The innate immune system plays an essential role in the host defense against infections by initially sensing and recognizing diverse microbial pathogens and directing adaptive immune responses to their infections. How does the innate immune system sense and recognize pathogens? The identification of pattern recognition receptors (PRRs) during the past decades has brought us into a new era to explore this fundamental question. PRRs are an array of germline-encoded multifunctional proteins that do not recognize specific pathogens but rather recognize conserved molecular patterns associated with various classes of pathogens, leading to pathogen-associated molecular patterns (PAMPs).1 Among the PRRs, there is a family of Toll-like receptors (TLRs), including TLR5, that recognize a wide variety of PAMPs and elicit innate immune responses. TLR5 is known to specifically sense and recognize flagellin, the major structural protein of bacterial flagella.2 It is conceivable that the immune system is equipped with TLR5 for the detection of flagellin in different cells, tissues and organs. However, the fundamental functions of TLR5 beyond the recognition of flagellin are only beginning to be appreciated. Studies on TLR5 in recent years have revealed different scenarios after the recognition of flagellin by TLR5 in the respiratory tract, gastrointestinal tract, liver or even in the dorsal root ganglion neurons.
TLR5 is expressed constitutively in epithelial cells and immune cells, such as monocytes and immature DCs. TLR5 is preferentially expressed on the apical side of respiratory epithelia in both mice and humans.3, 4 Thus, TLR5 can induce early signaling dedicated to protective innate immune responses against respiratory infection. For example, airway epithelium TLR5 can sense Pseudomonas aeruginosa and initiate an early host inflammatory reaction to clear the invading pathogen.5 Moreover, the early clearance of P. aeruginosa from the airways depends on flagellin-TLR5-MyD88-dependent signaling in respiratory epithelial cells.6
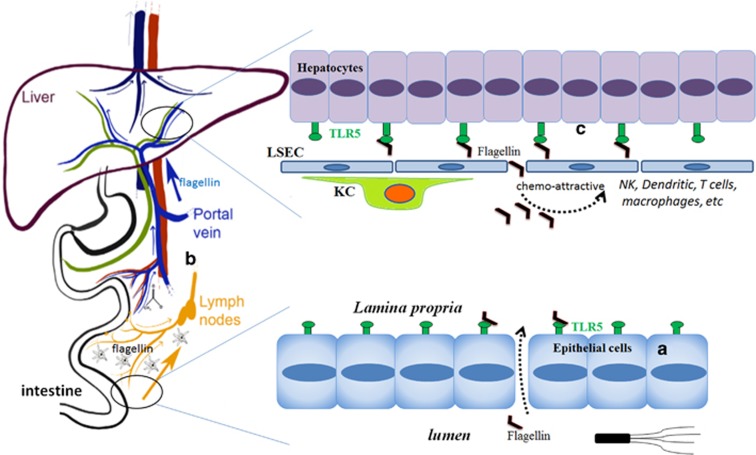
In contrast with the respiratory tract, TLR5 is expressed mostly on the basolateral side of intestinal epithelial cells and recognizes the bacterial locomotion component flagellin for detecting whether bacteria have crossed the gut epithelia1 (Figure 1a). The expression profile and subcellular distribution features of TLR5 in the gastrointestinal tract are in line with the prominent location of bacteria in the gastrointestinal tract and the requirements of the gut immune system to address gastrointestinal microbiota, in which commensal dissemination to extraintestinal organs, the overgrowth of toxigenic members or opportunistic pathogenic bacterial invasion and infection need to be constantly monitored and prevented. Accordingly, TLR5 is used by the mucosal immune system of the gut to detect flagellin for the surveillance of gastrointestinal microbiota. Thus, intestinal epithelial cell TLR5 senses the composition and localization of the intestinal microbiota to prevent diseases associated with intestinal inflammation.7 Aberrantly elevated TLR5 activation in response to bacterial flagellin might result in an impairment of the epithelial barrier integrity or the exacerbation and perpetuation of chronic gut inflammation.8 Improper bacterial flagellin recognition by TLR5 is also linked to changes in the gut microbiota composition, distorted adipose tissue metabolism and inflammation.9 As an ancient innate immune receptor, TLR5 is crucial for both immune homeostasis and protection against bacterial infection in mammals, birds, amphibians, fish and reptiles.10 Further investigation of the most primitive function of TLR5 will shed new light on the role of TLR5 in both sustaining gut immune homeostasis and the clearance of intestinal pathogenic bacteria.
