Abstract
The outcome of hepatitis B viral (HBV) infection is determined by the complex interactions between replicating HBV and the immune system. While the role of the adaptive immune system in the resolution of HBV infection has been studied extensively, the contribution of innate immune mechanisms remains to be defined. Here we examined the role of the interleukin-1 receptor/Toll-like receptor (IL-1R/TLR) signaling pathway in adaptive immune responses and viral clearance by exploring the HBV mouse model. Hydrodynamic injection with a replication-competent HBV genome was performed in wild-type mice (WT) and a panel of mouse strains lacking specific innate immunity component expression. We found higher levels of HBV protein production and replication in Tlr2−/−, Tlr23479−/−, 3d/Tlr24−/−, Myd88/Trif−/− and Irak4−/− mice, which was associated with reduced HBV-specific CD8+ T-cell responses in these mice. Importantly, HBV clearance was delayed for more than 2 weeks in 3d/Tlr24−/−, Myd88/Trif−/− and Irak4−/− mice compared to WT mice. HBV-specific CD8+ T-cell responses were functionally impaired for producing the cytokines IFN-γ, TNF-α and IL-2 in TLR signaling-deficient mice compared to WT mice. In conclusion, the IL-1R/TLR signaling pathway might contribute to controlling HBV infection by augmenting HBV-specific CD8+ T-cell responses.
Keywords: CD8+ T-cell response, Hepatitis B virus, IL-1R/TLR signaling pathway, Toll-like receptor
Introduction
Hepatitis B virus (HBV), a hepatotropic noncytopathic DNA virus, affects millions of chronically HBV-infected patients and causes fatal liver cirrhosis and hepatocellular carcinoma.1 The outcome of HBV infection is determined by complex interactions between replicating HBV and the host immune system. Specific CD8+ T-cell responses against HBV proteins play a major role in viral clearance but are functionally impaired in chronically HBV-infected patients.2 Restoration of HBV-specific CD8+ T-cell response in chronically HBV-infected patients may be a promising strategy to terminate HBV persistence. This concept has been successfully proven in the woodchuck model for chronic woodchuck hepatitis virus (WHV) infection.3, 4
Interleukin-1 receptor/Toll-like receptor (IL-1R/TLR) signaling is generally important for host immune responses against microbial infections and vaccine efficacy. For example, MyD88 was shown to be required for efficient pathogen clearance by inducing IFN-γ response or chemokines, which attract neutrophils, during both Mycobacterium avium and Escherichia coli infection.5, 6 In another study, MyD88 was shown to play an important role in the induction of bacteria-specific memory CD8+ T-cell responses during Listeria infection.7 The effectiveness of DNA vaccination generally requires the function of TLR98, 9 and vaccines with specific adjuvants based on monophosphoryl lipid A or flagellin TLR4 and TLR5, respectively.10, 11 MyD88 was shown to be essential for the induction of efficient CD8+ T-cell responses by a lipopeptide recombinant adenovirus prime/boost vaccine against herpes virus infection.12
Although the central role of adaptive immunity in controlling HBV infection is well established, the contribution of innate immunity in this regard remains largely unexplored. Recent studies have indicated that TLR-mediated innate immune responses contribute directly or indirectly to hepadnaviral replication regulation in both hepatocytes and animal models.13, 14, 15, 16, 17 Activation of TLR signaling pathways leads to the induction of type I interferons (IFNs) and inflammatory cytokines to trigger intracellular signaling pathways, which have been shown to inhibit hepadnaviral replication both in vitro or in vivo.13, 14, 15, 16, 17 As a bridge between innate and adaptive immunity, TLRs might play an important role in the induction of specific immune responses to HBV infection and HBV clearance.18, 19 For example, TLR2 is expressed in activated and memory CD4+ and CD8+ T cells and may serve as a co-stimulatory molecule to enhance T-cell proliferation, survival and effector functions.20, 21 Accordingly, the application of a TLR2 ligand into mice together with transferred tumor antigen (Ag)-specific CD8+ T cells resulted in increased efficacy in tumor models compared to that resulting from the application of CD8+ T cells alone.22 It has been shown that TLR2 engagement in CD8+ T cells increased T-bet transcription in a MyD88-Akt-mTOR- and protein kinase C-dependent manner.23 However, the importance of TLR functions for specific T-cell responses to HBV remains unclear. Accumulating evidence supports the hypothesis that HBV may interfere with TLR function and related IFN action. It has been consistently shown that TLR expression and function are reduced during HBV infection.15, 24, 25, 26 Recently, it was shown that HBV induces the host factor RUN domain Beclin-1-interacting cysteine-rich-containing (Rubicon) protein to antagonize IFN signaling and facilitate its own replication.27 However, no data have answered the question of whether reduced innate immunity impairs the induction of HBV-specific immune responses and HBV clearance. Here we examined HBV clearance and HBV-specific T-cell responses after hydrodynamic injection (HI) of an HBV infectious clone, pSM2, into wild-type mice (WT) and a panel of mouse strains with IL-1R/TLR signal pathway deficiency. Our results demonstrate that the IL-1R/TLR signaling pathway might be required for the priming of functional HBV-specific CD8+ T-cell responses in the HI model and for the suppression of HBV replication with subsequent HBV clearance.
Materials and methods
Mice
C57BL/6 WT mice 6–8 weeks of age were purchased from Harlan Winkelmann Laboratories (Borchen, Germany). Tlr2−/−, Tlr23479−/−, 3d/Tlr24−/−, Asc−/−, Myd88/Trif−/− and Irak4−/− mice on the C57BL/6 background were bred at the Institute of Medical Microbiology of the University Hospital of Essen. All animals were maintained according to the guidelines of the animal facility at the University Hospital of Essen. All the experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the district Government of Düsseldorf, Germany.
Plasmid and peptides
A replication-competent HBV clone harboring a head-to-tail tandem dimeric HBV genome, pSM2 (GenBank accession number: V01460), was provided by Dr Hans Will (Heinrich-Pette-Institute, Hamburg, Germany) and used previously in our lab.28 The peptide used in this study consisted of the Kb-restricted HBV Env190–197 epitope (VWLSVIWM) and the Kb-HBV Cor93–100 epitope (MGLKFRQL). The peptides and the synthetic lipopeptide Pam3CSK4 (TLR2/TLR1 ligand) were purchased from EMC Microcollections (Tübingen, Germany).
HI in mice
Male C57/BL6 mice and Tlr2−/−, Tlr23479−/−, 3d/Tlr24−/−, Asc−/−, Myd88/Trif−/− and Irak4−/− mice 8 weeks of age were hydrodynamically injected with 10 μg of pSM2 in a volume of phosphate-buffered saline (PBS) solution equivalent to 0.1 ml/g of their body weight through their tail veins within 8 s.29 The results of HI may be influenced by some factors, and therefore have significant inter-assay variation. Consequently, there are some differences between the WT control mice from experiment to experiment. For this reason, we used the control WT mice with each strain of deficient mice in parallel. The interpretation of a given experiment should be compared with the WT control group.
Isolation of lymphocytes from the blood, spleen and liver
Murine peripheral blood lymphocytes (PBLs) were isolated after lysis of red cells using erythrocyte lysis buffer (Qiagen, Hilden, Germany). Preparation of single cell suspensions of murine splenocytes (SPLs) was performed according to a protocol described previously.30 Mouse intrahepatic lymphocytes (IHLs) were isolated as described previously.31 In brief, the mouse liver was perfused immediately with 10 ml of PBS after killing. After perfusion, the liver was homogenized and digested with an enzyme solution containing 0.05% collagenase type IV (Sigma-Aldrich, St Louis, MO, USA), 0.002% DNAase I (Sigma-Aldrich) and 10% fetal bovine serum for 30 min. After digestion, the pellet was resuspended in 40% Percoll and centrifuged at 1000g without the breaks. After removing the debris and hepatocytes from the top layer, the IHLs in the pellet were collected, washed and subjected to further analysis.
