fortin DA, Trettel J, Levine ES (2004). Brief trains of action potentials enhance pyramidal neuron excitability via endocannabinoid-mediated suppression of inhibition. J Neurophysiol 92: 2105–2112. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D (2003). Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 83: 1017–1066. [DOI] [PubMed] [Google Scholar]
- Fride E, Gobshtis N, Dahan H, Weller A, Giuffrida A, Shabat B (2009). The endocannabinoid system during development: emphasis on perinatal events and delayed effects. Vitam Horm81: 139–158.. [DOI] [PubMed]
- Fu J, Bottegoni G, Sasso O, Bertorelli R, Rocchia W, Masetti M et al (2012). A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat Neurosci 15: 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Gee DG, Goff B, Flannery J, Telzer E, Humphreys KL et al (2016). Stimulus-elicited connectivity influences resting-state connectivity years later in human development: a prospective study. J Neurosci 36: 4771–4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetani S, Dipasquale P, Romano A, Righetti L, Cassano T, Piomelli D et al (2009). The endocannabinoid system as a target for novel anxiolytic and antidepressant drugs. Int Rev Neurobiol85, 57-72. [DOI] [PubMed]
- Gee DG, Casey BJ (2015). The impact of developmental timing for stress and recovery. Neurobiol Stress 1: 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M et al (2013). A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci 33: 4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Fetcho RN, Jing D, Li A, Glatt CE, Drysdale AT et al (2016). Individual differences in frontolimbic circuitry and anxiety emerge with adolescent changes in endocannabinoid signaling across species. Proc Natl Acad Sci USA 113: 4500–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Lovinger DM (2003). Emerging roles for endocannabinoids in long-term synaptic plasticity. Br J Pharmacol 140: 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González S, Bisogno T, Wenger T, Manzanares J, Milone A, Berrendero F et al (2000). Sex steroid influence on cannabinoid CB1 receptor mRNA and endocannabinoid levels in the anterior pituitary gland. Biochem Biophys Res Commun 270: 260–266. [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, Dang SS (2012). Minireview: endocannabinoids and gonadal hormones: bidirectional interactions in physiology and behavior. Endocrinology 153: 1016–1024. [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN (2009). Integration of endocannabinoid signaling into the neural network regulating stress-induced activation of the hypothalamic–pituitary–adrenal axis. Behavioral Neurobiology of the Endocannabinoid System. Springer: Berlin Heidelberg. pp 289-306. [DOI] [PubMed]
- Gray JM, Vecchiarelli HA, Morena M, Lee TTY, Hermanson DJ, Kim AB et al (2015). Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J Neurosci 35: 3879–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JM, Wilson CD, Lee TTY, Pittman QJ, Deussing JM, Hillard CJ et al (2016). Sustained glucocorticoid exposure recruits cortico-limbic CRH signaling to modulate endocannabinoid function. Psychoneuroendocrinology 66: 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O, Hill MN, McEwen BS, Holmes A (2013. a). Amygdala FAAH and anandamide: mediating protection and recovery from stress. Trends Pharmacol Sci 34: 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O, MacPherson KP, Cinar R, Gamble-George J, Sugden K, Williams B et al (2013. b). Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol Psychiatry 18: 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ (2008). Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry 63: 927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häring M, Guggenhuber S, Lutz B (2012). Neuronal populations mediating the effects of endocannabinoids on stress and emotionality. Neuroscience 204: 145–158. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F et al (2009). Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol Psychiatry 66: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR (2003). Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry 53: 494–501. [DOI] [PubMed] [Google Scholar]
- Harkany T, Guzmán M, Galve-Roperh I, Berghuis P, Devi LA, Mackie K (2007). The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci 28: 83–92. [DOI] [PubMed] [Google Scholar]
- Harkany T, Keimpema E, Barabás K, Mulder J (2008). Endocannabinoid functions controlling neuronal specification during brain development. Mol Cell Endocrinol 286(Suppl 1): S84–S90. [DOI] [PubMed] [Google Scholar]
- Heng L, Beverley JA, Steiner H, Tseng KY (2011). Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse 65: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa B, Rice KC (1991). Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 11: 563–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuera-Matas A, Ucha M, Ambrosio E (2015). Long-term consequences of perinatal and adolescent cannabinoid exposure on neural and psychological processes. Neurosci Biobehav Rev 55: 119–146. [DOI] [PubMed] [Google Scholar]