Figure 1.
TLR5 is in the intestine and liver for the recognition of flagellin and beyond. (a) TLR5 is expressed on the basolateral side of intestinal epithelial cells and functions in the normal surveillance of flagellated bacteria. (b) The liver receives the intestinal venous blood circulation and arterial supply and acts as a functional vascular firewall that clears commensals that have penetrated either the intestinal or systemic vascular circuits. (c) TLR5 is expressed at a low level by hepatocytes, which can be activated by flagellin specifically in the liver. TLR5 signaling in the liver modulates local immunity by secreting cytokines and chemo-attracting immunocytes, such as neutrophils, macrophages, NK cells, dendritic cells and T cells. These cells may help to eliminate bacteria directly and regulate specific immune responses.
The liver is a unique anatomical and immunological site because of its physiological roles in the metabolism of nutrients and the confluence of hepatic arterial and venous blood (Figure 1b). The liver is continually exposed to various pathogens with both systemic and gut origins. In light of this situation, the liver plays a role in immune surveillance for pathogen detection and host defense.11 The ability of liver immune sentinels to detect pathogens is also dependent on the array of PRRs expressed by liver sinusoidal endothelial cells (LSECs), hepatocytes, Kupffer cells (KCs), DCs and liver-resident lymphocytes. The liver expresses a wide array of PRRs, including TLRs. Given the anatomical location and functional features of the liver, the response to a number of TLR ligands in the liver often differs from the response observed in other tissues. This is represented mostly as the immunosuppressive response, which is speculated to be a mechanism used to avoid a constant state of liver inflammation induced by continuous exposure to bacteria-derived molecules, such as LPS, flagellin and so on. Although TLRs are expressed by most cells in the liver, including hepatocytes, LSECs, HSCs, KCs and lymphocytes, TLR5 is only expressed at low levels in hepatocytes, KCs and hepatic DCs; however, it is not expressed on LSECs.12 The role of TLR5 in the liver has been largely neglected because of its low expression in this organ, but in recent years, increasingly important activities of TLR5 in the liver have been observed (Figure 1c). For example, hepatocyte TLR5 was found to detect flagellin in the liver, promote bacterial clearance from the liver and protect the liver against chronic inflammatory diseases, although its precise mechanism has not yet been defined. In this detection and clearance process, the liver may act as a functional vascular firewall that clears commensals that have penetrated either the intestinal or systemic vascular circuits.13 Another study showed that the liver is the major organ that responds to the TLR5 agonist.14 Moreover, hepatocytes were found to be the key liver cell type that responds rapidly and directly to a flagellin analog but not to LPS, and the activation of liver TLR5 has been shown to play a central role in flagellin’s radioprotective and anticancer properties.14 The following study further demonstrated that the systemically administered flagellin analog generates protective antitumor T-cell immunity in the liver through coordination with CXCR3-expressing NK cells.15 These studies reveal that the effects of flagellin-TLR5 activation in the liver go far beyond the recognition of flagellin. In light of this, the protective effects and possible side effects of the activation of the TLR5-signaling pathway in the liver by flagellin should be evaluated more carefully, especially in regard to its proposed systemic use for tumor patients. At the least, the over-activation of TLR5 signaling in the liver should be avoided because liver injury induced by high doses of flagellin was observed in mice.16
In addition to the roles played by TLR5 in the respiratory tract, gastrointestinal tract and liver, a recent study on mechanical allodynia in neuropathic pain showed that the effect of TLR5 activation in neurons even works beyond its recognition of flagellin.17 This study found that TLR5 is expressed by peripheral sensory neurons, in which TLR5 mediates touch sensation and permits the rapid transport of certain small molecules across the neuronal membrane through an unknown mechanism.17 This introduces a promising new therapeutic strategy to treat persistent pain by targeting TLR5. On the other hand, it also suggests a more intriguing role for TLR5 as a gate-keeper for cellular ion channels or as a regulator of infection-caused tissue injury and pain.