Cell surface and intracellular cytokine staining of murine lymphocytes
Up to 1 × 106 isolated PBLs, SPLs and IHLs per well were plated in 96-well plates in 200 μl of complete RPMI 1640 medium. The cells were stimulated for 5 h at 37 °C with the selected CD8+ T-cell epitope peptide at a final concentration of 2 μg/ml in the presence of 2 μg/ml anti-CD28 antibody (clone 37.51; BD Pharmingen, Heidelberg, Germany) and 5 μg/ml brefeldin A (Sigma-Aldrich). Unstimulated cells and cells stimulated with the cytomegalovirus-derived peptide (YILEETSVM) served as negative controls. The cells were then incubated for 30 min at 4 °C with the anti-CD8 (clone 56.6-7; BD Pharmingen) and anti-CD4 (clone L3T4; BD Pharmingen) antibodies and 7-aminoactinomycin D (7AAD) (Becton Dickinson, Heidelberg, Germany) to exclude dead cells. After washing, intracellular cytokine staining was performed according to the manufacturer’s instructions using the Cytofix/Cytoperm Plus kit (BD Pharmingen) with the following antibodies: anti-IFN-γ (clone XMG1.2; BD Pharmingen), anti-TNF-α (clone MP6-XT22; eBioscience, Thermo Fisher Scientific, Waltham, MA, USA) and anti-IL-2 (clone JES6-5H4; eBioscience). The stained cells were analyzed on the FACSCalibur (Becton, Dickinson, Heidelberg, Germany) or NAVIOS Flow Cytometer (Beckman Coulter, Brea, CA, USA). The data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
Preparation of the peptide-loaded dimer and dimer staining
To stain the CD8+ T cells specific to the Kb-restricted HBV Env190–197 and Cor93–100 epitopes, recombinant soluble dimeric mouse H-2K[b]: Ig fusion proteins (DimerX I, BD Bioscience) were loaded with the respective peptides overnight and then used to stain mouse lymphocytes according to the technical instructions. The cells were first incubated with CD16/CD32 rat anti-mouse antibody (clone 2.4G2; BD Pharmingen) to block FcRs. Then, the cells were stained with anti-CD8, 7AAD and anti-PD-1 (clone J43; BD Pharmingen). After washing, dimer staining was performed by incubating the dimer and cells for 1.5 h at 4 °C. The cells were then washed and incubated with an anti-IgG1 antibody (clone 85.1; eBioscience) for 30 min at 4 °C. The stained samples were run on a FACSCalibur (Becton Dickinson) or NAVIOS Flow Cytometer (Beckman Coulter GmbH). The data were analyzed using FlowJo software. The percentage of specific CD8+ T cells in the liver was calculated based on the percentage of dimer+ CD8+ T cells within the CD8+ T-cell population of viable lymphocytes recovered from each liver.
Detection of serum HBV antigen and HBV DNA
The serum HBsAg and HBeAg levels were determined using the Architect System and HBsAg and HBeAg CMIA kits (Abbott Laboratories, Wiesbaden-Delkenheim, Germany) according to the manufacturer’s instructions. Serum HBV DNA was extracted using the QiAamp DNA Blood Mini kit (Qiagen) and quantified by real-time PCR using a Platinum SYBR Green Kit (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) as described.28
Statistical analyses
Statistical analyses were performed using GraphPad Prism software version 5 (GraphPad Software Inc., San Diego, CA, USA). Statistical differences were analyzed by the unpaired Student t-test and the Mann–Whitney test. P-values <0.05 were considered significant, and asterisks mark significant differences between the different groups (*indicates P<0.05, **indicates P<0.01, ***indicates P<0.001).
Results
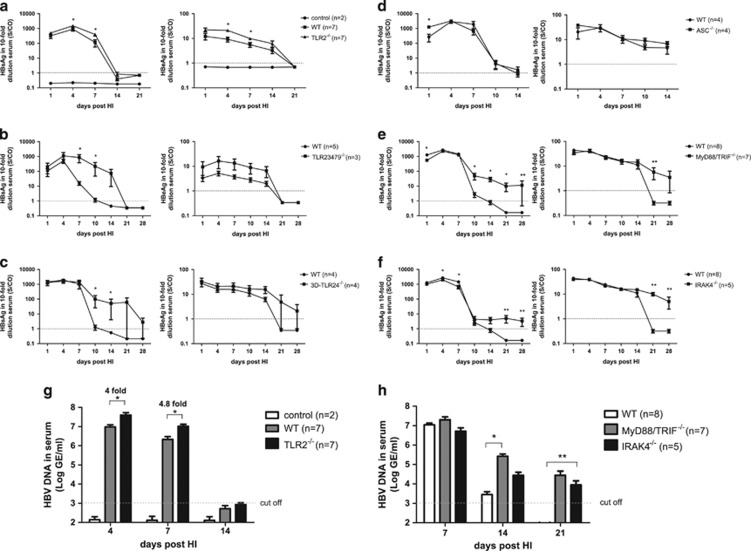
Deficiency in the IL-1R/TLR signaling pathway led to higher HBV replication and gene expression levels and partly delayed HBV clearance in the HI mouse model
To investigate the role of innate immunity in HBV clearance, we compared HBV gene expression and replication in WT, Tlr2−/−, Tlr23479−/−, 3d/Tlr24−/− and Asc−/− mice after HI of the HBV infectious clone pSM2. The 3d/Tlr24−/− mice lack TLR2 and TLR4 expression and express a neutralizing mutant of UNC93B1 (3D), which is involved in endoplasmic reticulum-endosome trafficking of endosomal TLRs, such as TLRs 3, 7, 9, 11, 12 and 13.32 Apoptosis-associated speck-like protein containing a CARD gene deficient (Asc−/−) mice lack an adapter protein central to the numerous nucleotide-binding domains and leucine-rich repeat receptor (NLR) encompassing inflammasomes, such as absent in melanoma 2 (AIM2)-like receptor inflammasomes.33 WT mice cleared HBsAg and HBeAg from the blood on days 14 and 21 after HI, respectively. Compared to WT mice, the levels of HBsAg, HBeAg and HBV DNA were significantly higher in the Tlr2−/− mice on days 4 and 7 after HI (Figures 1a and g). However, the Tlr2−/− mice cleared HBV markers from peripheral blood with a kinetic comparable to that of the controls. Thus, TLR2 contributed to the control of HBV replication but was not essential for viral clearance in the mice. In contrast, HBsAg clearance was delayed in the Tlr23479−/− (Figure 1b) and 3d/Tlr24−/− mice (Figure 1c) compared to WT mice, demonstrating the relevance of the TLR signaling pathway for HBV clearance in this model. The Asc−/− mice cleared HBsAg and HBeAg from the peripheral blood with the same kinetics as WT mice (Figure 1d), suggesting that apoptosis-associated speck-like protein containing a CARD gene (ASC)-dependent inflammasomes are not involved in the control of HBV replication. In general, the serum HBeAg levels were low in comparison with the HBsAg levels in the mouse HI model. Thus, the serum HBeAg level is not a sensitive marker and can only be used qualitatively for monitoring HBV infection levels. We compared the HBeAg levels between Tlr23479−/−, 3d/Tlr24−/− and WT mice and found that Tlr23479−/− and 3d/Tlr24−/− mice had higher HBeAg levels than WT mice (Figure 1). However, this difference was not statistically significant due to the low overall levels of HBeAg.
Figure 1.
