Antiretroviral therapy (ART) has increased the lifespan of HIV-infected individuals, which is now approaching that of the general population. However, cardiovascular disease (CVD), including myocardial infarction, stroke, heart failure, and arrhythmia, is still a main cause of mortality in HIV-positive population. The risk of CVD in people living with HIV is about 1.5–2-fold higher than that in the general population.1 Even in those successfully treated with ART, the incidence of CVD is still higher than that in uninfected individuals.2 Thus, in addition to the traditional risk factors associated with CVD, other factors (such as ART per se, inflammation, and immune activation caused by HIV infection) may be involved in CVD development.
Chronic immune activation, particularly of monocytes/macrophages, contributes to HIV pathogenesis, and disease progression. During HIV infection, monocytes can be activated by replicating virus, microbial translocation, pro-inflammatory cytokines, and activated platelets. Monocytes in human blood belong to three subsets: classical monocytes (CD14highCD16−), intermediate monocytes (CD14highCD16+), and non-classical monocytes (CD14dimCD16+). Classical monocytes possess the potential for phagocytosis. They differentiate into M1 macrophages under inflammatory or activation conditions and phagocytose native low-density lipoproteins (LDL) to form foam cells. Intermediate monocytes are a pro-inflammatory subset, which secrete large amounts of pro-inflammatory cytokines and express chemokine receptors associated with atherosclerosis (such as CCR2, CX3CR1, and CCR5). These cells generate reactive oxygen species (ROS) and are proangiogenic. Non-classical monocytes, also called ‘patrolling’ monocytes, patrol vascular surfaces, and migrate into atherosclerotic lesions. Subjects with acute coronary syndrome harbor increased numbers of non-classical and intermediate monocytes, which promote atherogenesis.3 These two subsets are also expanded in HIV-positive individuals, including those with primary or chronic HIV infection and those successfully treated with ART.4, 5 A high number of non-classical and intermediate monocytes is associated with the occurrence and progression of CVD events, independently of traditional risk factors and ART.3, 5, 6 The reasons for this are complicated and unclear. These two monocyte subsets represent an activated status of monocytes, accompanied by abundant CD16 expression. After primary HIV infection, endothelial cells become dysfunctional and express higher levels of adhesion molecules and chemokines to attract monocytes to the lesion site; the monocytes are then activated. Activated monocytes infiltrate into the intima (facilitated by CX3CL1) and differentiate into macrophages, which then phagocytose oxidized LDL (oxLDL) and form cholesterol-rich foam cells that ultimately develop into fibrous plaques. In addition, HIV infection impairs cholesterol efflux of monocytes and increases foam cell formation via the pro-atherogenic cytokine TNF-α and reduced expression of genes regulating cholesterol metabolism (for example, cholesterol transporter ABCA1 (ATP-binding cassette transporter A1)7). Notably, infliximab (a TNF-α antagonist) failed to reduce the incidence of advanced heart failure in an ATTACH trial.8
In addition to the above, monocytes can form monocyte-platelet aggregates (MPAs) with activated platelets via binding of P-selectin glycoprotein ligand-1 (PSGL-1) on monocytes to P-selectin (CD62P) on platelets. Upon injury, platelets are recruited to the lesion, where they become activated. Activated platelets recruit more platelets and eventually form a thrombus. At the same time, activated platelets secrete pro-inflammatory cytokines to activate endothelial cells and enhance monocyte recruitment, adhesion, and activation. Monocytes are activated through cell to cell contact or by cytokines. In a vicious circle, activated monocytes increase PSGL-1, CD86, CCR2, and CD11b expression and MPA formation, thereby attracting more monocytes to the lesion. MPA formation is accompanied by monocyte activation and platelet activation, along with increased cytokine and chemokine production and adhesion molecules expression. Platelet-derived chemokines (such as CXCL4, CCL5, and MIF) promote adhesion of monocytes to endothelial cells. Tumor growth factor β (TGF-β) produced by activated platelets increases CD16 expression and triggers classical monocytes to differentiate into intermediate or non-classical monocytes, possibly through the p38 MAPK pathway. Furthermore, platelet-derived chemokine platelet factor 4 (PF4) and CXCL12 can modulate monocyte survival, monocyte differentiation into macrophages, and foam cell formation. PF4 is present at high concentration around thrombi, at which the monocyte-platelet interaction occurs.9, 10 Platelets promote monocytes to release proteolytic enzymes, which degrade the extracellular matrix and destabilize plaques (Figure 1).
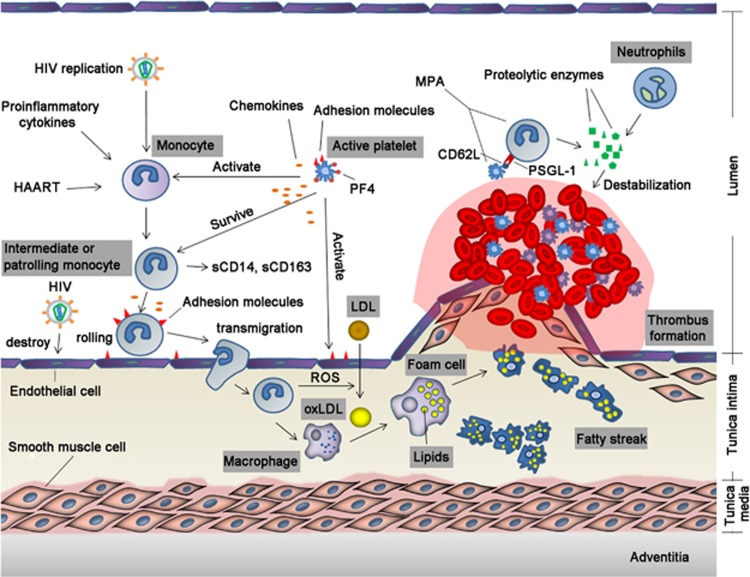
Figure 1.