It seems that more functional roles of TLR5 are waiting to be revealed. Many open questions regarding TLR5 beyond its recognition of flagellin remain to be answered. First, how does TLR5 act in the bidirectional communication between the intestine and liver under physiological conditions and in different diseases? Second, is there any immune communication between the lung and liver? If yes, does TLR5 play a role in the lung–liver axis? Third, what is the physiological role of TLR5 expressed in peripheral sensory neurons? Does TLR5 play roles in detecting tissue injury and related pain? These answers will open new doors for our further understanding of TLR5 and other TLRs.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Nos. 31300717 and 81461130019) and the Grants of Deutsche Forschungsgemeinschaft (TRR60 and GK1949).
Footnotes
The authors declare no conflict of interest.
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006; 124: 783–801. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001; 410: 1099–1103. [DOI] [PubMed] [Google Scholar]
- Cao Y, Zhang E, Yang J, Yang Y, Yu J, Xiao Y et al. Frontline science: nasal epithelial GM-CSF contributes to TLR5-mediated modulation of airway dendritic cells and subsequent IgA response. J Leukoc Biol 2017; 102: 575–587. [DOI] [PubMed] [Google Scholar]
- Wang R, Ahmed J, Wang G, Hassan I, Strulovici-Barel Y, Salit J et al. Airway epithelial expression of TLR5 is downregulated in healthy smokers and smokers with chronic obstructive pulmonary disease. J Immunol 2012; 189: 2217–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Louboutin JP, Weiner DJ, Goldberg JB, Wilson JM. Human airway epithelial cells sense Pseudomonas aeruginosa infection via recognition of flagellin by Toll-like receptor 5. Infect Immun 2005; 73: 7151–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anas AA, van Lieshout MH, Claushuis TA, de Vos AF, Florquin S, de Boer OJ et al. Lung epithelial MyD88 drives early pulmonary clearance of Pseudomonas aeruginosa by a flagellin dependent mechanism. Am J Physiol Lung Cell Mol Physiol 2016; 311: L219–L228. [DOI] [PubMed] [Google Scholar]
- Chassaing B, Ley RE, Gewirtz AT. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology 2014; 147: 1363–1377 e1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopetuso LR, Jia R, Wang XM, Jia LG, Petito V, Goodman WA et al. Epithelial-specific Toll-like receptor (TLR)5 activation mediates barrier dysfunction in experimental ileitis. Inflamm Bowel Dis 2017; 23: 392–403. [DOI] [PubMed] [Google Scholar]
- Pekkala S, Munukka E, Kong L, Pollanen E, Autio R, Roos C et al. Toll-like receptor 5 in obesity: the role of gut microbiota and adipose tissue inflammation. Obesity 2015; 23: 581–590. [DOI] [PubMed] [Google Scholar]
- Voogdt CG, Bouwman LI, Kik MJ, Wagenaar JA, van Putten JP. Reptile Toll-like receptor 5 unveils adaptive evolution of bacterial flagellin recognition. Sci Rep 2016; 6: 19046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol 2013; 14: 996–1006. [DOI] [PubMed] [Google Scholar]
- Wu J, Meng Z, Jiang M, Zhang E, Trippler M, Broering R et al. Toll-like receptor-induced innate immune responses in non-parenchymal liver cells are cell type-specific. Immunology 2010; 129: 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer ML, Slack E, de Gottardi A, Lawson MA, Hapfelmeier S, Miele L et al. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci Transl Med 2014; 6: 237ra266. [DOI] [PubMed] [Google Scholar]
- Burdelya LG, Brackett CM, Kojouharov B, Gitlin II, Leonova KI, Gleiberman AS et al. Central role of liver in anticancer and radioprotective activities of Toll-like receptor 5 agonist. Proc Natl Acad Sci USA 2013; 110: E1857–E1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackett CM, Kojouharov B, Veith J, Greene KF, Burdelya LG, Gollnick SO et al. Toll-like receptor-5 agonist, entolimod, suppresses metastasis and induces immunity by stimulating an NK-dendritic-CD8+ T-cell axis. Proc Natl Acad Sci USA 2016; 113: E874–E883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Liu F, Yang J, Zhong M, Zhang E, Li Y et al. Over-activation of TLR5 signaling by high-dose flagellin induces liver injury in mice. Cell Mol Immunol 2015; 12: 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZZ, Kim YH, Bang S, Zhang Y, Berta T, Wang F et al. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat Med 2015; 21: 1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]