The nucleotide-binding oligomerization domain-like receptor pyrin domain containing 3 (NLRP3) inflammasome is one of the pattern recognition receptors for host defense against pathogen-associated molecular patterns and danger-associated molecular patterns. The NLRP3 inflammasome is a best characterized as a multiprotein complex composed of NLRP3, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) adaptor, and pro-caspase-1. The NLRP3 inflammasome is activated by select activators or pathogen stimuli, and a conformational change of inflammasome-forming NOD-like receptor (NLR) enables NLR binding to ASC via pyrin domains. The adaptor ASC then conjugates with pro-caspase-1 through a caspase recruitment domain. Significant action of the NLRP3 inflammasome provides a molecular modification of pro-caspase-1, cleaving it into caspase-1 and thereby mediating IL-1β maturation and secretion from its precursor pro-IL-1β.1 NLRP3 inflammasome activators include whole pathogens, pathogen-associated molecules, environmental insults, and endogenous danger signals. However, it has been well documented that the signals controlling NLRP3 inflammasome activation by these activators are intracellular reactive oxygen species (ROS) or mitochondrial ROS.1, 2 The redox homeostasis associated transcription factor, known as nuclear factor E2-related factor 2 (Nrf2), has been further studied for its potential role in NLRP3 inflammasome activation.
After silencing Nrf2 expression via knock-out or knock-down assay, NLRP3 specific activators, such as alum, silica, cholesterol, and monosodium urate (MSU) crystals, or others such as ATP, nigericin or poly (dA:dT) failed to trigger IL-1β secretion in Nrf2-difienct bone marrow macrophages (BMMs) or human monocytes/macrophages (Figure 1).3, 4, 5 Accumulating evidence indicates that cytosolic ASC speck formation is diminished in similarly treated Nrf2−/− BMMs, suggesting that Nrf2 may promote the assembly of ASC speck.4 Another proposed mechanism, which has been far less studied, is that Nrf2 controls phagocytosis to mediate NLRP3 and IL-β secretion. Macrophages from Nrf2−/− mice generally impair phagocytosis and pathogen killing, resulting in promoting susceptibility to bacterial infection.6 It has also been shown that particular activators initiate NLRP3 inflammasome activation through frustrated phagocytosis,7 thus highlighting the crosstalk between Nrf2 and the NLRP3 inflammasome through the modulation of phagocytosis.
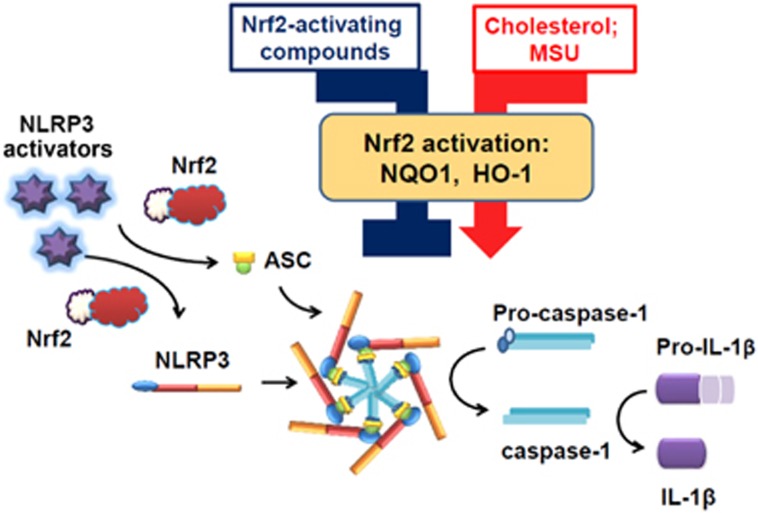
Figure 1.
Proposed mechanism of Nrf2-mediated NLRP3 inflammasome activation and IL-1β secretion. Nrf2 is required for NLRP3 activators to promote IL-1β secretion via ASC speck formation. Nrf2 activation by Nrf2-activating compounds or cholesterol/MSU promotes or inhibits IL-1β secretion. ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; HO-1, heme oxygenase-1; IL-1β, interleukin-1β MSU, monosodium urate; NLRP3, nucleotide-binding oligomerization domain-like receptor pyrin domain containing 3; NQO-1, NAD(P)H:quinone oxidoreductase 1.