IL-1R/TLR signaling pathway deficiency led to higher HBV replication and gene expression levels and partly delayed HBV clearance in the HI mouse model. The HBV plasmid pSM2 or PBS was hydrodynamically injected into WT C57BL/6 mice and Tlr2−/− (a), Tlr23479−/− (b), 3d/Tlr24−/− (c), Asc−/− (d), Myd88/Trif−/− (e) and Irak4−/− (f) mice. The control mice received PBS via HI. The serum levels of HBsAg, HBeAg and HBV DNA (g and h) were determined by ELISAs and real-time PCR at the indicated time points after HI. The bars represent the mean values and the standard errors of the means obtained for each group of mice. *P<0.05; **P<0.01. HBV, hepatitis B viral; HI, hydrodynamic injection; IL-1R/TLR, interleukin-1 receptor/Toll-like receptor; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; WT, wild type.
Next, we asked whether the TLR signaling pathways directly influenced HBV clearance in the HI model. MyD88, TRIF and IRAK4 are essential mediators of IL-1R/TLR signaling. Thus, we examined HBV gene expression and replication in Myd88/Trif−/− and Irak4−/− mice. Although WT mice cleared HBsAg and HBV DNA 14 days after HI, in both the Myd88/Trif−/− and Irak4−/− mice, the HBsAg and HBV DNA levels in the serum remained positive for more than 28 days after HI (Figures 1e, f and h). These results implied that the IL-1R/TLR signaling pathway significantly contributes to HBV clearance. Nevertheless, HBsAg and HBV DNA became undetectable in Myd88/Trif−/− and Irak4−/− mice on day 42 post HI (data not shown).
HBV-specific CD8+ T-cell responses after HI were impaired in TLR-deficient mice but not in Asc −/− mice
Recent studies demonstrated that adaptive immune responses against HBV are required for HBV clearance in the HI mouse model of HBV infection.34, 35, 36 IL-1R/TLR signaling was shown to be important for the initiation of proper adaptive immune responses.19 Therefore, we investigated whether the TLRs affected the adaptive immune responses to HBV after HI in the mice. In naive mice, the TLR deficiency did not affect the CD8+ and CD4+ T-cell frequencies or the activation of CD8+ T cells by anti-CD3 and anti-CD28 antibodies (Supplementary Figures S1A–C). However, CD8+ T-cell responses to the TLR2 ligand were absent in the Tlr2−/− mice (Supplementary Figure S1D and data not shown). These results suggested that TLR or TLR signaling deficiency did not lead to the intrinsic defective activation and function of CD8+ T cells.
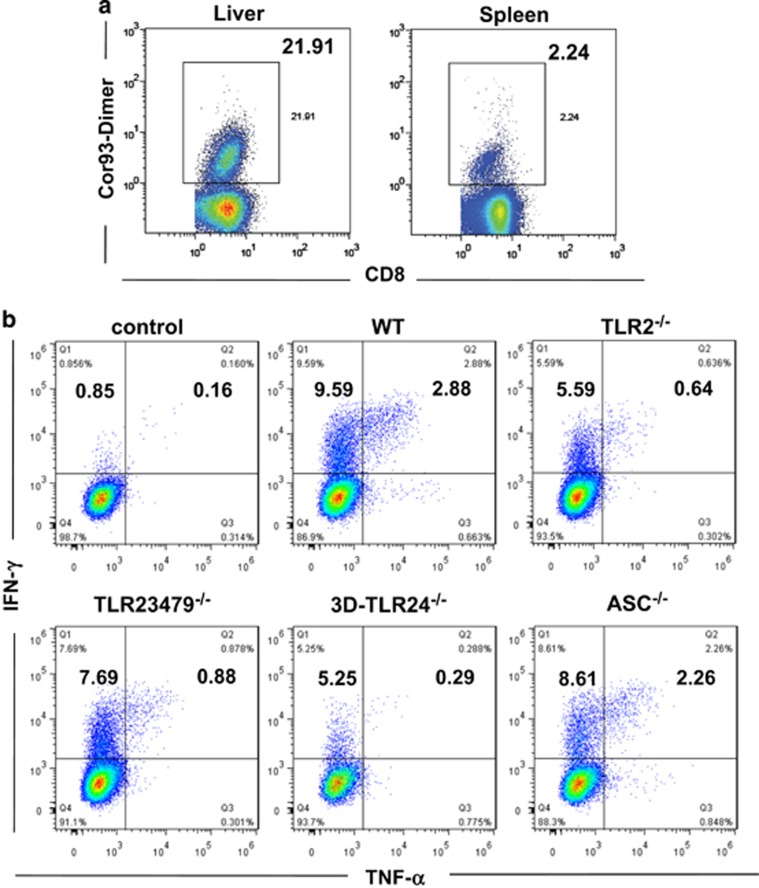
The HBcAg-specific CD8+ T-cell response has been shown to play a key role in the control of HBV replication and gene expression in the HI model.34 Therefore, we analyzed and compared the HBV-specific CD8+ T-cell responses in the different mouse strains after HI. The frequencies of intrahepatic and splenic HBcAg- and HBsAg-specific CD8+ T cells were determined by staining with the HBV Cor93–100 or Env190–197 peptide-loaded dimer. Because of high background with the HBV Env190–197 dimer staining (Supplementary Figure S2), we only showed the results from HBV Core93–100 dimer staining to describe HBV-specific CD8+ T cells in the HI mice (Figure 2a). The function of HBV-specific CD8+ T cells was analyzed by monitoring the frequencies of IFN-γ-, TNF-α- and IL-2-producing CD8+ T cells after ex vivo restimulation with specific HBV peptides (Figure 2b). Our previous study assessed a very low level of HBV-specific CD4+ T-cell response, with a frequency usually <0.1% of the CD4+ T-cell population in the livers of HBV HI mice.37 Thus, we did not continue the analysis of HBV-specific CD4+ T-cell responses by flow cytometry in this study.
Figure 2.
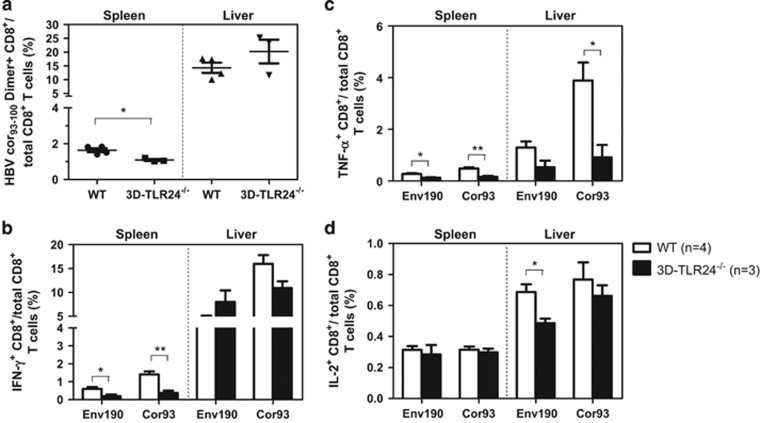
HBV-specific CD8+ T-cell responses in the HBV HI mouse model. Lymphocytes were isolated from the blood, spleens and livers of the mice on day 28 after HI. (a) The specific CD8+ T cells against the HBcAgCor93–100 epitope in the liver and spleen were detected by Cor93–100 peptide-loaded dimer staining. The control mice received PBS for HI. (b) The functionality of the HBV-specific CD8+ T cells was determined by intracellular cytokine staining after ex vivo stimulation with the peptides Env190–197 or Cor93–100 for 5 h. The intrahepatic HBV-specific CD8+ T cells against Cor93–100 from WT, Tlr2−/−, Tlr23479−/−, 3d/Tlr24−/− and Asc−/− mice are shown in the graph. HBV, hepatitis B viral; HI, hydrodynamic injection; PBS, phosphate-buffered saline.
