An understanding of the development and function of T helper (Th) 1, Th17 and regulatory T (Treg) cells is critical toward revealing pro-inflammatory immune responses, especially in autoimmune diseases. However, there is no standard protocol to monitor the development of these cells over time in vivo. This protocol details a method to generate Th1, Th17, and Treg cells in a T cell-induced colitis model in vivo and monitor their dynamic changes, which can be used for further investigations in their development from naive CD4+ T cells in specific gene knockouts and background strains. This protocol starts with the isolation of naive CD4+CD45RBhigh T cells from C57BL/6 IL-17Agfp mice followed by their i.p. injection into recombinase, activating gene-1-deficient (Rag1−/−) mice. At the indicated time points, mice are killed, and mesenteric lymphocytes and murine lamina propria mononuclear cells are isolated and subjected to flow cytometric analysis to assess the development of Th1, Th17, and Treg cells and Th2, Th9, Th22, and follicular T helper (Tfh) cells. This protocol can be completed within 8 weeks.
CD4+ T cells play a central role in the function of the immune system, particularly in adaptive immunity. They help the activity of other immune cells by releasing T cell cytokines. CD4+ T cells can be subdivided into lineages based on immunological functions, specific transcription factors, and cytokines: Th1, Th2, Th9, Th17, Th22, Tfh and Treg cells. Aberrant activation of Th1, Th17, Th22 and Tfh cells has been implicated in many autoimmune and inflammatory diseases, whereas excessive Th2 activity causes allergic diseases. Conversely, impaired function of Treg cells causes fatal inflammatory disorders both in human and mouse.1, 2
Although T helper and Treg cell development has been extensively studied in vitro,3, 4 their development in vivo is not as well understood. Here, we use the classical T cell transfer model of colitis originally described by Powrie5 to investigate the developmental characteristics of various T helper and Treg cells in vivo. Initially, Th1 cells were thought to dominate the pathogenesis of colitis.5 Recent studies indicate that Th17 cells are even more important than Th1 cells in this process.6 In total, this system could be used as a model to investigate the development of Th1/Th17 and Treg cells in vivo since CD4+ T cells isolated from mesenteric lymph nodes (MLN) and colonic lamina propria (LP) express specific phenotypes and functional characteristics of Th and Treg cells.7 Using Th17 reporter mice in a colitis model, we herein provide a detailed protocol and define the dynamics of T helper and Treg cell development in vivo.
Isolation of naive CD4+CD45RBhigh T cells from donor mice, injection of cells into Rag1 −/− mice
Splenic cells were collected from B6 IL-17Agfp wild type male mice (~8 weeks), and CD4+T cells were enriched by negative selection using magnetic bead-based cell isolation (Miltenyi AutoMACS).
Naive CD4+CD45RBhigh T cells were isolated from CD4+T cells via FACS cell sorting; the purity was >99%.
A total of 0.4 × 106 naive CD4+CD45RBhigh T cells was injected i.p. into Rag1 −/− male mice.
Monitoring disease progression, collecting tissues, sampling, and scoring for colitis
Disease progression was monitored by weighing each mouse. Gradual weight loss occurred at two weeks, and mild colitis was observed. After 4 weeks, mice developed signs of disease, such as loose stools, diarrhea, and a ‘hunched-over’ appearance.
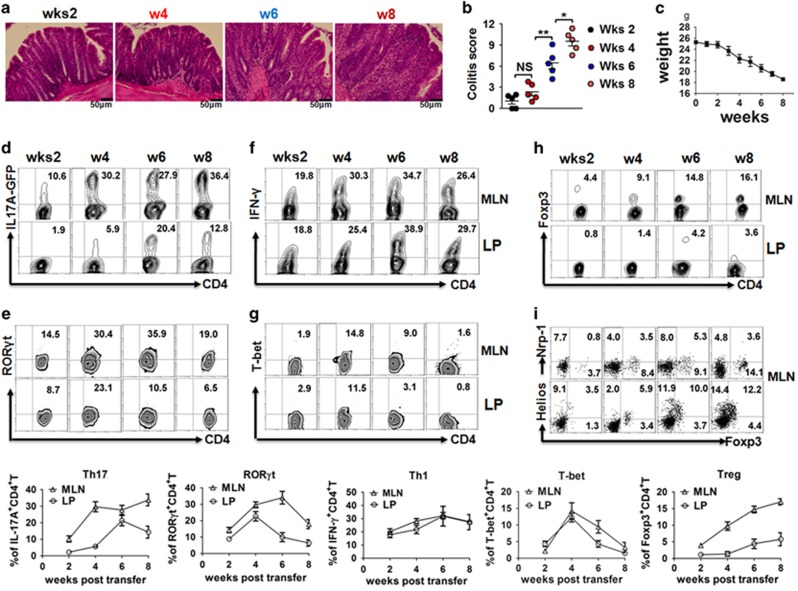
At 2, 4, 6 or 8 weeks post-injection, animals were killed and assessed for macroscopic evidence of colitis. 8 Colitis was clearly present at four weeks with severity gradually increasing through six weeks as evidenced by crypt branching, irregularity of size and shape, and an increase in chronic inflammatory cell numbers in the colon (Figures 1a and b).
