Abstract
Hepatitis C virus (HCV) infection is a major problem worldwide. HCV is not limited to liver disease but is frequently complicated by immune-mediated extrahepatic manifestations such as glomerulonephritis or vasculitis. A fatal complication of HCV-associated vascular disease is thrombosis. Polyriboinosinic:polyribocytidylic acid (poly (I:C)), a synthetic analog of viral RNA, induces a Toll-like receptor 3 (TLR3)-dependent arteriolar thrombosis without significant thrombus formation in venules in vivo. These procoagulant effects are caused by increased endothelial synthesis of tissue factor and PAI-1 without platelet activation. In addition to human umbilical endothelial cells (HUVEC), human mesangial cells (HMC) produce procoagulatory factors, cytokines and adhesion molecules after stimulation with poly (I:C) or HCV-containing cryoprecipitates from a patient with a HCV infection as well. Activated protein C (APC) is able to prevent the induction of procoagulatory factors in HUVEC and HMC in vitro and blocks the effects of poly (I:C) and HCV-RNA on the expression of cytokines and adhesion molecules in HMC but not in HUVEC. In vivo, protein C inhibits poly (I:C)-induced arteriolar thrombosis. Thus, endothelial cells are de facto able to actively participate in immune-mediated vascular thrombosis caused by viral infections. Finally, we provide evidence for the ability of protein C to inhibit TLR3-mediated arteriolar thrombosis caused by HCV infection.
Keywords: hepatitis C, thrombosis, toll-like receptor 3
INTRODUCTION
Infection with the hepatitis C virus (HCV) is a major problem worldwide. HCV infection is responsible for more than one million deaths per year resulting from liver cirrhosis and primary liver cancer.1 In addition, HCV infection is frequently complicated by a variety of autoimmune processes with extrahepatic manifestations, including glomerulonephtitis,2 cryoglobulinemia3, 4 and vasculitis,3, 4, 5, 6 which contribute significantly to morbidity and mortality. A severe complication of vasculitis is the occurrence of thrombotic blood vessel occlusions. Many viral infections are associated with the increased expression of procoagulatory factors, and direct or indirect activation of the endothelium by viruses results in alterations of the coagulation and the fibrinolytic systems.7 Thrombotic capillary occlusions lead to an activation of inflammatory processes, which in turn aggravate the course of vasculitis. Severe organ damage may result in the areas supplied by the involved blood vessels. Until now, therapeutic options have been very limited.
Toll-like receptors (TLRs) are an essential part of the innate immune system. TLRs recognize molecular patterns associated with microbial pathogens and induce an immune response.8 TLR3 specifically binds double-stranded RNA (dsRNA) of viral origin and polyriboinosinic:polyribocytidylic acid (poly (I:C)), a synthetic analog of viral dsRNA.9 Here, we demonstrate that activation of TLR3 causes arteriolar thrombosis in vivo. We investigated the mechanisms underlying this observation by analyzing the synthesis of procoagulatory factors in endothelial cells and platelet–endothelial interactions after stimulation with poly (I:C) and HCV-RNA isolated from patients infected with the HCV.
MATERIALS AND METHODS
Chemicals
Poly (I:C) was purchased from InvivoGen (San Diego, CA,USA), Ceprotin 1000 I.E. from Baxter Deutschland GmbH (Unterschleißheim, Germany), APC and fluorescein isothiocyanate (FITC)-dextran from Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany), and Fluoresbrite YG Carboxylate Microspheres 0.5 μm from Polysciences Europe GmbH (Eppelheim, Germany). All antibodies were purchased from BD Biosciences (San Jose, CA, USA): APC-Mouse anti-human CD62P-IgG1, APC-Mouse-IgG1-Isotyp-Control, FITC-anti-human PAC-1. ADP, thrombin-receptor activated peptide (TRAP-6) and collagen were purchased from Roche Diagnostics International (Risch-Rotkreuz, Switzerland). The cell culture media were from Sigma-Aldrich Chemie GmbH and the salts were from Applichem (Darmstadt, Germany).
In vivo experiments in mice
Animals and anesthesia
For animal experiments, we used wild-type C57BL/6 mice and TLR3−/− mice, which were purchased from Charles River, Sulzfeld, Germany. Short-term anesthesia was initiated by a single i.p. injection of 5 mg/kg midazolam (Ratiopharm, Ulm, Germany), 0.05 mg/kg fentanyl (CuraMED Pharma, Karlsruhe, Germany) and 0.5 mg/kg medetomidine hydrochloride (Pfizer, Berlin, Germany; produced by Orion Pharma, Espoo, Finland) diluted in 0.9% NaCl as described.10 The anesthesia was monitored by evaluating the response to pain. All surgical procedures were performed under these conditions. At the end of the experiments, the animals were killed by an overdose (2 g/kg) of sodium pentobarbital (Merial GmbH, Hallbergmoos, Germany). All experiments were conducted in accordance with the German animal protection law and approved by the district government of Upper Bavaria (approval reference number AZ 55.2-1-54-2532-172-13). The research conforms to Directive 2010/63/EU of the European Parliament. All experiments were approved by the University Ethics Review Board (Ethikkommission der Medizinischen Fakultät der Ludwig-Maximilians-Universität).
Intravital microscopy and surgical procedures for the cremaster muscle model
The cremaster muscle model was used to investigate thrombus formation in microcirculation in vivo. The surgery was performed as described previously.11 After anesthesia of wild-type or TLR3−/− C57BL/6 mice, all surgical procedures were conducted on a thermo-controlled plexi-glass stage to maintain body temperature at 37 °C with microscope cover slips. First, the left carotid artery was cannulated with a plastic catheter (0.28 mm ID, 0.61 mm AD; SIMS Portex, Hythe, UK) for taking blood samples to measure blood counts and for the application of several drugs and other solutions. Intravital fluorescence microscopy was performed using a modified microscope (Zeiss Axiotech Vario, Carl Zeiss Microscopy GmbH, München, Germany). Images were recorded with a digital camera (AxioCam HSm) and analyzed with AxioVision Rel. 4.6 (Carl Zeiss Microscopy GmbH).
Intravital assessment of arteriolar thrombosis
The cremaster muscle model was used to investigate arteriolar thrombosis in vivo by initiating thrombus formation using the light dye injury model.12 After the surgical procedures were finished, one to two arterioles per cremaster with a diameter of ~60 μm were chosen and the blood flow was measured by applying fluorescent beads (Fluoresbrite YG Carboxylate Microspheres 0.5 μm). The mean blood velocity (V-mean) was calculated as V-max/2 assuming laminar flow in the investigated sections of the arterioles. The wall shear rate (γ) was calculated based on the Newtonian definition: γ=8V-mean/diameter. Then, FITC-dextran (average mol. wt. 150 000 from Sigma-Aldrich Chemie GmbH) at a concentration of 5% in PBS+ was administered in doses of 1 μl/g mouse via the carotid artery catheter to perform fluorescent microscopy. Light with a wavelength of 450–490 nm was used to excite the fluorescent dye, which led to the emission of fluorescent light and to a release of reactive chemical molecules such as reactive oxygen species that were responsible for the injury of the endothelial cell layer. Two endpoints were defined: first, the onset of thrombus formation and second the time until total cessation of blood flow (occlusion time). The mice were treated 24 h before the start of the experiment with poly (I:C) 200 μg i.p. Ceprotin 1000 I.E. was administered via the carotid artery catheter in a dose of 1 μl/g mouse 30 min prior to initiation of the light dye injury model.