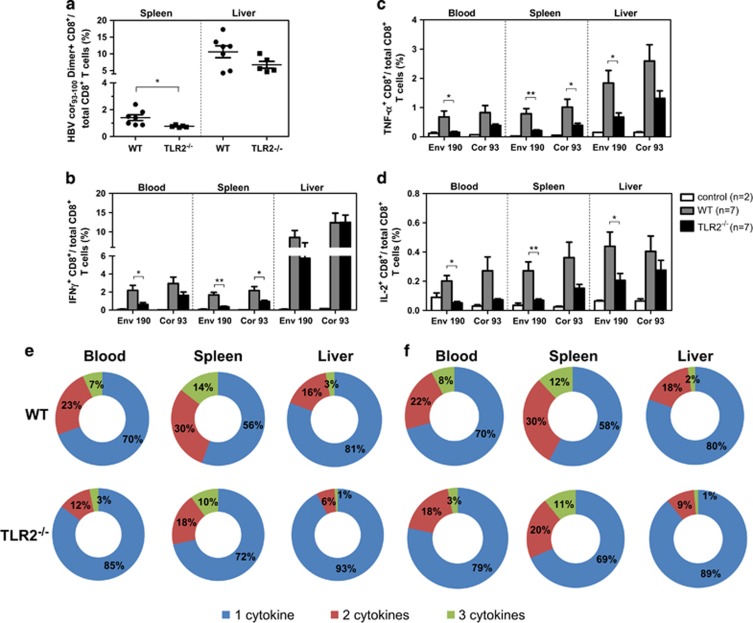
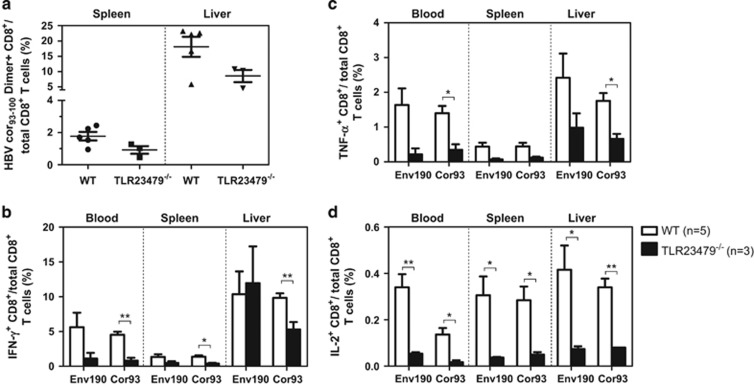
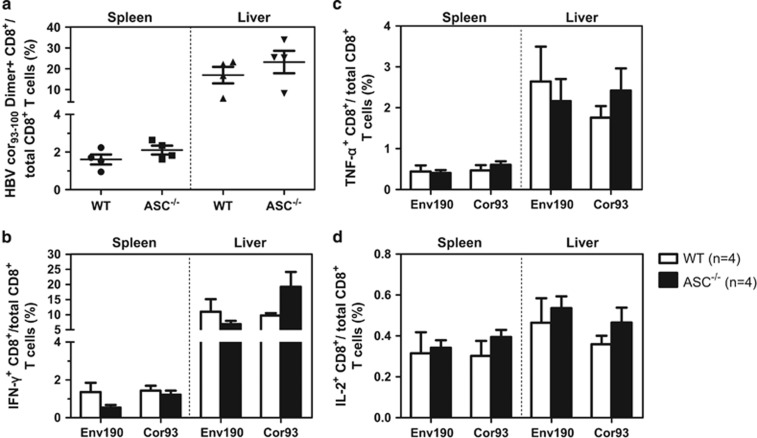
We first compared the HBV-specific CD8+ T-cell response in Tlr2−/− (Figure 3), Tlr23479−/− (Figure 4), 3d/Tlr24−/−(Figure 5) and Asc−/− (Figure 6) mice with that of WT mice. The frequency of HBcAg-specific CD8+ T cells was lower in the livers and spleens of Tlr2−/− mice compared to those of WT mice, but only the difference in the spleen was significant (Figure 3a). The frequency of cytokine-producing CD8+ T cells in the peripheral blood and spleen was also significantly reduced in the Tlr2−/− mice (Figures 3b–d). We further analyzed the multi-functionality of HBV-specific CD8+ T cells regarding their ability to produce single or multiple cytokines (Figures 3e and f). For both the HBsAg- and HBcAg-specific CD8+ T cells, the frequency of double- or triple-positive CD8+ T cells in the Tlr2−/− mice was decreased in the peripheral blood, the spleen and the liver compared to WT mice. Similarly, HBV-specific CD8+ T cells produced less cytokines in the Tlr23479−/− and 3d/Tlr24−/− mice compared to those in WT mice (Figures 4 and 5). Thus, TLR deficiency impaired the adaptive CD8+ T-cell response to HBV. These findings were consistent with higher serum levels of HBsAg and delayed HBsAg and HBeAg clearance in these mice. In contrast, the function of HBV-specific CD8+ T cells was intact in Asc−/− mice, and they cleared HBV with kinetics similar to that of WT mice (Figure 6), indicating that the ASC-dependent inflammasomes are not essential for HBV clearance in the HI mouse model.
Figure 3.
Comparison of HBV-specific CD8+ T-cell responses in WT and Tlr2−/− mice after HI. The HBV plasmid pSM2 was hydrodynamically injected into WT C57BL/6 and Tlr2−/− mice. The WT mice received PBS as a control. Lymphocytes were isolated from the blood, spleens and livers of the mice on day 21 after HI. The specific CD8+ T cells against the HBcAg Cor93–100 epitope in the liver and spleen were detected by Cor93–100 peptide-loaded dimer staining. (a) The functionality of HBV-specific CD8+ T cells was determined by intracellular cytokine staining for IFN-γ, TNF-α and IL-2 after in vitro stimulation with peptides Env190–197 and Cor93–100 for 5 h (b–d). The multi-functionality of specific CD8+ T cells to Env190–197 (e) and Cor93–100 (f) was judged by the percentage of single-, double- and triple-positive CD8+ T cells for the cytokines IFN-γ, TNF-α and IL-2. The bars represent the mean values and the standard errors of the means. *P<0.05; **P<0.01. HBV, hepatitis B viral; HI, hydrodynamic injection; PBS, phosphate-buffered saline; WT, wild type.
Figure 4.
Comparison of HBV-specific CD8+ T-cell responses in WT and Tlr23479−/− mice after HI. The HBV plasmid pSM2 was hydrodynamically injected into WT C57BL/6 mice and Tlr23479−/− mice. Lymphocytes were isolated from the blood, spleens and livers of the mice on day 28 after HI. The specific CD8+ T cells against the HBcAg Cor93–100 epitope in the liver and spleen were detected by Cor93–100 peptide-loaded dimer staining. (a) The functionality of HBV-specific CD8+ T cells was determined by intracellular cytokine staining after in vitro stimulation with peptides Env190–197 and Cor93–100 for 5 h (b–d). HBV, hepatitis B viral; HI, hydrodynamic injection; WT, wild type.
Figure 5.
Comparison of HBV-specific CD8+ T-cell responses in WT and 3d/Tlr24−/− mice after HI. The HBV plasmid pSM2 was hydrodynamically injected into WT C57BL/6 and 3d/Tlr24−/− mice. Lymphocytes were isolated from the spleens and livers of the mice on day 28 after HI. The specific CD8+ T cells against the HBcAg Cor93–100 epitope in the liver and spleen were detected by Cor93–100 peptide-loaded dimer staining. (a) The functionality of HBV-specific CD8+ T cells was determined by intracellular cytokine staining after in vitro stimulation with peptides Env190–197 and Cor93–100 for 5 h (b–d). HBV, hepatitis B viral; HI, hydrodynamic injection; WT, wild type.