Figure 1.
Th1, Th17 and Treg cell development in colitis model. C57BL/6 Rag1−/− mice are injected i.p. with 0.4 × 106 naive CD4+CD45RBhigh T cells from C57BL/6 IL-17Agfp donors. Mice are killed at different indicated times (2–8 weeks). (a) Representative plots of proximal colon sections from the indicated experimental groups (200x magnification, scale bars, 50 μM); (b) Colitis score and (c) weight curve are shown; (d) Frequency of IL-17A+CD4+T cells, (e) transcription factor RORγt, (f) IFN-γ+CD4+T cells, (g) T-bet, (h) Foxp3+CD4+T cells, (i) Neuropilin-1+ and Helios+ CD4+T cells among CD4+T cells from mesenteric lymph node (MLN) and colonic lamina propria (LP) in recipient mice. The summary data are shown at the bottom. The data indicate the mean±s.e.m. of two separated experiments. Each group consists of five mice. *P<0.05 versus control group. **P<0.01 versus control group. NS, not significant.
T helper and Treg cells staining
Mesenteric lymphocytes and colonic lamina propria mononuclear cells from recipient mice were isolated at different time points (2, 4, 6 and 8 weeks).
T cell subsets stained with fluorochrome-labeled or isotype antibodies were analyzed for the percentages of T helper and Treg cells by flow cytometry.
Development of Th1 and Th17 cells in vivo in colitis model
The use of IL-17Agfp reporter cells provided the distinct advantage that we could accurately identify Th17 cells without extensive cellular disruptions caused by fixation and permeabilization, which provided a more flexible and accurate analysis. We revealed that substantial numbers of Th17 cells are present in MLN, as early as two weeks post transfer and that the frequencies increase over time, reaching peak levels at four weeks. Although Th17 frequencies are slightly lower in LP than in MLN, interestingly, Th17 cells peak earlier in MLN than in LP (Figure 1d). It is noted that the expression levels of retinoic acid-related orphan receptor gamma t (RORγt)+ cells parallel Th17 cell development in both MLN and LP, even though RORγt levels are reduced at six weeks (Figure 1e).
While Th1 cells are no longer considered to be the cell type responsible for pathogenicity in colitis, our study nevertheless demonstrated that IFN-γ+Th1 cells gradually develop and that their levels are similar or even higher compared with Th17 cells in both MLN and LP (Figure 1f). As expected, the Th1 cell transcription factor T-bet+ could be identified in cells found both in MLN and LP. Unlike RORγt, T-bet+ cells peak at four weeks and rapidly reduce and disappear 6–8 weeks later (Figure 1g), suggesting different stabilities of Th1 and Th17 cells in this model.
Foxp3+ Treg cell development in vivo in colitis model
Foxp3+ Treg cells play an important role in the prevention of autoimmune diseases.9 We characterized the dynamics of Foxp3+ Treg cell development in vivo since it had not been previously systematically defined. Unlike Th1 and Th17 cells, Treg cells are almost undetectable in MLN at two weeks, but they start to appear at 4 weeks and gradually increase until 8 weeks. However, Treg cells are less developed in LP (Figure 1h).