Mouse blood cell counts
Mouse blood was collected via a carotid catheter and EDTA used as an anti-coagulant. Blood cell counts were measured using a Beckman Coulter Counter (Beckman Coulter, München, Germany).
In vitro experiments in human endothelial and mesangial cells
Cell culture
Human umbilical vein endothelial cells (HUVEC) were isolated and cultured as previously described13 according to the Declaration of Helsinki. The procedure was approved by the University Ethics Review Board (Ethikkommission der Medizinischen Fakultät der Ludwig-Maximilians-Universität). Written informed consent for the collection and generation of the cell lines was obtained. Human microvascular endothelial cells (HMEC) were provided by Ades et al.14 and were cultured in M199 media supplement with 10% fetal calf serum, 10% endothelial growth media (PromoCell, Heidelberg, Germany) and 1% penicillin/streptomycin as described previously.15 Human mesangial cells (HMC) were cultivated as described.16
Preparation of HCV RNA
HCV-RNA containing cryoprecipitates were isolated from a patient with a HCV-associated mixed cryoglobulinemia with a high viral load during routine plasmapheresis treatment and centrifuged as described previously.17 The procedure was approved by the University Ethics Review Board (Ethikkommission der Medizinischen Fakultät der Ludwig-Maximilians-Universität). Written informed consent for the isolation of HCV-RNA-containing cryoprecipitates was obtained. The concentration of HCV used for stimulation was 100 × 106 geq/ml, confirmed by reverse transcription–PCR (RT–PCR). For HCV stimulation, confluent HMECs in six-well plates were used; once the virus was added, the plates were centrifuged at 1000g for 45 min to allow for efficient viral infection. Subsequent stimulation was performed as indicated.
In vitro experiments on human platelets, whole blood and PRP
Clotting assay
To assess the influence of prothrombotic molecules, the clotting time (CT) of human whole blood after the administration of cell lysate material was analyzed using thromboelastometry (ROTEM delta, Tem Innovations GmbH, München, Germany), as described.10 HMECs were stimulated with poly (I:C) or tumor necrosis factor alpha (TNFα) (positive control) as indicated, then the cells were lysed with 15 μM n-Octyl-β-D-glucopyranosid and centrifuged at 1200g for 10 min at 4 °C. The supernatants were used as activators of coagulation. Human whole blood samples were treated with 10% Na-citrate to prevent coagulation. After administration of 20 μl CaCl2 and 20 μl of cell lysate material, the time until clot development (CT) was measured.
Platelet aggregation
To assess the platelet aggregation in human platelet-rich plasma (PRP), we used the turbidimetric method described by Born.18 Human PRP was isolated by centrifugation of citrated whole blood samples of healthy human volunteers at 150g. The procedure was approved by the University Ethics Review Board (Ethikkommission der Medizinischen Fakultät der Ludwig-Maximilians-Universität). Written informed consent was obtained from platelet donors. For the photometric assessment of platelet aggregation, a two-channel aggregometer (ChronoLog 490-2D, Havertown, PA, USA) was used. Platelet aggregation was initiated by the administration of ADP, TRAP-6 and collagen under continuous stirring at 1000 r.p.m. at 37 °C. Written consent was obtained from the platelet donors.
Assessment of plasmatic coagulation
Parameters of the plasmatic coagulation were assessed by thromboelastometry. According to the manufacturer’s instructions, 20 μl of 200 mmol/l CaCl2 for re-calcification and 20 μl of the respective activation reagent (recombinant tissue factor for the extrinsic system and partial thromboplastin phospholipid made of rabbit brain for the intrinsic system) were poured into the pre armed reaction cups, which had been pre-warmed. Then, 300 μl of citrated (3.13% sodium citrate) mouse whole blood was added. Measurements were recorded for at least 10 min, and the CT (time to onset of clot formation) and clot-formation time (CFT; time from onset of clot formation to a clot firmness of 20 mm) were analyzed for both extrinsic and intrinsic activation.
Real-time PCR analysis
Endothelial cells (HMEC, HUVEC) were incubated with poly (I:C) as indicated. RNA isolation and real-time PCR was performed as described previously.16 Commercially available pre-developed TaqMan reagents were used for the human target genes tissue factor, PAI-1, IL-6 and ICAM-1 (Applied Biosystems, Waltham, MA, USA), and GAPDH was used as a reference housekeeping gene. All measurements were performed in duplicate.
FACS analysis
FACS analysis was used to measure the surface expression of P-selectin and the activated fibrinogen receptor GPIIbIIIa, as described previously.10 Human platelets were isolated from the whole blood of healthy human volunteers. Whole blood samples were treated with 10% Na-citrate for anti-coagulation. After centrifugation at 150g for 15 min, the PRP was separated and diluted in thrombocyte buffer at a ratio of 1:2. Iloprost at a ratio of 1:1000 was needed to inhibit platelet activation ahead of schedule. A second centrifugation was performed at 600g over 10 min. The platelet pellet was resuspended in thrombocyte buffer ('washed platelets'). For dyeing, the platelets were pooled and diluted to 25 000 platelets per μl. Thrombin was used for platelet activation at a concentration of 2 U/ml for 3 min. Incubation with antibodies was performed for 20 min at 37 °C. For the detection of P-selectin, APC mouse anti-human CD62P-IgG1 (BD; #550888) was used, and for the activated fibrinogen receptor GPIIbIIIa, FITC anti-human PAC-1 (BD; #340507) was used. An APC mouse IgG1 isotype control (BD; #555751) was used as an isotope control. Prior to FACS analysis, the platelets were fixed with 0.5% formalin for 15 min at room temperature. FACS analysis was performed with a BD FACScanto II flow cytometer (BD Biosciences).
Statistical analysis
Statistical analysis of the data was performed using Student’s t-test, one-way analysis of variance (ANOVA) or an ANOVA on rank test as appropriate. The results are shown as the means±s.e.m. As a cutoff for significance, an error probability level of *P<0.05 and **P<0.01 was chosen.