Figure 6.
Comparison of HBV-specific CD8+ T-cell responses in WT and Asc−/− mice after HI. The HBV plasmid pSM2 was hydrodynamically injected into WT C57BL/6 and Asc−/− mice. Lymphocytes were isolated from the spleens and livers of the mice on day 14 after HI. The specific CD8+ T cells against the HBcAg Cor93–100 epitope in the livers and spleens were detected by Cor93–100 peptide-loaded dimer staining. (a) The functionality of HBV-specific CD8+ T cells was determined by intracellular cytokine staining after in vitro stimulation with peptides Env190–197 and Cor93–100 for 5 h (b–d). HBV, hepatitis B viral; HI, hydrodynamic injection; WT, wild type.
We tried to detect HBV-specific CD8+ T-cell responses in deficient and WT mice at earlier time points (days 7 and 14 after HI) to generate data on T-cell priming. HBV-specific CD8+ T-cell responses were not detected in the liver, spleen and blood on day 7 by dimer staining or intracellular cytokine staining. However, on day 14 post HI, HBV-specific CD8+ T-cell responses were detected in the liver and blood but not in the spleen. HBV-specific CD8+ T-cell responses were impaired in Tlr2−/−, Tlr23479−/−, 3d/Tlr24−/−, Myd88/Trif−/− and Irak4−/− mice compared with those of WT mice (data not shown), suggesting that the defect in HBV-specific CD8+ T-cell responses in these IL-1R/TLR signaling-deficient mice may be due to inefficient priming of HBV-specific CD8+ T cells at earlier time points of infection.
Our results implicate that IL-1R/TLR signaling might play an important role in the induction of specific CD8+ T-cell responses against HBV and mediate HBV clearance. However, HBV-specific CD8+ T cells accumulated in the liver of Tlr2−/− mice despite reduced numbers of T cells in peripheral organs, which might explain why HBV clearance still occurred in the Tlr2−/− mice within 14 days (Figure 3).
Impaired HBV-specific CD8+ T-cell responses in Myd88/Trif−/− and Irak4−/− mice were associated with decreased T-cell function and higher PD-1 expression
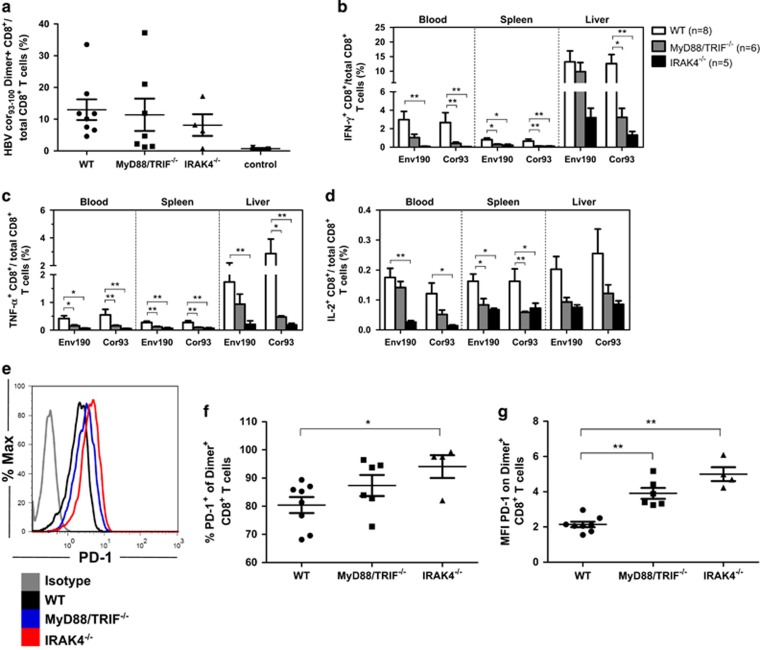
HBV clearance in Myd88/Trif−/− and Irak4−/− mice after HI was delayed compared to that in WT, Tlr2−/−, Tlr23479−/− and 3d/Tlr24−/− mice, as shown in the previous section. Thus, HBV-specific CD8+ T cells may also be functionally impaired in Myd88/Trif−/−and Irak4−/− mice after HI. The frequency of HBcAg-specific CD8+ T cells in the liver was equal in WT, Myd88/Trif−/− and Irak4−/− mice (Figure 7a). However, HBV-specific CD8+ T cells in the peripheral blood, spleen and liver were functionally impaired and produced less cytokines, including IFN-γ-, TNF-α- and IL-2, in both the Myd88/Trif−/− and Irak4−/− mice compared with those of WT mice (Figures 7b–d). Multi-functional analysis of both HBsAg- and HBcAg-specific CD8+ T cells showed that the frequency of double- or triple-positive CD8+ T cells in Myd88/Trif−/− and Irak4−/− mice was decreased in the peripheral blood, the spleen and the liver compared to WT mice (Supplementary Figure S3). These results imply that HBV-specific CD8+ T cells were functionally impaired in the absence of MyD88/TRIF and IRAK4, consistent with the delayed clearance of HBV in Myd88/Trif−/− and Irak4−/− mice.
Figure 7.
Impaired HBV-specific CD8+ T-cell responses in Myd88/Trif−/− and Irak4−/− mice was associated with decreased T-cell function and higher PD-1 expression. (a) Lymphocytes were isolated from the blood, spleens and livers of the mice on day 28 after HI. The specific CD8+ T cells against the HBcAg Cor93–100 epitope in the livers and spleens were detected by Cor93–100 peptide-loaded dimer staining. The control mice received PBS. (b–d) The functionality of HBV-specific CD8+ T cells was determined by intracellular cytokine staining after ex vivo stimulation with peptides Env190–197 and Cor93–100 for 5 h. (e–g) Detection of PD-1 expression on intrahepatic HBcAg-specific CD8+ T cells. *P<0.05; **P<0.01. HBV, hepatitis B viral; HI, hydrodynamic injection; WT, wild type.
Recent studies have suggested that PD-1 is involved in the induction and maintenance of CD8+ T-cell tolerance and energy.38, 39 As shown in Figures 7e–g, most of the intrahepatic HBcAg-specific CD8+ T cells were PD-1 positive in WT, Myd88/Trif−/− and Irak4−/− mice. The frequency of PD-1+ HBcAg-specific CD8+ T cells was significantly higher in Irak4−/− mice but normal in Myd88/Trif−/− mice compared to WT mice. However, when we analyzed the PD-1 expression at a single cell level, we found that the mean fluorescence intensity of PD-1 was significantly higher in intrahepatic HBcAg-specific CD8+ T cells in the Myd88/Trif−/− and Irak4−/− mice compared with that of WT mice.
The functional impairment of HBV-specific CD8+ T cells may due to the insufficient infiltration or activation of antigen-presenting cells (APCs) in the liver in the absence of IL-1R/TLR signaling. Thus, we investigated the frequency and expression of co-stimulatory molecules on the intrahepatic APC-like macrophages, DCs and B cells on day 7 after HI (Supplementary Figure S4). The results showed that there was no significant difference in the APC frequency among the WT, Myd88/Trif−/− and Irak4−/− mice (Supplementary Figure S4A). Furthermore, the expression of co-stimulatory molecules, such as CD40, CD80 and MHC I, was nearly equal to that of the intrahepatic APCs from different mice (Supplementary Figures S4B–D). These results indicated that IL-1R/TLR signal pathway deficiency does not result in impaired recruitment and activation of APCs in the liver after HI.