Recent studies have suggested that Neuropilin-1(Nrp-1) and Helios expression may help distinguish natural Treg from induced Treg subsets.10, 11 With this in mind, we also analyzed the phenotypic characteristics of these Treg cells. As shown in Figure 1i, very few MLN Foxp3+ cells express Nrp-1, indicating that these cells belong to the induced Treg subset. We also found that these cells express Helios in levels similar to that reported for natural Treg cells. It is not surprising that Helios may not be a specific marker for natural Treg cells.12
We identified the characteristics of the differentiation and development of Th1, Th17 and Treg cells in vivo using dynamic analysis. MLN are the most ideal tissue to analyze the above three subset developments in vivo. Th1 and Th17 cells emerge at two weeks and reach peak levels at four weeks. These data suggest that a 4-week period of observation is sufficient for Th1 and Th17 cell development in this model. Treg cells emerge a little later with peak levels appearing in the late stage of disease. It is possible that the late appearance of Treg cells represents a compensatory feedback to dampen the pro-inflammatory effects of Th1 and Th17 cells. While the Treg cells can not completely prevent disease, it is possible that disease progression will be exacerbated if these Treg cells are absent.13
Th2, Th9, Th22 and Tfh cells are less detectable
We also analyze the developmental ability of Th2, Th9, Th22 and Tfh cells since they also play important roles in different immune responses. As expected, the levels of these cells are much lower in either MLN or LP in colitis (Supplementary Figures 1a–c). Thus, colitis may not be an ideal model to determine the differentiation and mechanisms of these cell subsets in vivo. As reported previously, Tfh cells were significantly increased in mice immunized with keyhole limpet hemocyanin (KLH) protein in vivo,14 suggesting that the KLH model could be utilized as an alternative for monitoring the development of Tfh cells. In total, an obvious advantage is that naive CD4+CD45RBhigh T cells from any gene knockout mice on B6 background can be transferred into B6 Rag1−/− mice, and the role of suspected gene(s) on Th17, Th1, and Treg cell development and colitis inflammation could be identified. For instance, transfer of CD4+CD45RBhigh T cells isolated from RORγt KO mice into Rag1−/− recipients fails to induce colitis, suggesting that the Th17 cell subset is crucial for disease induction in the colitis model.15 Taken together, our data have demonstrated the dynamic changes and differentiation features of Th1, Th17, Treg and other T helper cells in an adoptive transfer model of colitis in vivo. This protocol can provide important information on the differentiation and development of T helper and Treg subsets at different time points during disease induction or under the influence of novel gene(s).
Acknowledgments
This work is supported in part by grants from the National Key R&D Program of China (2017YFA0105800), NIH R01 AR059103, NIH STAR award, NIH R61 AR073049, National Natural Science Foundation of China (81671611, 81701600), Guangdong Province Innovative Research Program Project (2011Y035), and Zhejiang Provincial Natural Science Foundation of China (LY14H100002).
Footnotes
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website (http://www.nature.com/cmi)
The authors declare no conflict of interest.
Supplementary Material
References
- Hu G, Liu Z, Zheng C, Zheng SG. Antigen-non-specific regulation centered on CD25+foxp3+ treg cells. Cell Mol Immunol 2010; 7: 414–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Ni X, Pan X, Lu H, Lu Y, Zhao J et al. Human cd39hi regulatory T cells present stronger stability and function under inflammatory conditions. Cell Mol Immunol 2017; 14: 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Guo Z, Ju W, Ryffel B, He X, Zheng SG. The development and function of follicular helper t cells in immune responses. Cell Mol Immunol 2012; 9: 375–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZM, Wang KP, Ma J, Guo Zheng S. The role of all-trans retinoic acid in the biology of foxp3+ regulatory t cells. Cell Mol Immunol 2015; 12: 553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45rbhi CD4+ T cells. Immunity 1994; 1: 553–562. [DOI] [PubMed] [Google Scholar]
- Coccia M, Harrison OJ, Schiering C, Asquith MJ, Becher B, Powrie F et al. Il-1beta mediates chronic intestinal inflammation by promoting the accumulation of il-17a secreting innate lymphoid cells and CD4(+) th17 cells. J Exp Med 2012; 209: 1595–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Thalhamer T, Franca RF, Xiao S, Wang C, Hotta C et al. Galectin-9-cd44 interaction enhances stability and function of adaptive regulatory T cells. Immunity 2014; 41: 270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izcue A, Hue S, Buonocore S, Arancibia-Carcamo CV, Ahern PP, Iwakura Y et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity 2008; 28: 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Tang J, Chen W, Li Q, Nie J, Lin F et al. Inflammation negatively regulates foxp3 and regulatory T-cell function via dbc1. Proc Natl Acad Sci USA 2015; 112: E3246–E3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced foxp3+ T reg cells. J Exp Med 2012; 209: 1723–1742, S1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y et al. Expression of helios, an ikaros transcription factor family member, differentiates thymic-derived from peripherally induced foxp3+ T regulatory cells. J Immunol 2010; 184: 3433–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurek E, Cebula A, Wojciech L, Pietrzak M, Rempala G, Kisielow P et al. Differences in expression level of helios and neuropilin-1 do not distinguish thymus-derived from extrathymically-induced CD4+foxp3+ regulatory T cells. PLoS One 2015; 10: e0141161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Su W, Lin X, Guo Z, Wang J, Zhang Q et al. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of th1 and th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum 2013; 65: 1181–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD et al. Bcl6 mediates the development of t follicular helper cells. Science 2009; 325: 1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppkes M, Becker C, Ivanov II, Hirth S, Wirtz S, Neufert C et al. Rorgamma-expressing th17 cells induce murine chronic intestinal inflammation via redundant effects of il-17a and il-17f. Gastroenterology 2009; 136: 257–267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.