- Hill MN, Bierer LM, Makotkine I, Golier JA, Galea S, McEwen BS et al (2013). Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology 38: 2952–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ et al (2009). Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic–pituitary–adrenal axis. Neuropsychopharmacology 34: 2733–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Miller GE, Carrier EJ, Gorzalka BB, Hillard CJ (2009). Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology 34: 1257–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Karatsoreos IN, Hillard CJ, McEwen BS (2010). Rapid elevations in limbic endocannabinoid content by glucocorticoid hormones in vivo. Psychoneuroendocrinology 35: 1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL, Gallopin T, Férézou I, Cauli B, Rossier J, Schweitzer P et al (2007). Functional CB1 receptors are broadly expressed in neocortical gabaergic and glutamatergic neurons. J Neurophysiol 97: 2580–2589. [DOI] [PubMed] [Google Scholar]
- Hill MN, Tasker JG (2012). Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience 204: 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Wilkison DM, Edgemond WS, Campbell WB (1995). Characterization of the kinetics and distribution of N-arachidonylethanolamine (anandamide) hydrolysis by rat brain. Biochim Biophys Acta 1257: 249–256. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA et al (2002). International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54: 161–202. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S (2003). Convergence and interaction of hippocampal and amygdalar projections within the prefrontal cortex in the rat. J Neurosci 23: 9987–9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras-Aswad D, DiNieri JA, Harkany T, Hurd YL (2009). Neurobiological consequences of maternal cannabis on human fetal development and its neuropsychiatric outcome. Eur Arch Psychiatry Clin Neurosci 259: 395–412. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M (2009). Endocannabinoid-mediated control of synaptic transmission. Physiol Rev 89: 309–380. [DOI] [PubMed] [Google Scholar]
- Katona I, Freund TF (2008). Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med 14: 923–930. [DOI] [PubMed] [Google Scholar]
- Katona I, Freund TF (2012). Multiple functions of endocannabinoid signaling in the brain. Ann Rev Neurosci 35: 529–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry 62: 593–602. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ (2011). Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex 21: 1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugmann M, Klippenstein V, Leweke FM, Spanagel R, Schneider M (2011). Cannabinoid exposure in pubertal rats increases spontaneous ethanol consumption and NMDA receptor associated protein levels. Int J Neuropsychopharmacol 14: 505–517. [DOI] [PubMed] [Google Scholar]
- Kolodny RC, Masters WH, Kolodner RM, Toro G (1974). Depression of plasma testosterone levels after chronic intensive marihuana use. N Engl J Med 290: 872–874. [DOI] [PubMed] [Google Scholar]
- Kumar MS, Chen CL (1983). Effect of an acute dose of delta 9-THC on hypothalamic luteinizing hormone releasing hormone and met-enkephalin content and serum levels of testosterone and corticosterone in rats. Subst Alcohol Actions Misuse 4: 37–43. [PubMed] [Google Scholar]
- Lee TT-Y, Gorzalka BB (2012). Timing is everything: evidence for a role of corticolimbic endocannabinoids in modulating hypothalamic–pituitary–adrenal axis activity across developmental periods. Neuroscience 204: 17–30. [DOI] [PubMed] [Google Scholar]
- Lee TTY, Hill MN (2013). Age of stress exposure modulates the immediate and sustained effects of repeated stress on corticolimbic cannabinoid CB1 receptor binding in male rats. Neuroscience 249: 106–114. [DOI] [PubMed] [Google Scholar]
- Lee TT-Y, Hill MN, Hillard CJ, Gorzalka BB (2013). Temporal changes in N-acylethanolamine content and metabolism throughout the peri-adolescent period. Synapse 67: 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Heimer H, Giedd JN, Lein ES, Šestan N, Weinberger DR et al (2014). Adolescent mental health—opportunity and obligation. Science 346: 547–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TT-Y, Hill MN, Hillard CJ, Gorzalka BB (2015). Disruption of peri-adolescent endocannabinoid signaling modulates adult neuroendocrine and behavioral responses to stress in male rats. Neuropharmacology 99: 89–97. [DOI] [PubMed] [Google Scholar]