RESULTS
Poly (I:C) induces a TLR3-dependent arteriolar occlusion in vivo
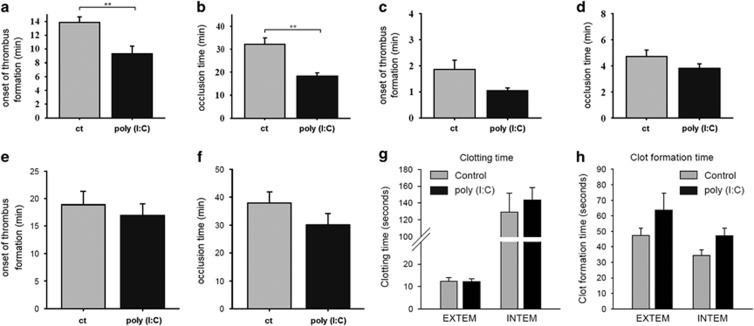
To test whether poly (I:C), a synthetic analog of viral dsRNA, has an effect on thrombus formation in arterioles or venules in vivo, C57Bl/6wt mice were systemically treated with poly (I:C) (200 μg i.p.) or injected with a control treatment. Intravital microscopy in a light dye injury model of the cremaster muscle was used as described to determine the onset of thrombus formation and the occlusion time. Thrombus formation in arterioles of the poly (I:C)-treated group occurred significantly earlier (at 9.33±1.1 min) than in the control group (at 13.9±0.8 min) (Figure 1a). In addition, the occlusion time in the arterioles of the poly (I:C)-treated group was 19.0±1.8 min, which was significantly shorter than in the control group, at 36.4±3.9 min (Figure 1b). The observation of thrombus formation in the venules yielded comparable results, but the differences in the onset of thrombus formation (1.0±0.1 min vs 1.9±0.4 min; poly (I:C) vs control) and the occlusion time (3.8±0.3 min vs 4.7±0.5 min; poly (I:C) vs control) were not significant (Figures 1c and d). When the onset of thrombus formation and the occlusion time were studied in the arterioles of TLR3−/− mice, no significant difference was seen in the control and the poly (I:C)-treated groups (Figures 1e and f).
Figure 1.
Poly (I:C) accelerated thrombus formation and the occlusion time in arterioles in vivo. C57/Bl6wt mice were treated with 200 μg poly (I:C) i.p. About 24 h before the light dye injury model of the cremaster muscle was performed as described in the Materials and methods section. The onset of thrombus formation and the occlusion time was measured in minutes in the arterioles (a and b) and venules (c and d) in the poly (I:C)-treated and control (ct) groups (n=11–12, **P<0,01. Mean±s.e., statistics with t-test (sigma plot)). (e and f) The onset of thrombus formation and the occlusion time was measured in the arterioles of TLR3−/− mice (n=4, **P<0.01. Mean±s.e., statistics with t-test (sigma plot)). The extrinsic (EXTEM) or intrinsic (INTEM) coagulation cascades were activated under control or poly (I:C)-stimulated conditions as described in the Materials and methods section and the clotting time (g) and clot-formation time (h) were analyzed. (n=4, statistics with t-test (sigma plot)). Comparable results were obtained in two series of independent experiments. Poly (I:C), polyriboinosinic:polyribocytidylic acid.
In addition, the parameters of plasmatic coagulation were assessed in an ex vivo/in vitro assay by thromboelastometry, as described in the Materials and methods section. Poly (I:C) treatment did not significantly affect the CT (the time from stimulation to onset of clot formation), neither on activation of the extrinsic nor on activation of the intrinsic coagulation cascade, indicating no significant difference in the levels or activity of the coagulation factors. However, blood from poly (I:C)-treated animals showed a tendency toward a prolonged CFT (the time for the formation of a solid clot), which can be explained by the lower platelet count in poly (I:C)-treated animals (see Table 1), as this parameter is dependent on platelet number and function (Figures 1g and h).
Table 1. Baseline hematologic and hemodynamic parameters.
| Plt. count 103/μl | WBC, 103/μl | Arteriole shear rate, s−1 | |
|---|---|---|---|
| WT+vehicle | 1126±51 | 7.5±2.3 | 760±62 |
| WT+vehicle+poly (l:C) | 783±112 | 1.7±0.4 | 839±75 |
| WT+protein C | 1074±162 | 9.3±2.6 | 926±118 |
| WT+protein C+poly (l:C) | 751±227 | 0.7±0.3 | 796±71 |
| TLR3−/− +vehicle | 1389±153 | 8.6±4.4 | 649±95 |
| TLR3−/− +poly (l:C) | 828±115 | 0.7±0.1 | 807±72 |
Abbreviations: Plt, platelets; WBC, white blood cell; WT, wild type.
Poly (I:C) increases the endothelial synthesis of prothrombotic factors and accelerates clotting
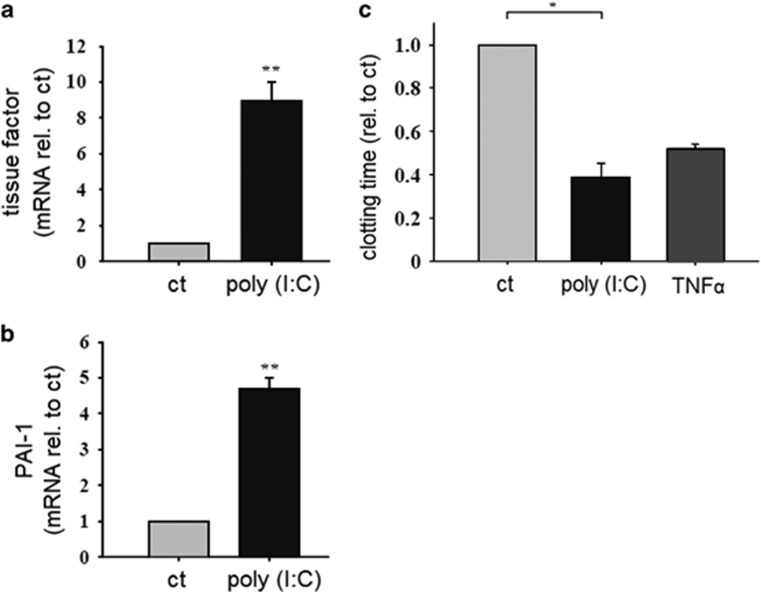
To investigate the mechanism underlying poly (I:C)-induced arteriolar occlusion in vivo, we tested whether poly (I:C) has an effect on the endothelial production of prothrombotic molecules. HMECs were stimulated with poly (I:C) (10 μg/ml) for 12 h and the expression of the prothrombotic factors tissue factor and PAI-1 was analyzed by RT–PCR (Figures 2a and b). To ensure that the prothrombotic molecules would be functional, the clotting of human whole blood was investigated in an endothelial-dependent clotting assay by thromboelastometry, as described in the Materials and methods section. HMEC were incubated for 24 h with poly (I:C) (10 μg/ml) and then lysed. Afterwards, the cell lysates were used to initiate the clotting of human whole blood and the time until beginning of clot formation was measured. The CFT for the cell lysate material treated with poly (I:C) was significantly shorter than the CFT for the control group. TNFα was used as a positive control and decreased the CT by an amount that was comparable to the poly (I:C)-induced decrease of the CT (Figure 2c).
Figure 2.
Effect of poly (I:C) on the endothelial expression of procoagulatory factors and clotting time. HMEC were stimulated with poly (I:C) (10 μg/ml) for 12 h and the expression of tissue factor (a) and PAI-1 (b) was analyzed by RT–PCR (n=4, *P<0.05. mean±s.e., statistics with t-test (sigma plot); rel. to ct, relative to control). Comparable results were obtained in two series of independent experiments. (c) HMEC were stimulated with poly (I:C) (10 μg/ml) or TNFα (5 ng/ml) as a positive control for 24 h and then lysed. Whole blood samples were stimulated with cell lysates and the clotting time was analyzed as described in the Materials and methods section (n=5–6, *P<0.05. mean±s.e., statistics with t-test (sigma plot); rel. to ct, relative to control). Comparable results were obtained in two series of independent experiments. HMEC, human microvascular endothelial cell; poly (I:C), polyriboinosinic:polyribocytidylic acid; RT–PCR, reverse transcription–PCR. **P<0.01.
