The United States is in the grip of an opioid misuse and overdose crisis. Progression from misuse to opioid use disorder (OUD) is marked by compulsive drug-taking and clinically significant impairment. Behavioral interventions along with treatment with OUD pharmacotherapeutics, such as buprenorphine, mitigates withdrawal, reduces mortality, opioid intake, and opioid-seeking behaviors, as well as improves psychosocial functioning (Volkow et al, 2014). Medication-assisted treatment is an important adjunct to the proper management of OUD patients, yet additional opportunities exist to enhance the probability of extended abstinence and recovery from OUD.
Dysregulation of the limbic-corticostriatal circuitry that subserves reward and adaptive behaviors contributes to OUD development as well as the vulnerability to relapse during abstinence. Serotonin (5-HT) neurotransmission confers modulatory control over this circuitry, particularly through the 5-HT2C receptor (5-HT2CR) (for review) (Cunningham and Anastasio, 2014). Selective 5-HT2CR agonists, which lack intrinsic abuse liability, curb self-administration of psychostimulants (eg, cocaine, nicotine) as well as associated sensitivity to drug-associated cues and impulsivity in preclinical studies (for review) (Cunningham and Anastasio, 2014). These data sparked a clinical trial that demonstrated that the FDA-approved anti-obesity medication and first-in-class 5-HT2CR agonist lorcaserin (Belviq) increased abstinence from smoking (Shanahan et al, 2016). Furthermore, the efficacy and safety of lorcaserin for cocaine use disorder is presently under study (https://clinicaltrials.gov/ct2/show/NCT03007394). We have recently demonstrated the efficacy of lorcaserin to suppress intake of the semisynthetic opioid oxycodone as well as associated cue reactivity in both abstinence and extinction-reinstatement paradigms, effects which were blocked by the selective 5-HT2CR antagonist SB242084 (Neelakantan et al, 2017). Lorcaserin pretreatment also decreases opioid-induced behavioral sensitization and the physical signs of naloxone-precipitated withdrawal following chronic opioid exposure in mice (Wu et al, 2015). Thus, the non-opioid medication lorcaserin acts as a 5-HT2CR agonist to influence aspects of opioid-evoked behaviors, suggesting that the 5-HT2CR system may play a key role in the shared mechanisms for addiction and relapse vulnerability across psychostimulant and opioid drug classes.
The development of pain medications with analgesic efficacy, but reduced abuse liability, remains a high priority. Of equally high priority is the identification of therapeutic interventions that increase the maintenance of abstinence after cessation of opioid intake, even in high drug cue environments. A selective 5-HT2CR agonist, such as lorcaserin, may provide a new avenue to add value to the outcomes of medication-assisted treatment in OUD (Figure 1). The next step is to expand the present data set to definitively test the hypotheses that lorcaserin inhibits opioid withdrawal and countermands stress- and/or opioid-triggered relapse events, for example. It is also possible that low doses of lorcaserin administered in combination with a partial μ-opioid agonist, with limited abuse liability but efficacy to suppress opioid-induced euphoria and withdrawal may reduce relapse risk via regulation of two signaling pathways. The neurochemical mechanisms and sites of action for 5-HT2CR systems to control opioid-related behaviors are also of interest. Finally, preclinical studies to evaluate low-dose combinations of a partial μ-opioid agonist plus lorcaserin will provide insight into the potential for translational value in clinical trials geared to reduce the devastation of opioid overdose and OUD.
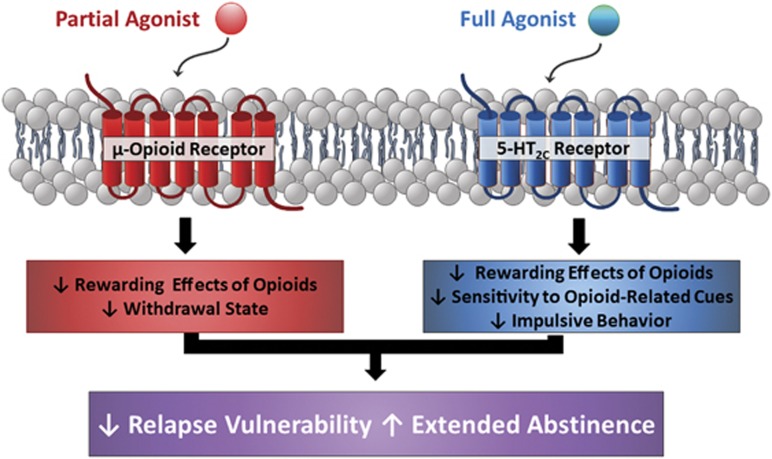
Figure 1.
Either a μ-opioid receptor partial agonist, a full 5-HT2C receptor (5-HT2CR) agonist, or a combination may afford gains in reducing relapse vulnerability and extending abstinence in opioid use disorder (OUD). Partial agonist actions at the μ-opioid receptor reduce the rewarding effects of opioids and withdrawal. The selective 5-HT2CR full agonist lorcaserin suppresses oxycodone intake and associated cue reactivity as well as impulsivity. Low-dose combinations of a μ-opioid receptor partial agonist plus the non-opioid lorcaserin may provide an additional new avenue to support recovery in OUD patients. While the brain locus for a potential receptor–receptor interaction is unknown, both the μ-opioid receptor and 5-HT2CR are co-expressed in nodes of the limbic-corticostriatal circuitry engaged in drug reward and relapse vulnerability. The FDA-approved OUD medication buprenorphine is a μ-opioid receptor partial agonist, but is not selective given its complex actions at δ-, κ-, and nociceptin/opioid receptor-like receptors (NOP or ORL-1) (Lutfy and Cowan, 2004). Thus, other μ-opioid partial agonists may be needed to test the hypothesis that low-dose combinations with lorcaserin may add value in OUD medication-assisted therapy.
Funding and disclosure
Drs Moeller and Cunningham are supported by grants NIDA U54 DA038999 and NIDA P50 DA033935. Dr Moeller is an uncompensated consultant for INDIVIOR and receives research support from Boehringer-Ingelheim. Dr Cunningham declares no conflict of interest.
Acknowledgments
We thank Ms Christina Merritt for the design of the figure.
References
- Cunningham KA, Anastasio NC (2014). Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology 76 Pt B: 460–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Cowan A (2004). Buprenorphine: a unique drug with complex pharmacology. Curr Neuropharmacol 2: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakantan H, Holliday ED, Fox RG, Stutz SJ, Comer SD, Haney M et al (2017). Lorcaserin suppresses oxycodone self-administration and relapse vulnerability in rats. ACS Chem Neurosci 8: 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan WR, Rose JE, Glicklich A, Stubbe S, Sanchez-Kam M (2016). Lorcaserin for smoking cessation and associated weight gain: a randomized 12-week clinical trial. Nicotine Tob Res 2016 Nov 4. ppi: ntw301. (e-pub ahead of print). [DOI] [PubMed]
- Volkow ND, Frieden TR, Hyde PS, Cha SS (2014). Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med 370: 2063–2066. [DOI] [PubMed] [Google Scholar]
- Wu X, Pang G, Zhang YM, Li G, Xu S, Dong L et al (2015). Activation of serotonin 5-HT(2C) receptor suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in heroin-treated mice. Neurosci Lett 607: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]