- Lee TT-Y, Hill MN, Lee FS (2016). Developmental regulation of fear learning and anxiety behavior by endocannabinoids: endocannabinoids, development and anxiety. Genes Brain Behav 15: 108–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S-L, Alger BE, McCarthy MM (2014). Developmental increase in hippocampal endocannabinoid mobilization: role of metabotropic glutamate receptor subtype 5 and phospholipase C. J Neurophysiol 112: 2605–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long LE, Lind J, Webster M, Weickert CS (2012). Developmental trajectory of the endocannabinoid system in human dorsolateral prefrontal cortex. BMC Neurosci 13: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM (2008). Presynaptic modulation by endocannabinoids. In: Südhof TC, Starke K (eds). Pharmacology of Neurotransmitter Release. Springer: Berlin Heidelberg, pp 435-477. [DOI] [PubMed]
- Lu H-C, Mackie K (2016). An introduction to the endogenous cannabinoid system. Biol Psychiatry 79: 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman DI, Cheetham A, Yücel M (2015). Cannabis and adolescent brain development. Pharmacol Ther 148: 1–16. [DOI] [PubMed] [Google Scholar]
- MacCarrone M, Bari M, Rienzo MD, Finazzi-Agrò A, Rossi A (2003). Progesterone activates fatty acid amide hydrolase (FAAH) promoter in human T lymphocytes through the transcription factor ikaros. Evidence for a synergistic effect of leptin. J Biol Chem 278: 32726–32732. [DOI] [PubMed] [Google Scholar]
- MacCarrone M, De Felici M, Bari M, Klinger F, Siracusa G, Finazzi-Agrò A (2000). Down-regulation of anandamide hydrolase in mouse uterus by sex hormones. Eur J Bioche 267: 2991–2997. [DOI] [PubMed] [Google Scholar]
- MacCarrone M, Valensise H, Bari M, Lazzarin N, Romanini C, Finazzi-Agrò A (2001). Progesterone up-regulates anandamide hydrolase in human lymphocytes: role of cytokines and implications for fertility. J Immunol 166: 7183–7189. [DOI] [PubMed] [Google Scholar]
- MacCarrone M, Guzmán M, Mackie K, Doherty P, Harkany T (2014). Programming of neural cells by (endo)cannabinoids: from physiological rules to emerging therapies. Nat Rev Neurosci 15: 786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K (2005). Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol 168: 299–325. [DOI] [PubMed] [Google Scholar]
- Mackie K (2006). Mechanisms of CB1 receptor signaling: endocannabinoid modulation of synaptic strength. Int J Obes 30: S19–S23. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A et al (2003). CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302: 84–88. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B (1999). Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci 11: 4213–4225. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI (1990). Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature346: 561–564.. [DOI] [PubMed]
- McCallum J, Kim JH, Richardson R (2010). Impaired extinction retention in adolescent rats: effects of D-cycloserine. Neuropsychopharmacology 35: 2134–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA (2013). The endocannabinoid system and the brain. Ann Rev Psychol 64: 21–47. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He J, Burstein M, Swanson SA, Avenevoli S, Cui L et al (2010). Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Study-Adolescent Supplement (NCS-A). J Am Acad Child Adoles Psychiatry 49: 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Ferres-Torres R, Mas M (1978). The effects of puberty and castration on hippocampal dendritic spines of mice: a Golgi study. Brain Res 155: 108–112. [DOI] [PubMed] [Google Scholar]
- Morena M, Patel S, Bains JS, Hill MN (2016). Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology 41: 80–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM (2006). Maternal presence serves as a switch between learning fear and attraction in infancy. Nat Neurosci 9: 1004–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder J, Aguado T, Keimpema E, Barabás K, Rosado CJB, Nguyen L et al (2008). Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc Natl Acad Sci 105: 8760–8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy LL, Rodriguez de Fonseca F, Steger RW (1991). delta 9-Tetrahydrocannabinol antagonism of the anterior pituitary response to estradiol in immature female rats. Steroids 56: 97–102. [DOI] [PubMed] [Google Scholar]
- Nguyen QH, Wagner EJ (2006). Estrogen differentially modulates the cannabinoid-induced presynaptic inhibition of amino acid neurotransmission in proopiomelanocortin neurons of the arcuate nucleus. Neuroendocrinology 84: 123–137. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Sodhi J, Juraska JM (2002). Ovarian hormones after postnatal day 20 reduce neuron number in the rat primary visual cortex. J Neurobiol 52: 312–321. [DOI] [PubMed] [Google Scholar]
- O’Donnell P (2011). Adolescent onset of cortical disinhibition in schizophrenia: insights from animal models. Schizophr Bull 37: 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea M, McGregor IS, Mallet PE (2006). Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar long-lasting deficits in object recognition and reduced social interaction in rats. J Psychopharmacol 20: 611–621. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ (2004). Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology 145: 5431–5438. [DOI] [PubMed] [Google Scholar]