Poly (I:C) does not influence platelet aggregation and activation
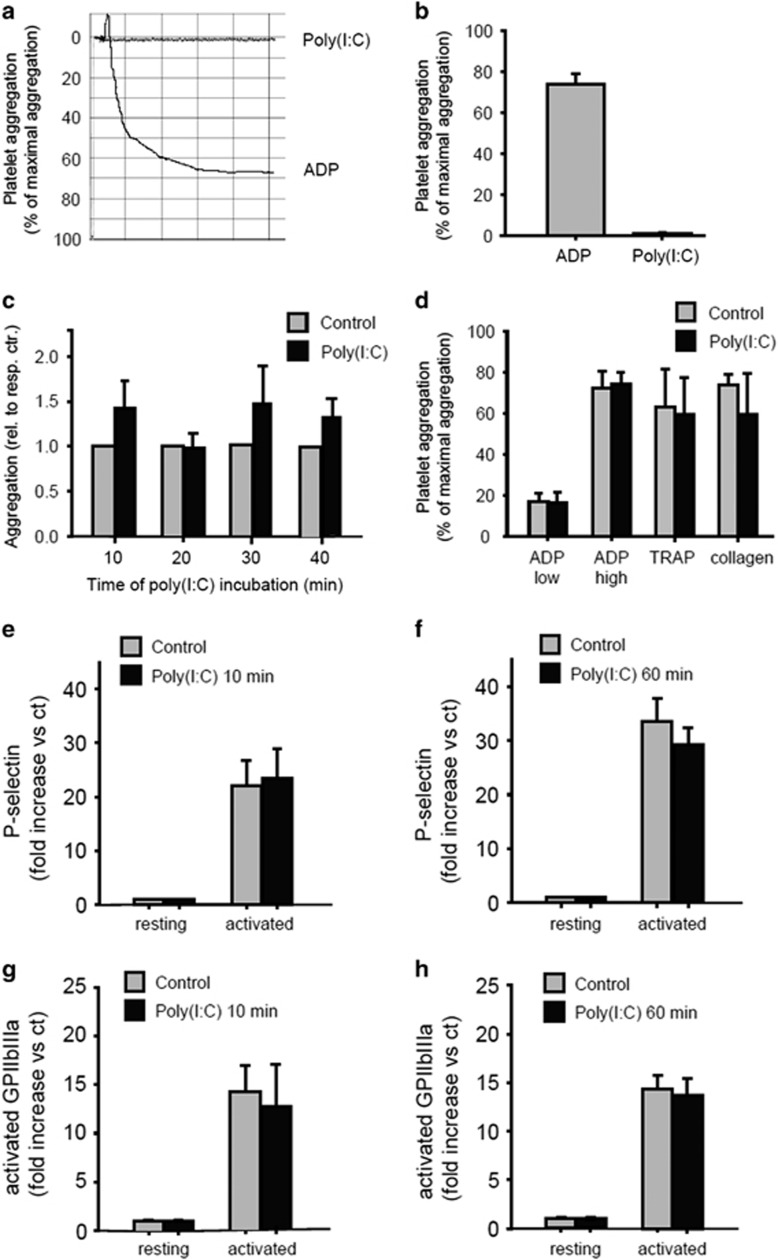
To evaluate the direct effects of poly (I:C) on platelets, we assessed platelet aggregation in vitro in human PRP using light transmission aggregometry (the method by Born)18 as described in the Materials and methods section. Incubation of PRP with 10 μg/ml poly (I:C) did not affect the ability of the platelets to form aggregates. ADP (10 μmol/l) was used as a positive control (Figures 3a and b). When PRP was incubated with poly (I:C) for different time intervals (10, 20, 30, 45 min), ADP-dependent platelet aggregation remained unchanged (Figure 3c). In addition, there was no significant difference in platelet aggregation when HMECs were incubated with poly (I:C) for 15 min in the presence of the known platelet activator ADP at a low concentration (5 μM), ADP at a high concentration (10 μM), TRAP (20 μM) or collagen (10 μg/ml) (Figure 3d). Because activated platelets usually have increased surface expression of P-selectin and the activated fibrinogen receptor GPIIbIIIa, human platelets were isolated, stimulated with poly (I:C) and analyzed by FACS with a monoclonal antibody against P-selectin and GPIIbIIIa as described in the Materials and methods section. When the platelets were incubated with poly (I:C) alone or in the presence of the known platelet activator thrombin for different time intervals (10, 60 min), no difference in the surface expression of P-selectin and GPIIbIIIa was found (Figures 3e–h).
Figure 3.
Poly (I:C) did not influence platelet aggregation and activation. Light transmission aggregometry (the method by Born)18 was performed in platelet-rich-plasma (PRP) from healthy human volunteers, as described in the Materials and methods section. The percent light transmission of platelet-rich plasma (PRP) was compared with platelet poor plasma (PPP) on stimulation with poly (I:C) (10 μg/ml) or ADP (10 μM) (a and b). PRP was incubated with poly (I:C) (10 μg/ml) for different time intervals (10, 20, 30, 45 min) and ADP-dependent (5 μM) platelet aggregation was analyzed (c). PRP was incubated with poly (I:C) (10 μg/ml) for 15 min in the presence of ADP at a low concentration (5 μM), ADP at a high concentration (10 μM), thrombin-receptor-activated peptide (TRAP) (20 μM) or collagen (10 μg/ml) and platelet aggregation was analyzed (n=4, P>0.05, mean±s.e., statistics with t-test (sigma plot)) Comparable results were obtained in two series of independent experiments. (d) Human platelets were isolated as described in the Materials and methods section. The platelets were stimulated with poly (I:C) (10 μg/ml) for different time intervals (10, 60 min) alone or in the presence of thrombin (2 U/ml) and FACS analysis with a monoclonal antibody against P-selectin (e and f) and GPIIbIIIa (g and h) was performed (n=3–4, P>0.05, mean±s.e., statistics with one-way ANOVA (sigma plot)). Comparable results were obtained in two series of independent experiments. ANOVA, analysis of variance; HMEC, human microvascular endothelial cell; poly (I:C), polyriboinosinic:polyribocytidylic acid.