Discussion
In this study, we investigated the role of innate immunity in the control of HBV replication in the HBV infection mouse model based on HI. Compared with WT mice, Tlr2−/−, Tlr23479−/−, 3d/Tlr24−/−, Myd88/Trif−/− and Irak4−/− mice showed higher serum levels of HBsAg and delayed clearance of HBsAg after HI. Consistently, HBV-specific CD8+ T-cell responses in these mice were reduced and functionally impaired, implying that the IL-1R/TLR signal pathway might play a significant role in the control of HBV replication. Our study suggested that the IL-1R/TLR signaling pathway might significantly contribute to controlling HBV replication, gene expression and HBV clearance. Impairment of HBV-specific CD8+ T-cell responses in Myd88/Trif−/− and Irak4−/− mice might enable HBV to replicate at higher levels and markedly delay viral clearance in the host.
Interestingly, although the Tlr2−/− mice had a significantly impaired specific CD8+ T-cell response to HBV, like that of the Tlr23479−/−, 3d/Tlr24−/−, Myd88/Trif−/− and Irak4−/− mice, they could still clear HBV with the same kinetics as WT mice. The major difference in the Tlr2−/− mice and the other IL-1R/TLR signaling-deficient mice was the accumulated frequency of IFN-γ-producing HBV-specific CD8+ T cells in the liver despite reduced frequencies in the peripheral organs compared with WT mice. The explanation for this difference would be that IFN-γ-producing CD8+ T cells are critical for HBV clearance, and therefore, sufficient IFN-γ-producing CD8+ T cells were recruited to the liver and produced IFN-γ in Tlr2−/− mice, resulting in HBV clearance. The importance of IFN-γ for HBV clearance in the HI mouse model was demonstrated in our previous study by applying the TLR3 ligand PolyI:C.40 Tlr23479−/− mice could still clear HBV, though with slightly delayed kinetics. Even in the Myd88/Trif−/− and Irak4−/− mice, which completely lack IL-1R/TLR signaling, prolonged HBV replication was found only in some of the mice. Thus, single TLR deficiency, as tested and shown here, had little to no influence on HBV clearance. Therefore, single TLR deficiency does not generally impair the initiation of adaptive immunity.
Innate immune deficiency was found in patients with chronic hepatitis B viral infection. For example, their TLR expression and host cell responsiveness to TLR ligands were decreased.24, 25, 26 Though TLR-mediated innate immunity may generally not be activated during acute HBV infection, especially in the early phase, it could be supportive for the initiation of an HBV-specific immune response in the host.41, 42 Reduced TLR expression and impaired TLR signaling during chronic HBV infection likely weakens specific immune responses to HBV.
Using the HBV HI mouse model, Yang et al. first demonstrated that host factors, such as CD4+ and CD8+ T cells, NK cells, Fas, IFN-γ, IFN receptors and TNF receptor 1, are required for elimination of the HBV transcriptional template from the liver. HBcAg93-specific CD8+ T cells are key for mediating HBV clearance from the liver.34, 43 Our study consistently showed that efficient HBV-specific CD8+ T cells play significant roles in the control and clearance of HBV in the HI model. We found that the frequency and functional quality of HBV-specific CD8+ T cells is important for viral control and clearance.
Recently, Tzeng et al.44 demonstrated that TNF-α but not IFN receptors, RIG-1, MDA-5, MYD88, NLRP3, ASC and IL-1 receptor (IL-1R) is required for HBV clearance using an HBV HI mouse model. IL-1R and MYD88 deficiency did not influence HBV clearance in mice. Despite of some differences in the models, the basic findings are consistent with those of our present study. The previous studies showed that HI of the pAAV/HBV1.2 plasmid led to persistent viral replication in mice and resulted in a weak T-cell response, which was only detectable by the ELISpot assay and not by flow cytometry.40, 44 This not only limits the characterization of specific T-cell responses in mice, but may also influence the experimental outcomes. For example, Tzeng et al. found that IFN receptor deficiency did not influence the clearance of HBV. However, Yang et al.34 came to a contradictory conclusion with another HBV clone, as a strong induction of specific T-cell responses was observed.
To answer the question of whether high PD-1 expression in HBV-specific CD8+ T cells contributed to the dysfunction of CD8+ T cells in Myd88/Trif−/− and Irak4−/− mice, we performed a PD-1/PD-L1 pathway blocking experiment with T cells from these mice. However, the PD-L1 antibody treatment did not enhance the function of HBV-specific CD8+ T cells from Myd88/Trif−/− or Irak4−/− mice (data not shown). Thus, PD-1 expression is apparently not the main reason for CD8+ T-cell dysfunction in the knockout mice. Rather, the insufficient priming or helper functions of the HBV-specific CD8+ T cells in Myd88/Trif−/− and Irak4−/− mice might lead to both functional impairment and higher expression of PD-1 in these cells. One must keep in mind that our study was performed during acute infection. While it is well accepted that PD-1 inhibits the function of antigen-specific T cells during chronic viral infection, its role during acute viral infection is less well defined. We have actually shown that PD-1 is an activation marker in CD8+ T cells rather than an exhaustion marker during acute retroviral infection,45 but it can also contribute to cytotoxic T lymphocyte immune escape.46 Additionally, one study demonstrated that antigen-specific CD8+ T cells expressed higher levels of PD-1 in the absence of CD4+ T cells during acute infection.47 Therefore, we suggest that the higher levels of PD-1 expression in HBV-specific CD8+ T cells was attributed to insufficient priming or helper functions in the Myd88/Trif−/− and Irak4−/− mice compared to WT mice, but this is not the mechanistic reason for the CD8+ T-cell dysfunction. The intrahepatic activation and function of APC-like macrophages, DCs and B cells are intact in the Myd88/Trif−/− and Irak4−/− mice compared to WT mice. This implied that the insufficient priming of HBV-specific CD8+ T cells was not due to activation of the APCs. However, Myd88/Trif and IRAK4 deficiency might lead to the impaired induction of inflammatory cytokines, such as IL-1β and IL-18, and other genes relevant for adaptive immunity. Some of these cytokines have immunomodulatory properties that can affect T-cell proliferation and function. Deficiency in the IL-1R/TLR signaling pathway changes these innate immune effector functions, likely impacting T-cell responses.
Our novel findings might add some evidence for the importance of innate immunity in the host immune control of HBV infection. HBV has been initially described as a stealth virus, as no pronounced innate immune responses were found in the livers of HBV-infected chimpanzees during the early phase of acute HBV infection.41 However, recent studies have demonstrated the activation of host innate immunity by HBV or WHV to a measurable extent in humans,42, 48 woodchucks49 and cultured cells.50, 51 It will be important to elucidate the role of innate immune responses for HBV clearance in such experimental systems of natural infection.
According to the data available, there is no doubt that TLR signaling contributes to HBV clearance. Clinical studies have revealed that TLR2/4 and TLR3 signaling are strongly impaired in immune cells from chronically HBV-infected patients,25, 52 indicating a strong interaction between HBV products and TLR2/4 and TLR3 signaling. Activation of the TLR3 pathway has been shown to induce interferon production and lead to viral inhibition.14 In the woodchuck model, the expression of TLR2/4 was inversely correlated with viral titers and modulated by antiviral therapy in chronically infected woodchucks.15 We have also shown that TLR2 activation inhibits hepadnaviral replication in hepatocytes 53 and reverses the tolerogenic properties of liver sinusoidal endothelial cells.54 Thus, TLR2/4 and TLR3 signaling plays a vital role in HBV control. There is not yet evidence of whether TLR5, TLR7, TLR8 and TLR9 are involved in HBV control. However, our recent study with the TLR9 ligand in the woodchuck model clearly demonstrated that TLR9 activation may contribute to viral inhibition but is not sufficient to completely control hepadnaviral infection.55 TLR7 activation may be a promising way to inhibit HBV replication, though TLR7 signaling is apparently not involved in HBV control and not impaired in patients with chronic HBV infection (Huang et al., manuscript in preparation, personal communication).