- Pattwell SS, Bath KG, Casey BJ, Ninan I, Lee FS (2011). Selective early-acquired fear memories undergo temporary suppression during adolescence. Proc Natl Acad Sci 108: 1182–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD et al (2012). Altered fear learning across development in both mouse and human. Proc Natl Acad Sci 109: 16318–16323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Liston C, Jing D, Ninan I, Yang RR, Witztum J et al (2016). Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nat Commun 7: 11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli D, Xiao F, Gouveia AM, Ferreira JG, Vinson GP (2006). Adrenarche in the rat. J Endocrinol 191: 301–308. [DOI] [PubMed] [Google Scholar]
- Piomelli D (2003). The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4: 873–884. [DOI] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS (2000). Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci 20: 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver SM, Haughwout SP, Keller A (2013). Adolescent cannabinoid exposure permanently suppresses cortical oscillations in adult mice. Neuropsychopharmacology 38: 2338–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Realini N, Rubino T, Parolaro D (2009). Neurobiological alterations at adult age triggered by adolescent exposure to cannabinoids. Pharmacol Res 60: 132–138. [DOI] [PubMed] [Google Scholar]
- Reich CG, Mihalik GR, Iskander AN, Seckler JC, Weiss MS (2013). Adolescent chronic mild stress alters hippocampal CB1 receptor-mediated excitatory neurotransmission and plasticity. Neuroscience 253: 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard J, Vitalis T, Rame M, Krebs M-O, Lenkei Z, Le Pen G et al (2016). Chronic cannabinoid exposure during adolescence leads to long-term structural and functional changes in the prefrontal cortex. Eur Neuropsychopharmacol 26: 55–64. [DOI] [PubMed] [Google Scholar]
- Riebe CJ, Pamplona F, Kamprath K, Wotjak CT (2012). Fear relief—toward a new conceptual frame work and what endocannabinoids gotta do with it. Neuroscience 204: 159–185. [DOI] [PubMed] [Google Scholar]
- Rodríguez de Fonseca F, Cebeira M, Ramos JA, Martín M, Fernández-Ruiz JJ (1994). Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci 54: 159–170. [DOI] [PubMed] [Google Scholar]
- Rodríguez de Fonseca FR, Ramos JA, Bonnin A, Fernández-Ruiz JJ (1993). Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport 4: 135–138. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, Chhua N, McPherson CR, McEwen BS (2004). Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology 79: 125–132. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, McEwen BS (2005). Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology 80: 387–393. [DOI] [PubMed] [Google Scholar]
- Romeo RD, McEwen BS (2006). Stress and the adolescent brain. Ann NY Acad Sci 1094: 202–214. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD et al (2006). Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology 147: 1664–1674. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E et al (2006). Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Map 27: 973–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Parolaro D (2008). Long lasting consequences of cannabis exposure in adolescence. Mol Cell Endocrinol 286: S108eS113. [DOI] [PubMed] [Google Scholar]
- Rubino T, Parolaro D (2016). The impact of exposure to cannabinoids in adolescence: insights from animal models. Biol Psychiatry 79: 578–585. [DOI] [PubMed] [Google Scholar]
- Rubino T, Prini P, Piscitelli F, Zamberletti E, Trusel M, Melis M et al (2015). Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiol Dis 73: 60–69. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Castiglioni C, Guidali C, Viganó D, Marras E et al (2008). Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb Cortex 18: 1292–1301. [DOI] [PubMed] [Google Scholar]
- Ruehle S, Rey AA, Remmers F, Lutz B (2012). The endocannabinoid system in anxiety, fear memory and habituation. J Psychopharmacol 26: 23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales-Carbonell C, Rueda-Orozco PE, Soria-Gómez E, Buzsáki G, Marsicano G, Robbe D (2013). Striatal GABAergic and cortical glutamatergic neurons mediate contrasting effects of cannabinoids on cortical network synchrony. Proc Natl Acad Sci 110: 719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M (2008). Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict Biol 13: 253–263. [DOI] [PubMed] [Google Scholar]
- Schneider M, Drews E, Koch M (2005). Behavioral effects in adult rats of chronic prepubertal treatment with the cannabinoid receptor agonist WIN 55,212-2. Behav Pharmacol 16: 447–453. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Raghanti MA, Stimpson CD, Spocter MA, Uddin M, Boddy AM et al (2010). Inhibitory interneurons of the human prefrontal cortex display conserved evolution of the phenotype and related genes. Proc R Soc Lond B Biol Sci 277: 1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF (2002). A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci 99: 8394–8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LThe Behavioral Neuroscience of Adolescence. WW Norton & Company: New York, NY, 2010. [Google Scholar]