APC is able to reduce the expression of procoagulatory factors in human endothelial and mesangial cells
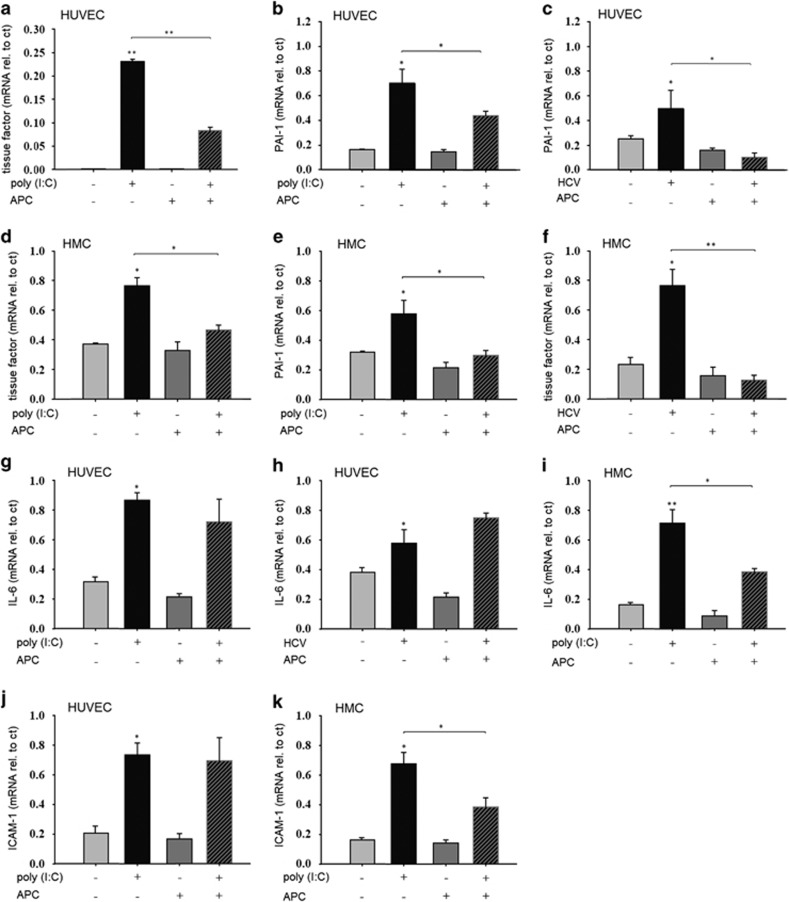
Because we could demonstrate that poly (I:C) induces the expression of procoagulatory factors, tissue factor and PAI-1 in endothelial cells, and hypothesized that these effects were responsible for thrombus formation and vessel occlusion in vivo, possible therapeutic interventions were tested. HUVEC were incubated with poly (I:C) (10 μg/ml) for 24 h in the presence or absence of APC (100 nM), then the expression of tissue factor and PAI-1 was measured by RT–PCR. APC had no significant effect on the basal expression of tissue factor or PAI-1. As expected, poly (I:C) significantly increased the expression of both factors, which could be prevented by simultaneous treatment with APC (Figures 4a and b). When HUVECs were stimulated with HCV-RNA-containing cryoprecipitates as described, PAI-1 expression was increased, and this effect was as well-blocked by APC treatment (Figure 4c). In addition to immune-mediated vascular disease, HCV infection is frequently associated with glomerulonephritis. Therefore, we also evaluated the effect of a stimulation with poly (I:C)- or HCV-containing cryoprecipitates on the expression of tissue factor and PAI-1 in HMCs. We previously demonstrated the TLR3-dependent expression of proinflammatory cytokines, chemokines and adhesion molecules in HCV-associated renal disease.16, 17, 19 When HMC were stimulated with poly (I:C) (10 μg/ml) for 24 h, the expression of tissue factor and PAI-1 was increased. APC alone had no effect on the basal expression of these factors but was able to block their poly (I:C)-dependent induction significantly (Figures 4d and e). Furthermore, HCV-RNA-containing cryoprecipitates increased the mesangial expression of tissue factor, which could also be prevented by APC treatment (Figure 4f). We have previously demonstrated a TLR3-dependent increase in the endothelial expression of cytokines, chemokines and adhesion molecules on treatment with poly (I:C) and HCV-RNA.20 To test whether these effects could also be blocked by APC treatment, HUVECs were stimulated with poly (I:C) in the presence or absence of APC. APC alone had no effect on the basal expression of the selected targets, IL-6 and ICAM-1. As expected, poly (I:C) significantly increased the expression of IL-6 and ICAM-1, but simultaneous treatment with APC did not prevent this effect (Figures 4g and j). In addition, HCV-RNA-containing cryoprecipitates increased the expression of IL-6, which again could not be prevented by APC treatment (Figure 4f). In HMC, poly (I:C) stimulation increased the expression of IL-6 and ICAM-1. In contrast to the observation in HUVEC, APC treatment of HMC was able to prevent the poly (I:C)-induced expression of both IL-6 and ICAM-1 (Figures 4i and k).
Figure 4.
Activated Protein C reduces the expression of procoagulatory factors in human endothelial and mesangial cells. Human umbilical vein endothelial cells (HUVEC) were stimulated without and with poly (I:C) (10 μg/ml) or HCV-RNA-containing cryoprecipitates (HCV) as described for 24 h in the presence or absence of activated Protein C (APC) (100 nM) and the expression of tissue factor (a), PAI-1 (b and c), IL-6 (g and h) and ICAM-1 (j) was analyzed by RT–PCR. Human mesangial cells (HMC) were stimulated without and with poly (I:C) (10 μg/ml) or HCV-RNA-containing cryoprecipitates (HCV) for 24 h in the presence or absence of activated protein C (APC) (100 nM) and the expression of tissue factor (d and f), PAI-1 (e), IL-6 (i) and ICAM-1 (k) was analyzed by RT–PCR (n=4, *P<0.05. mean±s.e., statistics with t-test (sigma plot); rel. to ct, relative to control). Comparable results were obtained in two series of independent experiments. RT–PCR, reverse transcription–PCR. **P<0.01.
Protein C is able to prevent poly (I:C)-dependent effects on thrombus formation and vessel occlusion in arterioles in vivo
As we could demonstrate that poly (I:C) leads to a TLR3-dependent thrombus formation and arterial vessel occlusion in vivo by increasing the endothelial production of procoagulatory factors without the activation of platelets, and the poly (I:C)-dependent effect on the synthesis of procoagulatory factors could be prevented by APC in vitro, we next tested whether protein C could be able to prevent poly (I:C)-induced vessel occlusion in vivo. Again, the light dye injury model was performed as described. Treatment with poly (I:C) i.p. significantly decreased the onset of thrombus formation and the occlusion time in arterioles as expected. When protein C (100 U/kg) was given i.a. 30 min prior to injury application, the onset of thrombus formation and the occlusion time were significantly delayed. Protein C alone had no effect on basal thrombus formation or the occlusion time (Figure 5).
Figure 5.
Protein C is able to prevent the poly (I:C)-induced effects on thrombus formation and vessel occlusion in arterioles in vivo. C57/Bl6wt mice were treated with 200 μg poly (I:C) i.p. for 24 h before the light dye injury of the cremaster muscle was performed as described in the Materials and methods section. Protein C (100 U/kg) was given i.a. 30 min before the start of the injury. The onset of thrombus formation (a) and the occlusion time (b) was measured in minutes in the arterioles (n=5, *P<0.05, mean±s.e., statistics with one-way ANOVA (sigma plot)). (c) Representative images of thrombus formation in cremaster muscle arterioles on light dye injury. White arrows indicate the sites where thrombus material hinders blood flow and leads to vessel occlusion. ANOVA, analysis of variance; poly (I:C), polyriboinosinic:polyribocytidylic acid.