Given the importance of TLR-mediated innate immunity for the initiation of specific immune responses, TLR activation may be an additional option for combined immunotherapeutic approaches for the treatment of chronic HBV infection. Several studies have shown that the therapeutic activation of TLR2, TLR7, TLR8 and TLR9 could enhance HBV-specific T- or B-cell responses in the liver and lead to HBV clearance in murine models.40, 56, 57, 58 More importantly, a TLR7 ligand, GS-9620, has been examined and showed strong antiviral effects against HBV by inducing IFN responses in woodchuck and chimpanzee models.16, 59 Recently, the TLR8 ligand ssRNA40 was found to selectively activate liver-residential innate immune cells to produce high levels of the antiviral cytokine IFN-γ not only in healthy human livers, but also in chronically HBV- or HCV-infected livers.60 These studies implied that TLR agonists facilitate the restoration of HBV-specific immune responses and thereby achieve viral clearance in chronically HBV-infected patients.18
Acknowledgments
We thank Thekla Kemper for excellent technical assistance. This work was supported in part by grants from the Deutsche Forschungsgemeinschaft (DFG Transregio TRR60 and GRK1045/2).
Footnotes
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website (http://www.nature.com/cmi)
The authors declare no conflict of interest.
Supplementary Material
References
- Dienstag JL. Hepatitis B virus infection. N Engl J Med 2008; 359: 1486–1500. [DOI] [PubMed] [Google Scholar]
- Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol 2005; 5: 215–229. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang E, Ma Z, Wu W, Kosinska A, Zhang X et al. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS Pathog 2014; 10: e1003856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosinska AD, Zhang E, Johrden L, Liu J, Seiz PL, Zhang X et al. Combination of DNA prime—adenovirus boost immunization with entecavir elicits sustained control of chronic hepatitis B in the woodchuck model. PLoS Pathog 2013; 9: e1003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng CG, Scanga CA, Collazo-Custodio CM, Cheever AW, Hieny S, Caspar P et al. Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll-like receptor 2 (TLR2)- and TLR4-deficient animals. J Immunol 2003; 171: 4758–4764. [DOI] [PubMed] [Google Scholar]
- Lebeis SL, Bommarius B, Parkos CA, Sherman MA, Kalman D. TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J Immunol 2007; 179: 566–577. [DOI] [PubMed] [Google Scholar]
- Tam MA, Wick MJ. MyD88 and interferon-alpha/beta are differentially required for dendritic cell maturation but dispensable for development of protective memory against Listeria. Immunology 2009; 128: 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor D, Dubuquoy C, Gaboriau V, Lefevre F, Charley B, Riffault S. TLR9 pathway is involved in adjuvant effects of plasmid DNA-based vaccines. Vaccine 2005; 23: 1258–1264. [DOI] [PubMed] [Google Scholar]
- Pavlenko M, Leder C, Moreno S, Levitsky V, Pisa P. Priming of CD8+ T-cell responses after DNA immunization is impaired in TLR9- and MyD88-deficient mice. Vaccine 2007; 25: 6341–6347. [DOI] [PubMed] [Google Scholar]
- Pouliot K, Buglione-Corbett R, Marty-Roix R, Montminy-Paquette S, West K, Wang S et al. Contribution of TLR4 and MyD88 for adjuvant monophosphoryl lipid A (MPLA) activity in a DNA prime-protein boost HIV-1 vaccine. Vaccine 2014; 32: 5049–5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CT, Hong SH, Sin JI, Vu HV, Jeong K, Cho KO et al. Flagellin enhances tumor-specific CD8(+) T cell immune responses through TLR5 stimulation in a therapeutic cancer vaccine model. Vaccine 2013; 31: 3879–3887. [DOI] [PubMed] [Google Scholar]
- Zhang X, Dervillez X, Chentoufi AA, Badakhshan T, Bettahi I, Benmohamed L. Targeting the genital tract mucosa with a lipopeptide/recombinant adenovirus prime/boost vaccine induces potent and long-lasting CD8+ T cell immunity against herpes: importance of MyD88. J Immunol 2012; 189: 4496–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogawa M, Robek MD, Furuichi Y, Chisari FV. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol 2005; 79: 7269–7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Lu M, Meng Z, Trippler M, Broering R, Szczeponek A et al. Toll-like receptor-mediated control of HBV replication by nonparenchymal liver cells in mice. Hepatology 2007; 46: 1769–1778. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ma Z, Liu H, Liu J, Meng Z, Broering R et al. Role of Toll-like receptor 2 in the immune response against hepadnaviral infection. J Hepatol 2012; 57: 522–528. [DOI] [PubMed] [Google Scholar]
- Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM et al. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology 2013; 144: 1508–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Jiang D, Ma D, Chang J, Dougherty AM, Cuconati A et al. Activation of pattern recognition receptor-mediated innate immunity inhibits the replication of hepatitis B virus in human hepatocyte-derived cells. J Virol 2009; 83: 847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Zhang E, Yang D, Lu M. Contribution of Toll-like receptors to the control of hepatitis B virus infection by initiating antiviral innate responses and promoting specific adaptive immune responses. Cell Mol Immunol 2015; 12: 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul WE. Bridging innate and adaptive immunity. Cell 2011; 147: 1212–1215. [DOI] [PubMed] [Google Scholar]
- Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci USA 2004; 101: 3029–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottalorda A, Verschelde C, Marcais A, Tomkowiak M, Musette P, Uematsu S et al. TLR2 engagement on CD8 T cells lowers the threshold for optimal antigen-induced T cell activation. Eur J Immunol 2006; 36: 1684–1693. [DOI] [PubMed] [Google Scholar]
- Geng D, Zheng L, Srivastava R, Velasco-Gonzalez C, Riker A, Markovic SN et al. Amplifying TLR-MyD88 signals within tumor-specific T cells enhances antitumor activity to suboptimal levels of weakly immunogenic tumor antigens. Cancer Res 2010; 70: 7442–7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng D, Zheng L, Srivastava R, Asprodites N, Velasco-Gonzalez C, Davila E. When Toll-like receptor and T-cell receptor signals collide: a mechanism for enhanced CD8 T-cell effector function. Blood 2010; 116: 3494–3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvanathan K, Skinner NA, Thompson AJ, Riordan SM, Sozzi V, Edwards R et al. Regulation of Toll-like receptor-2 expression in chronic hepatitis B by the precore protein. Hepatology 2007; 45: 102–110. [DOI] [PubMed] [Google Scholar]
- Chen Z, Cheng Y, Xu Y, Liao J, Zhang X, Hu Y et al. Expression profiles and function of Toll-like receptors 2 and 4 in peripheral blood mononuclear cells of chronic hepatitis B patients. Clin Immunol 2008; 128: 400–408. [DOI] [PubMed] [Google Scholar]
- Huang Z, Ge J, Pang J, Liu H, Chen J, Liao B et al. Aberrant expression and dysfunction of TLR2 and its soluble form in chronic HBV infection and its regulation by antiviral therapy. Antiviral Res 2015; 118: 10–19. [DOI] [PubMed] [Google Scholar]
- Wan Y, Cao W, Han T, Ren S, Feng J, Chen T et al. Inducible Rubicon facilitates viral replication by antagonizing interferon production. Cell Mol Immunol 2017; 14: 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang E, Ma Z, Pei R, Jiang M, Schlaak JF et al. Modulation of hepatitis B virus replication and hepatocyte differentiation by microRNA-1. Hepatology 2011; 53: 1476–1485. [DOI] [PubMed] [Google Scholar]