- Sturman DA, Moghaddam B (2011). The neurobiology of adolescence: changes in brain architecture, functional dynamics, and behavioral tendencies. Neurosci Biobehav Rev 35: 1704–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC Jr (1995). Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res 89: 167–172. [DOI] [PubMed] [Google Scholar]
- Thomases DR, Cass DK, Tseng KY (2013). Periadolescent exposure to the NMDA receptor antagonist MK-801 impairs the functional maturation of local GABAergic circuits in the adult prefrontal cortex. J Neurosci 33: 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER (2006). Mapping brain maturation. Trends Neurosci 29: 148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trettel J, Levine ES (2002). Cannabinoids depress inhibitory synaptic inputs received by layer 2/3 pyramidal neurons of the neocortex. J Neurophysiol 88: 534–539. [DOI] [PubMed] [Google Scholar]
- Trezza V, Cuomo V, Vanderschuren LJ (2008). Cannabis and the developing brain: insights from behavior. Eur J Pharmacol 585: 441–452. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK (2009). The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res 204: 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutahara NM, Weems YS, Arreguin-Arevalo JA, Nett TM, LaPorte ME, Uchida J et al (2011). Effects of endocannabinoid 1 and 2 (CB1; CB2) receptor agonists on luteal weight, circulating progesterone, luteal mRNA for luteinizing hormone (LH) receptors, and luteal unoccupied and occupied receptors for LH in vivo in ewes. Prostagland Other Lipid Mediat 94: 17–24. [DOI] [PubMed] [Google Scholar]
- Ueda N, Tsuboi K, Uyama T, Ohnishi T (2011). Biosynthesis and degradation of the endocannabinoid 2-arachidonoylglycerol. BioFactors 37: 1–7. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E (2009). The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc Natl Acad Sci 106: 9866–9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W (2011). The development of neural synchrony and large-scale cortical networks during adolescence: relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophr Bull 37: 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS et al (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci 26: 4415–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel Z, Barg J, Levy R, Saya D, Heldman E, Mechoulam R (1993). Anandamide, a brain endogenous compound, interacts specifically with cannabinoid receptors and inhibits adenylate cyclase. J Neurochem 61: 352–355. [DOI] [PubMed] [Google Scholar]
- Wang L, Mori W, Cheng R, Yui J, Hatori A, Ma L et al (2016). Synthesis and preclinical evaluation of sulfonamido-based [11C-Carbonyl]-carbamates and ureas for imaging monoacylglycerol lipase. Theranostics 6: 1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener N, Koch M (2009). Behavioural disturbances and altered Fos protein expression in adult rats after chronic pubertal cannabinoid treatment. Brain Res 1253: 81–91. [DOI] [PubMed] [Google Scholar]
- Wenger T, Gerendai I, Fezza F, González S, Bisogno T, Fernandez-Ruiz J et al (2002). The hypothalamic levels of the endocannabinoid, anandamide, peak immediately before the onset of puberty in female rats. Life Sci 70: 1407–1414. [DOI] [PubMed] [Google Scholar]
- Wenger T, Ledent C, Csernus V, Gerendai I (2001). The central cannabinoid receptor inactivation suppresses endocrine reproductive functions. Biochem Biophys Res Commun 284: 363–368. [DOI] [PubMed] [Google Scholar]
- Williams E-J, Walsh FS, Doherty P (2003). The FGF receptor uses the endocannabinoid signaling system to couple to an axonal growth response. J Cell Biol 160: 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Kujawa A, Lu LH, Fitzgerald DA, Klumpp H, Fitzgerald KD et al (2016). Age-related changes in amygdala–frontal connectivity during emotional face processing from childhood into young adulthood. Hum Brain Map 37: 1684–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamberletti E, Beggiato S, Steardo L Jr, Prini P, Antonelli T, Ferraro L et al (2014). Alterations of prefrontal cortex GABAergic transmission in the complex psychotic-like phenotype induced by adolescent delta-9-tetrahydrocannabinol exposure in rats. Neurobiol Dis 63: 35–47. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM (2010). Developmental alteration of endocannabinoid retrograde signaling in the hippocampus. J Neurophysiol 103: 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurolo E, Iyer AM, Spliet WGM, Van Rijen PC, Troost D, Gorter JA et al (2010). CB1 and CB2 cannabinoid receptor expression during development and in epileptogenic developmental pathologies. Neuroscience 170: 28–41. [DOI] [PubMed] [Google Scholar]