We next analyzed the platelet count, white blood count and arteriolar shear rate, as described in the Materials and methods section. Poly (I:C) significantly decreased both the platelet count in the WT (783 × 103/μl±112 × 103/μl vs 1126 × 103/μl±51 × 103/μl; poly (I:C) vs control) and knockout mice (828 × 103/μl±115 × 103/μl vs 1389 × 103/μl±153 × 103/μl; poly (I:C) vs control) and the white blood cell count in the WT (1.7 × 103/μl±0.4 × 103/μl vs 7.5 × 103/μl±2.3 × 103/μl; poly (I:C) vs control) and knockout mice (0.7 × 103/μl±0.1 × 103/μl vs 8.6 × 103/μl±4.4 × 103/μl; poly (I:C) vs control). The effects of poly (I:C) on both the platelet and the white blood cell count were comparable in the WT and knockout groups. When the arteriolar shear rate was analyzed, no significant difference was found between the groups. Protein C treatment had no significant effect on the platelet count, the white blood count or the arteriolar shear rate. Table 1 shows the baseline hematologic and hemodynamic parameters.
DISCUSSION
In this study, we aimed to define the pathomechanism of immune-mediated vascular thrombosis in viral infections and to identify possible therapeutic approaches. Because HCV infection is the most prevalent viral disease associated with vasculitis, we performed in vivo experiments in mice using poly (I:C), a synthetic analog of viral RNA and a ligand for the viral receptor TLR3, and in vitro experiments in human endothelial cells using HCV-RNA-containing cryoprecipitates.
For our in vivo experiments, we used the cremaster model of vascular injury in mice. Application of poly (I:C), used because of the species restriction of HCV, led to arteriolar thrombus formation without affecting the venules. In TLR3−/− mice, this prothrombotic effect of poly (I:C) was abolished. This observation suggests that the effect of poly (I:C) on arteriolar thrombus formation could be directly mediated by receptor activation of the innate immune receptor TLR3. Endothelial cells are key regulators of coagulation; they are able to produce and present the anti-coagulant markers thrombomodulin and anti-thrombin, as well as procoagulant factors, including tissue factor and PAI-1.21 Viremia leads to the activation of endothelial cells, and a locally procoagulatory status can result. In addition, both in vitro and in vivo studies have shown that a variety of viruses with known prothrombotic effects are able to directly infect endothelial cells.22, 23, 24, 25, 26, 27, 28 Several mechanisms may be involved in converting endothelial cells from an anti-coagulant to a procoagulant phenotype during viral infections:21 (i) a decrease in the heparin sulfate proteoglycan synthesis and the expression of thrombomodulin leads to a reduced activation of protein C;29 (ii) an increase in endothelial synthesis of procoagulatory factors enhances platelet binding to the activated endothelium;29 and (iii) an increase in binding sites for inflammatory cells, such as granulocytes and platelets, promotes a prothrombotic condition, as these cells produce procoagulant cytokines.30, 31 The impact of inflammation on the coagulation system, which results in the stimulation of coagulation and a simultaneously decreased synthesis of anti-coagulants and suppression of fibrinolysis, is broadly accepted.32 In particular, an increased expression of tissue factor is found, which leads to the activation of the extrinsic coagulation pathway, the downregulation of APC and the inhibition of fibrinolysis.33 Furthermore, proinflammatory cytokines such as IL-6 or TNFα are known to increase the production of von Willebrand factor, finally resulting in platelet activation.34, 35, 36
To analyze which of these mechanisms could actually be responsible for virally induced thrombosis in our model, we performed in vitro experiments in HMEC and HUVEC. For the activation of the RNA-specific viral receptor TLR3, we used poly (I:C), and we found increased endothelial expression of the procoagulant tissue factor and PAI-1. Stimulation with HCV-RNA-containing cryoprecipitates from patients with HCV infection and cryoglobulinemia was also able to induce the expression of procoagulatory factors in endothelial cells. Furthermore, both poly (I:C) and HCV-RNA enhanced the expression of selected proinflammatory cytokines and the adhesion molecule ICAM-1. The functional relevance of these results was confirmed using an endothelial-dependent clotting assay performed in human whole blood. Significantly accelerated clot formation occurred when cell lysate material from cells treated with poly (I:C) was used.
In addition to producing procoagulatory factors, endothelial cells are also able to interact with platelets and thus induce coagulation. Platelets mainly interact with the endothelium via integrins or surface glycoproteins, such as P-selectin or GPIIbIIIa.37 To evaluate the direct effects of poly (I:C) on platelets, we assessed platelet aggregation in vitro using human PRP. Stimulation of platelets with poly (I:C) neither increased the platelet surface expression of P-selectin or GPIIbIIIa nor activated endothelial–platelet interaction, as shown by functional assays.
Therefore, in vitro experiments confirmed the induction of a prothrombotic condition in human endothelial cells on the activation of viral receptors, which is dependent on the induction of procoagulatory and proinflammatory mediators and not platelet aggregation or activation.
Immune-mediated glomerulonephritis is another relevant complication of HCV infection in addition to virus-associated vascular thrombosis or vasculitis. HMCs play a key role in the pathogenesis of HCV-associated glomerulonephritis. We have previously demonstrated that the activation of mesangial TLR3 by viral RNA-nucleotides increases the expression of cytokines, chemokines and adhesion molecules, as well as PAI-1.16, 17, 19, 38 In severe forms of proliferative or crescentic glomerulonephritis, fibrin deposits are observed and correlate with the severity of glomerular lesions, as well as the impairment of renal function.39, 40 The resulting glomerular sclerosis and tubulointerstitial fibrosis ultimately lead to the loss of renal function and progression toward end-stage renal failure.41 A disturbed balance of fibrinolytic and procoagulatory factors is a characteristic finding during these processes.42 Against this background, we here tested the effect of stimulation with poly (I:C) and HCV-RNA from patients with HCV infection on the mesangial expression of procoagulatory factors. Both induced the expression of tissue factor and PAI-1.
Therapeutic options in vascular complications of viral infections to date are limited to supportive measures. APC is an endogenous protein that is able to modulate both inflammation and coagulation. Protein C is synthesized in the liver, circulates as an inactive protease precursor and is activated by the thrombin–thrombomodulin complex to form APC on endothelial cells.43 APC is able to promote fibrinolysis mainly through the inhibition of PAI-1 activity. In addition, APC inactivates factor Va and factor VIIa, thus reducing thrombin generation. Furthermore, anti-inflammatory properties result from the inhibition of neutrophil chemotaxis by suppression of TNFα, IL-6 and IL-8 production.43, 44, 45 The recombinant human APC (rhAPC; also called drotrecogin alfa) promotes fibrinolysis and inhibits thrombosis.46 As it modulates both the procoagulant and inflammatory responses, which are believed to contribute to multisystem organ dysfunction, it was hypothesized to have beneficial effects in patients with sepsis.45 However, rhAPC has not been confirmed to improve survival in patients with severe sepsis or septic shock. For these reasons, drotrecogin alfa was withdrawn from the market.47, 48 However, as we hypothesized that the increased endothelial production of procoagulatory factors such as tissue factor and PAI-1 are the main cause of TLR3-mediated thrombus formation, we expected APC to have beneficial effects on the procoagulatory status resulting from the activation of viral receptors. For our in vivo experiments in mice, we used protein C instead of APC, as it is expected to be activated to APC by endogenous thrombin as soon as it is injected in the blood circulation. Protein C significantly reduced the poly (I:C)-induced onset of arteriolar thrombus formation and occlusion. When protein C was given alone, no effect on thrombus formation or the occlusion time was observed. For in vitro experiments, we had to use APC instead of protein C because of the inherent lack of thrombin. APC was able to block both the poly (I:C)- and the HCV-RNA-dependent induction of the endothelial expression of procoagulatory factors. Similarly, the poly (I:C)- and HCV-RNA-induced increase in procoagulatory factor expression could be reduced by APC in HMC.