Effect of activated monocytes in the occurrence of atherosclerosis in HIV-infected individuals. Endothelial cells are activated by HIV or injury, and platelets are recruited and activated to form a thrombus. Activated endothelial cells and platelets express adhesion molecules and secrete chemokines, which recruit and activate monocytes. Monocytes activated by endothelial cells, platelets, and other factors transmigrate into the intima and differentiate into macrophages that engulf oxLDL and form foam cells. Activated monocytes interact with activated platelets to form MPA, which further stimulates thrombus formation and increases production of proteolytic enzymes, resulting in plaque destabilization and rupture. HIV, human immunodeficiency virus; ART, antiretroviral therapy; PF4, platelet factor 4; LDL, low-density lipoprotein; oxLDL, oxidized LDL; PSGL-1, P-selectin glycoprotein ligand-1; ROS, reactive oxygen species; MPA, monocyte-platelet aggregate.
Formation of MPAs and the interaction between monocytes and platelets are highly correlated with CVD events.11 Studies show that MPA levels are elevated in those with ischemic heart failure, as well as in those with primary and chronic HIV infection, regardless of ART.4, 11 MPA levels in the three monocyte subsets show a positive correlation with sCD163 levels in HIV-infected individuals, indicating an association between increased formation of MPA and monocyte activation status.4 These studies indicate that the interaction between monocytes and platelets plays a key role in CVD events, and that MPA is a sensitive and appropriate marker for monitoring monocyte and platelet activation.
The levels of sCD14 and sCD163, two soluble markers of monocyte activation, are elevated in HIV-positive individuals.4, 12 sCD14 is a receptor for lipopolysaccharide (LPS) and a marker of microbial translocation. sCD14 is also associated with coronary stenosis, coronary artery calcium, and progression of subclinical atherosclerosis in HIV-infected individuals.12, 13, 14 sCD163 is a scavenger receptor derived from ecto-domain shedding of membrane CD163. sCD163 is recognized as a marker for atherosclerosis in HIV-negative populations and is associated with coronary artery calcium, mixed plaques, coronary stenosis, calcified plaques, and non-calcified coronary plaques in HIV-positive individuals.12, 15 Furthermore, there is a dose-dependent relationship between sCD163 levels and the coronary artery calcium score.9
Taken together, current researches reveal that increased numbers of non-classical and intermediate monocytes in HIV-infected individuals are associated with adverse HIV-related CVD events, suggesting that monocyte activation plays an essential role in atherogenesis. MPA, sCD14, and sCD16 are all sensitive markers of monocyte activation. A better understanding of the underlying mechanism(s) and relationship(s) between monocyte activation and CVD, and identification of sensitive and reliable markers for monocyte activation, will improve monitoring and intervention of HIV-related CVD.
Acknowledgments
This work was financially supported by the State Key Laboratory of Infectious Disease Prevention and Control (2015SKLID506) and the National Science and Technology Major Project of China (2017ZX10202101-003, 2017ZX10301101-001, and 2017ZX09309008-003).
Footnotes
The authors declare no conflict of interest.
References
- Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med 2012; 13: 453–468. [DOI] [PubMed] [Google Scholar]
- Hanna DB, Post WS, Deal JA, Hodis HN, Jacobson LP, Mack WJ et al. HIV infection is associated with progression of subclinical carotid atherosclerosis. Clin Infect Dis 2015; 61: 640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow DC, Kagihara JM, Zhang G, Souza SA, Hodis HN, Li Y et al. Non-classical monocytes predict progression of carotid artery bifurcation intima-media thickness in HIV-infected individuals on stable antiretroviral therapy. HIV Clin Trials 2016; 17: 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Duan Z, Li D, Wang Z, Ren L, Shen T et al. Higher levels of circulating monocyte-platelet aggregates are correlated with viremia and increased sCD163 levels in HIV-1 infection. Cell Mol Immunol 2015; 12: 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, Musselwhite LW et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood 2012; 120: 4599–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JV, Hullsiek KH, Singh A, Wilson E, Henry K, Lichtenstein K et al. Immunologic predictors of coronary artery calcium progression in a contemporary HIV cohort. AIDS 2014; 28: 831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisa A, Hearps AC, Angelovich TA, Pereira CF, Zhou J, Shi MD et al. Monocytes from HIV-infected individuals show impaired cholesterol efflux and increased foam cell formation after transendothelial migration. AIDS 2015; 29: 1445–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 2003; 107: 3133–3140. [DOI] [PubMed] [Google Scholar]
- Scheuerer B, Ernst M, Durrbaum-Landmann I, Fleischer J, Grage-Griebenow E, Brandt E et al. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood 2000; 95: 1158–1166. [PubMed] [Google Scholar]
- Chatterjee M, von Ungern-Sternberg SN, Seizer P, Schlegel F, Buttcher M, Sindhu NA et al. Platelet-derived CXCL12 regulates monocyte function, survival, differentiation into macrophages and foam cells through differential involvement of CXCR4-CXCR7. Cell Death Dis 2015; 6: e1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley BJ, Shantsila E, Tapp LD, Lip GY. Increased formation of monocyte-platelet aggregates in ischemic heart failure. Circ Heart Fail 2013; 6: 127–135. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Mustfa N. Hot tub Legionella pneumonia outbreak. Eur Respir J 2014; 44: 1379–1381. [DOI] [PubMed] [Google Scholar]
- Longenecker CT, Jiang Y, Orringer CE, Gilkeson RC, Debanne S, Funderburg NT et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS 2014; 28: 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis 2012; 206: 1558–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis 2013; 208: 1737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]