Several publications demonstrate that Nrf2-activating compounds, such as tertiary butylhydroquinone, sulforaphane, and xanthohumol, can suppress IL-1β secretion by up-regulating Nrf2-mediated NAD(P)H:quinone oxidoreductase 1 (NQO-1), heme oxygenase-1 (HO-1), and glutamate cysteine ligase expression.8, 9, 10 These antioxidant responses further scavenge ROS, resulting in maintenance of the thioredoxin/thioredoxin interaction protein system and down-regulation of ROS-priming NF-κB signaling. It seems that these Nrf2-activating compounds indirectly control IL-1β secretion because it is unclear whether cytosolic Nrf2 or its repressor Keap-1 directly changes the conformation of the NLRP3 inflammasome and interacts with one of the NLRP3 inflammasome components. In some cases, NLRP3 inflammasome activators, such as cholesterol, induce Nrf2-dependent genes, including peroxiredoxin-1, NQO-1 and HO-1,3 and MSU, which can trigger Nrf2 translocation into the nucleus with increasing mRNA levels of superoxide dismutase and HO-1.5 MSU-induced lysosomal disruption and p62/SQSTM1 accumulation are indicated as the major causes of Nrf2 activation.5 Furthermore, IL-1β secretion was also suppressed via silencing of HO-1 or by using HO-1 inhibitors (ZnPP and SnPP).5, 11 On the basis of these findings, Nrf2 activation and HO-1 expression are indicated as redox homeostasis factors, as well as pro-inflammatory factors in IL-1β/NLRP3 inflammasome activation.
It is unclear whether Nrf2 activation towards preventive or aggressive inflammation occurs when macrophages are treated with Nrf2-activating compounds in cholesterol or MSU-challenged macrophages. This issue is important for patients suffering from hyperlipidemia and gout if they attempt to alleviate the attacks with a daily intake of Nrf2-activating phytochemicals. Although our previous results have illustrated that green tea polyphenolics, such as epigallocatechin gallate, which are known as Nrf2-activating phytochemicals, significantly reduced MSU-induced IL-1β secretion and NLRP3 inflammasome activation,12 the role of Nrf2 on MSU-induced inflammation still has not be clarified.
In conclusion, IL-1β secretion is inhibited by Nrf2 silencing, suggesting that the presence of Nrf2 is required for NLRP3 inflammasome activation. Nrf2 activation by Nrf2-activating compounds or cholesterol/MSU has the opposite effects on NLRP3 inflammasome activation. Nrf2-activating compounds down-regulate the NLRP3 inflammasome, whereas cholesterol/MSU up-regulate the NLRP3 inflammasome. Therefore, the therapeutic strategy of targeting Nrf2 activation in cholesterol-induced atherosclerosis or MSU crystal-induced acute gout remains to be investigated.
Footnotes
The authors declare no conflict of interest.
References
- Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol 2010; 10: 210–215. [DOI] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011; 469: 221–225. [DOI] [PubMed] [Google Scholar]
- Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J et al. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol 2011; 41: 2040–2051. [DOI] [PubMed] [Google Scholar]
- Zhao C, Gillette DD, Li X, Zhang Z, Wen H. Nuclear factor E2-related factor-2 (Nrf2) is required for NLRP3 and AIM2 inflammasome activation. J Biol Chem 2014; 289: 17020–17029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhang JJ, Cheng YT, Ho CY, Yen GC. Monosodium urate crystals trigger Nrf2- and heme oxygenase-1-dependent inflammation in THP-1 cells. Cell Mol Immunol 2015; 12: 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy NM, Suryanarayana V, Kalvakolanu DV, Yamamoto M, Kensler TW, Hassoun PM et al. Innate immunity against bacterial infection following hyperoxia exposure is impaired in NRF2-deficient mice. J Immunol 2009; 183: 4601–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 2008; 9: 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang X, Ding Y, Zhou W, Tao L, Lu P et al. Nuclear factor E2-related factor-2 negatively regulates NLRP3 inflammasome activity by inhibiting reactive oxygens species-induced NLRP3 priming. Antioxid Redox Signal 2017; 26: 28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H, Liu Q, Wen Z, Feng H, Deng X, Ci X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol 2017; 12: 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Shang H, Chen YQ, Pan LL, Bhatia M, Sun J. Sulforaphane protects pancreatic acinar cell injury by modulating Nrf2-mediated oxidative stress and NLRP3 inflammatory pathway. Oxid Med Cell Longev 2016; 2016: 7864150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel B, Larsen R, Gallo D, Chin BY, Harris C, Mannam P et al. Macrophages sense and kill bacteria through carbon monoxide-dependent inflammasome activation. J Clin Invest 2014; 124: 4926–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhang JJ, Lu CC, Yen GC. Epigallocatechin gallate inhibits urate crystals-induced peritoneal inflammation in C57BL/6 mice. Mol Nutr Food Res 2016; 60: 2297–2303. [DOI] [PubMed] [Google Scholar]