Because of the known ability of APC to suppress proinflammatory cytokines in immune cells,49, 50 we tested whether APC could also prevent the poly (I:C)-dependent induction of cytokines and chemokines in endothelial cells. As expected, stimulation with both poly (I:C) and HCV-RNA increased the expression of selected cytokines and chemokines in HUVEC. However, in contrast to the effects of APC on the poly (I:C)- and HCV-RNA-induced expression of procoagulatory factors in HUVEC, APC was not able to suppress the poly (I:C)- and HCV-induced expression of cytokines and adhesion molecules in endothelial cells. Only in HMC, APC was able to block the poly (I:C)-dependent induction of cytokines and adhesion molecules.
From these results, we infer that protein C has the ability to prevent thrombosis in the setting of viral infection by suppressing endothelial-derived procoagulant mediators. Beyond that, APC might have an additional beneficial effect in the prevention of virally induced glomerulonephritis, as it is able to reduce the inflammatory response of mesangial cells.
There are two significant differences between our experimental approaches in immune-mediated thrombosis and the protocols used in the anti-inflammatory and fibrinolytic treatment of patients with sepsis. The advantage of the application of protein C instead of APC is the preserved regulation of protein C by the endogenous thrombin–thrombomodulin system. In the clinical setting, a lower risk of bleeding should result.
In summary, we observe the induction of a TLR3-dependent arteriolar thrombosis in mice treated with poly (I:C) as an analog of viral RNA. In vitro studies in endothelial cells suggest that prothrombotic effects depend on the increased expression of procoagulatory factors on poly (I:C) treatment. The clinical relevance of the data is corroborated by experiments performed with HCV-RNA-containing immune precipitates from a patient with HCV infection and cryoglobulinemia. Our results demonstrate that there is no significant involvement of platelet-derived factors. Protein C in vivo and APC in vitro are able to prevent arteriolar thrombosis due to their inhibitory effect on the endothelial production of procoagulatory factors such as tissue factor and PAI-1.
Because APC reduced the poly (I:C)- and HCV-RNA-induced mesangial synthesis of both procoagulatory and proinflammatory factors, and HMCs play a key role in the pathogenesis of immune-mediated glomerulonephritis, further studies should be performed to evaluate the possible therapeutic benefits of protein C treatment in virus-associated glomerulonephritis.
Acknowledgments
This work was supported by grants WO 1716/1-1 from the Deutsche Forschungsgemeinschaft (DFG), a grant from the Else Kröner-Fresenius-Stiftung and the Wilhelm-Vaillant-Stiftung to MW and a grant from the Friedrich Baur Stiftung to JP.
Footnotes
The authors declare no conflict of interest.
References
- Poynard T, Yuen MF, Ratziu V, Lai CL. Viral hepatitis C. Lancet 2003; 362: 2095–2100. [DOI] [PubMed] [Google Scholar]
- Perico N, Cattaneo D, Bikbov B, Remuzzi G. Hepatitis C infection and chronic renal diseases. Clin J Am Soc Nephrol 2009; 4: 207–220. [DOI] [PubMed] [Google Scholar]
- Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med 1992; 327: 1490–1495. [DOI] [PubMed] [Google Scholar]
- Ferri C, Greco F, Longombardo G, Palla P, Moretti A, Marzo E et al. Antibodies to hepatitis C virus in patients with mixed cryoglobulinemia. Arthritis Rheum 1991; 34: 1606–1610. [DOI] [PubMed] [Google Scholar]
- Cacoub P, Fabiani FL, Musset L, Perrin M, Frangeul L, Leger JM et al. Mixed cryoglobulinemia and hepatitis C virus. Am J Med 1994; 96: 124–132. [DOI] [PubMed] [Google Scholar]
- Vassilopoulos D, Calabrese LH. Hepatitis C virus infection and vasculitis: implications of antiviral and immunosuppressive therapies. Arthritis Rheum 2002; 46: 585–597. [DOI] [PubMed] [Google Scholar]
- van Gorp EC, Suharti C, ten Cate H, Dolmans WM, van der Meer JW, ten Cate JW et al. Review: infectious diseases and coagulation disorders. J Infect Dis 1999; 180: 176–186. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2001; 2: 675–680. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by toll-like receptor 3. Nature 2001; 413: 732–738. [DOI] [PubMed] [Google Scholar]
- Pircher J, Merkle M, Wörnle M, Ribeiro A, Czermak T, Stampnik Y et al. Prothrombotic effects of tumor necrosis factor alpha in vivo are amplified by the absence of TNF-alpha receptor subtype 1 and require TNF-alpha receptor subtype 2. Arthritis Res Ther 2012; 14: R225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannell H, Pircher J, Chaudhry DI, Alig SK, Koch EG, Mettler R et al. ARNO regulates VEGF-dependent tissue responses by stabilizing endothelial VEGFR-2 surface expression. Cardiovasc Res 2012; 93: 111–119. [DOI] [PubMed] [Google Scholar]
- Rumbaut RE, Slaff AR, Burns AR. Microvascular thrombosis models in venules and arterioles in vivo. Microcirculation 2005; 12: 259–274. [DOI] [PubMed] [Google Scholar]
- Sohn HY, Krotz F, Gloe T, Keller M, Theisen K, Klauss V et al. Differential regulation of xanthine and NAD(P)H oxidase by hypoxia in human umbilical vein endothelial cells. Role of nitric oxide and adenosine. Cardiovasc Res 2003; 58: 638–646. [DOI] [PubMed] [Google Scholar]
- Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC et al. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol 1992; 99: 683–690. [DOI] [PubMed] [Google Scholar]
- Mannell H, Hellwig N, Gloe T, Plank C, Sohn HY, Groesser L et al. Inhibition of the tyrosine phosphatase SHP-2 suppresses angiogenesis in vitro and in vivo. J Vasc Res 2008; 45: 153–163. [DOI] [PubMed] [Google Scholar]
- Wörnle M, Schmid H, Banas B, Merkle M, Henger A, Roeder M et al. Novel role of toll-like receptor 3 in hepatitis C-associated glomerulonephritis. Am J Pathol 2006; 168: 370–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle M, Ribeiro A, Wörnle M. TLR3 dependent regulation of cytokines in human mesangial cells: a novel role for IP-10 and TNFa in hepatitis C associated glomerulonephritis. Am J Physiol Renal Physiol 2011; 301: 57–69. [DOI] [PubMed] [Google Scholar]
- Born G. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 1962; 194: 927–929. [DOI] [PubMed] [Google Scholar]