- Yin Y, Wu C, Song J, Wang J, Zhang E, Liu H et al. DNA immunization with fusion of CTLA-4 to hepatitis B virus (HBV) core protein enhanced Th2 type responses and cleared HBV with an accelerated kinetic. PLoS One 2011; 6: e22524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Hilken G, Kruppenbacher J, Kemper T, Schirmbeck R, Reimann J et al. Immunization of woodchucks with plasmids expressing woodchuck hepatitis virus (WHV) core antigen and surface antigen suppresses WHV infection. J Virol 1999; 73: 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispe IN. Isolation of mouse intrahepatic lymphocytes. Curr Protoc Immunol 2001; Chapter 3: Unit 3 21. [DOI] [PubMed] [Google Scholar]
- Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 2008; 452: 234–238. [DOI] [PubMed] [Google Scholar]
- Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev 2015; 265: 6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PL, Althage A, Chung J, Maier H, Wieland S, Isogawa M et al. Immune effectors required for hepatitis B virus clearance. Proc Natl Acad Sci USA 2010; 107: 798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang B, Huang S, Song Z, Wu J, Zhang E et al. Immunosuppressive drugs modulate the replication of hepatitis B virus (HBV) in a hydrodynamic injection mouse model. PLoS One 2014; 9: e85832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YJ, Wu HL, Chen DS, Chen PJ. Hepatitis B virus nucleocapsid but not free core antigen controls viral clearance in mice. J Virol 2012; 86: 9266–9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Wu C, Shi H, Gong Z, Zhang E, Wang H et al. Coexistence of hepatitis B virus quasispecies enhances viral replication and the ability to induce host antibody and cellular immune responses. J Virol 2014; 88: 8656–8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochweller K, Anderton SM. Kinetics of costimulatory molecule expression by T cells and dendritic cells during the induction of tolerance versus immunity in vivo. Eur J Immunol 2005; 35: 1086–1096. [DOI] [PubMed] [Google Scholar]
- Haspot F, Fehr T, Gibbons C, Zhao G, Hogan T, Honjo T et al. Peripheral deletional tolerance of alloreactive CD8 but not CD4 T cells is dependent on the PD-1/PD-L1 pathway. Blood 2008; 112: 2149–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Huang S, Zhao X, Chen M, Lin Y, Xia Y et al. Poly(I:C) treatment leads to interferon-dependent clearance of hepatitis B virus in a hydrodynamic injection mouse model. J Virol 2014; 88: 10421–10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci USA 2004; 101: 6669–6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn C, Peppa D, Khanna P, Nebbia G, Jones M, Brendish N et al. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology 2009; 137: 1289–1300. [DOI] [PubMed] [Google Scholar]
- Kosinska AD, Pishraft-Sabet L, Wu W, Fang Z, Lenart M, Chen J et al. Low hepatitis B virus-specific T-cell response in males correlates with high regulatory T-cell numbers in murine models. Hepatology 2017; 66: 69–93. [DOI] [PubMed] [Google Scholar]
- Tzeng HT, Tsai HF, Chyuan IT, Liao HJ, Chen CJ, Chen PJ et al. Tumor necrosis factor-alpha induced by hepatitis B virus core mediating the immune response for hepatitis B viral clearance in mice model. PLoS One 2014; 9: e103008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinskyy G, Myers L, Dietze KK, Gibbert K, Roggendorf M, Liu J et al. Virus-specific CD8+ T cells upregulate programmed death-1 expression during acute friend retrovirus infection but are highly cytotoxic and control virus replication. J Immunol 2011; 187: 3730–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmetzyanova I, Drabczyk M, Neff CP, Gibbert K, Dietze KK, Werner T et al. PD-L1 expression on retrovirus-infected cells mediates immune escape from CD8+ T cell killing. PLoS Pathog 2015; 11: e1005224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse S, Tsai CY, Molloy MJ, Allie SR, Zhang W, Yagita H et al. Recall responses by helpless memory CD8+ T cells are restricted by the up-regulation of PD-1. J Immunol 2009; 182: 4244–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisicaro P, Valdatta C, Boni C, Massari M, Mori C, Zerbini A et al. Early kinetics of innate and adaptive immune responses during hepatitis B virus infection. Gut 2009; 58: 974–982. [DOI] [PubMed] [Google Scholar]
- Guy CS, Mulrooney-Cousins PM, Churchill ND, Michalak TI. Intrahepatic expression of genes affiliated with innate and adaptive immune responses immediately after invasion and during acute infection with woodchuck hepadnavirus. J Virol 2008; 82: 8579–8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosel M, Quasdorff M, Wiegmann K, Webb D, Zedler U, Broxtermann M et al. Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatology 2009; 50: 1773–1782. [DOI] [PubMed] [Google Scholar]
- Lucifora J, Durantel D, Testoni B, Hantz O, Levrero M, Zoulim F. Control of hepatitis B virus replication by innate response of HepaRG cells. Hepatology 2010; 51: 63–72. [DOI] [PubMed] [Google Scholar]
- Huang YW, Lin SC, Wei SC, Hu JT, Chang HY, Huang SH et al. Reduced Toll-like receptor 3 expression in chronic hepatitis B patients and its restoration by interferon therapy. Antivir Ther 2013; 18: 877–884. [DOI] [PubMed] [Google Scholar]
- Zhang X, Meng Z, Qiu S, Xu Y, Yang D, Schlaak JF et al. Lipopolysaccharide-induced innate immune responses in primary hepatocytes downregulates woodchuck hepatitis virus replication via interferon-independent pathways. Cell Microbiol 2009; 11: 1624–1637. [DOI] [PubMed] [Google Scholar]
- Liu J, Jiang M, Ma Z, Dietze KK, Zelinskyy G, Yang D et al. TLR1/2 ligand-stimulated mouse liver endothelial cells secrete IL-12 and trigger CD8+ T cell immunity in vitro. J Immunol 2013; 191: 6178–6190. [DOI] [PubMed] [Google Scholar]
- Meng Z, Zhang X, Pei R, Zhang E, Kemper T, Vollmer J et al. Combination therapy including CpG oligodeoxynucleotides and entecavir induces early viral response and enhanced inhibition of viral replication in a woodchuck model of chronic hepadnaviral infection. Antiviral Res 2016; 125: 14–24. [DOI] [PubMed] [Google Scholar]
- Huang LR, Wohlleber D, Reisinger F, Jenne CN, Cheng RL, Abdullah Z et al. Intrahepatic myeloid-cell aggregates enable local proliferation of CD8(+) T cells and successful immunotherapy against chronic viral liver infection. Nat Immunol 2013; 14: 574–583. [DOI] [PubMed] [Google Scholar]
- Lan P, Zhang C, Han Q, Zhang J, Tian Z. Therapeutic recovery of hepatitis B virus (HBV)-induced hepatocyte-intrinsic immune defect reverses systemic adaptive immune tolerance. Hepatology 2013; 58: 73–85. [DOI] [PubMed] [Google Scholar]
- Lv S, Wang J, Dou S, Yang X, Ni X, Sun R et al. Nanoparticles encapsulating hepatitis B virus cytosine-phosphate-guanosine induce therapeutic immunity against HBV infection. Hepatology 2014; 59: 385–394. [DOI] [PubMed] [Google Scholar]
- Menne STB, Liu KH, Ascenzi MA, Baldwin BH, Bellezza CA, Cote PJ et al. Antiviral efficacy and induction of an antibody response against surface antigen with the TLR7 agonist GS-9620 in the woodchuck model of chronic HBV infection. J Hepatol 2011; 54: S441. [Google Scholar]
- Jo J, Tan AT, Ussher JE, Sandalova E, Tang XZ, Tan-Garcia A et al. Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog 2014; 10: e1004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.