- Merkle M, Ribeiro A, Köppel S, Pircher J, Mannell H, Roeder M et al. TLR3-dependent immune regulatory functions of human mesangial cells. Cell Mol Immunol 2012; 9: 334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher J, Czermak T, Merkle M, Mannell H, Krötz F, Ribeiro A et al. Hepatitis C virus induced endothelial inflammatory response depends on the functional expression of TNFα receptor subtype 2. PLoS One 2014; 9: e113351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeijenbier M, van Wissen M, van de Weg C, Jong E, Gerdes VE, Meijers JC et al. Review: viral infections and mechanisms of thrombosis and bleeding. J Med Virol 2012; 84: 1680–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R, König W. Respiratory syncytial virus infection of human lung endothelial cells enhances selectively intercellular adhesion molecule-1 expression. J Immunol 2005; 174: 7359–7367. [DOI] [PubMed] [Google Scholar]
- Gavrilovskaya IN, Gorbunova EE, Mackow ER. Pathogenic hantaviruses direct the adherence of quiescent platelets to infected endothelial cells. J Virol 2010; 84: 4832–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro CM, Crowson AN, Dawood M, Nuovo GJ. Parvoviral infection of endothelial cells and its possible role in vasculitis and autoimmune disease. J Rheumatol 2000; 29: 1227–1235. [PubMed] [Google Scholar]
- Mason A, Wick M, White H, Perrillo R. Hepatitis B virus replication in diverse cell types during chronic hepatitis B virus infection. Hepatology 1993; 18: 781–789. [DOI] [PubMed] [Google Scholar]
- Poland SD, Rice GP, Dekaban GA. HIV-1 infection of human brain-derived microvascular endothelial cells in vitro. J Acquir Immune Defic Syndr Hum Retrovirol 1995; 8: 437–445. [DOI] [PubMed] [Google Scholar]
- Squizzato A, Gerdes VE, Büller HR. Effects of human cytomegalovirus infection on the coagulation system. Thromb Haemost 2005; 93: 403–410. [DOI] [PubMed] [Google Scholar]
- Visseren FL, Bouwman JJ, Bouter KP, Diepersloot RJ, de Groot PH, Erkelens DW. Procoagulant activity of endothelial cells after infection with respiratory viruses. Thromb Haemost 2000; 84: 319–324. [PubMed] [Google Scholar]
- Nicholson AC, Hajjar DP. Herpesviruses and thrombosis: activation of coagulation on the endothelium. Clin Chim Acta 1999; 286: 23–29. [DOI] [PubMed] [Google Scholar]
- Nicholson AC, Hajjar DP. Herpesvirus in atherosclerosis and thrombosis: etiologic agents or ubiquitous bystanders? Arterioscler Thromb Vasc Biol 1998; 18: 339–348. [DOI] [PubMed] [Google Scholar]
- Sutherland MR, Friedman HM, Pryzdial EL. Thrombin enhances herpes simplex virus infection of cells involving protease-activated receptor 1. J Thromb Haemost 2007; 5: 1055–1061. [DOI] [PubMed] [Google Scholar]
- Lipinski S, Bremer L, Lammers T, Thieme F, Schreiber S, Rosenstiel P. Coagulation and inflammation. Molecular insights and diagnostic implications. Hamostaseologie 2011; 31: 94–102. [DOI] [PubMed] [Google Scholar]
- Petäjä J. Inflammation and coagulation. An overview. Thromb Res 2011; 127: 34–37. [DOI] [PubMed] [Google Scholar]
- Levi M, van der Poll T, Büller HR. Bidirectional relation between inflammation and coagulation. Circulation 2004; 109: 2698–2704. [DOI] [PubMed] [Google Scholar]
- Opal SM. Interactions between coagulation and inflammation. Scand J Infect Dis 2003; 35: 545–554. [DOI] [PubMed] [Google Scholar]
- Schouten M, Wiersinga WJ, Levi M, van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol 2008; 83: 536–545. [DOI] [PubMed] [Google Scholar]
- Passos AM, Treitinger A, Spada C. An overview of the mechanisms of HIV-related thrombocytopenia. Acta Haematol 2010; 124: 13–18. [DOI] [PubMed] [Google Scholar]
- Wörnle M, Roeder M, Sauter M, Merkle M, Ribeiro A. Effect of dsRNA on mesangial cell synthesis of plasminogen activator inhibitor type 1 and tissue plasminogen activator. Nephron Exp Nephrol 2009; 113: 57–65. [DOI] [PubMed] [Google Scholar]
- Kincaid-Smith P. Coagulation and renal disease. Kidney Int 1972; 2: 183–190. [DOI] [PubMed] [Google Scholar]
- Nield GH, Cameron JS. Primary glomerulonephritis. In: Remuzzi G, Rossi EC (eds). Haemostasis and the Kidney. Butterworth: London, UK. 1989. pp 56–64. [Google Scholar]
- Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 1992; 20: 1–17. [DOI] [PubMed] [Google Scholar]
- Grandaliano G, Gesualdo L, Ranieri E, Monno R, Schena FP. Tissue factor, plasminogen activator inhibitor-1, and thrombin receptor expression in human crescentic glomerulonephritis. Am J Kidney Dis 2000; 35: 726–738. [DOI] [PubMed] [Google Scholar]
- Houston G, Cuthbertson BH. Activated protein C for the treatment of severe sepsis. Clin Microbiol Infect 2009; 15: 319–324. [DOI] [PubMed] [Google Scholar]
- Abraham E, Laterre PF, Garg R, Levy H, Talwar D, Trzaskoma BL et al. Administration of drotrecogin alfa (activated) in early stage severe sepsis (ADDRESS) Study Group. N Engl J Med 2005; 353: 1332–1341. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Margolis BD, Shanies HM, Ely EW, Wheeler AP, Levy H et al. Extended evaluation of recombinant human activated protein C United States Trial (ENHANCE US): a single-arm, phase 3B, multicenter study of drotrecogin alfa (activated) in severe sepsis. Chest 2004; 125: 2206–2216. [DOI] [PubMed] [Google Scholar]
- Gabre J, Chabasse C, Cao C, Mukhopadhyay S, Siefert S, Bi Y et al. Activated protein C accelerates venous thrombus resolution through heme oxygenase-1 induction. J Thromb Haemost 2014; 12: 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí-Carvajal AJ, SolàI I, Lathyris D, Cardona AF. Human recombinant activated protein C for severe sepsis. Cochrane Database Syst Rev 2012; 3: CD004388. [DOI] [PubMed] [Google Scholar]
- FDA Drug Safety Communication: Voluntary Market Withdrawal of Xigris [(Drotrecogin alfa (activated)] due to failure to show a survival benefit http://www.fda.gov/Drugs/DrugSafety/ucm277114.htm Accessed on 25 October 2011.
- Cao C, Gao Y, Li Y, Antalis TM, Castellino FJ, Zhang L. The efficacy of activated protein C in murine endotoxemia is dependent on integrin CD11b. J Clin Invest 2010; 120: 1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson DA, Toltl LJ, Beaudin S, Liaw PC. Modulation of monocyte function by activated protein C, a natural anticoagulant. J Immunol 2006; 177: 2115–2122. [DOI] [PubMed] [Google Scholar]