Tlymphocytes are generated from hematopoietic stem cells in bone marrow, mature in the thymus and migrate to peripheral lymphoid tissues. The thymus is composed of the cortex and medulla, and there are many immature thymocytes in the cortex and more mature thymocytes are present in the medulla. Developing thymocytes expressing T-cell receptor (TCR) αβ chains generated by TCR gene rearrangement selectively differentiate only when they are determined to be useful cells by judgement of the strength of interaction between their TCR and peptide antigens presented on major histocompatibility complex (MHC) molecules of cortical thymic epithelial cells (cTECs). Cells unable to recognize peptide-MHC ligands cannot receive survival signals, and only cells that can recognize peptide-MHC ligands with an appropriate affinity proliferate and differentiate into mature T cells. This checkpoint mechanism is called positive selection. On the other hand, cell death is induced in cells that show strong responses to peptide-MHC ligands, a mechanism that is called negative selection. Although the mechanism that produces different responses depending on the affinity between TCR and peptide-MHC ligands has not been unclear, the mitogen-activated protein kinase (MAPK) pathway, and strength and localization of downstream signals in TCR stimulation are likely involved in regulation of the selection process of T cells.1 For example, p38, c-Jun NH2-terminal kinase (JNK), and extracellular signal-regulated kinase 5 (ERK5) are important MAPK signals for negative selection but are not required for positive selection. On the other hand, phosphorylation of ERK1/2 is important for positive selection but not for negative selection. It is also known that localization of phosphorylated ERK differs between positive selection and negative selection. Stimuli of negative selection induce phosphorylated ERK closer to the plasma membrane than stimuli of positive selection do. As a new mechanism not dependent on the MAPK pathway, Adoro et al.2 recently reported that an adequate input of TCR promotes the survival of positively selected thymocytes via the stabilization of one of the pro-survival factors, B-cell lymphoma 2 (Bcl-2).
Endosomal sorting complex required for transport (ESCRT) is a system necessary for separating membranes, forming and transporting vesicles, and forming multivesicular bodies (MVBs). Multiple ESCRT proteins are involved in this system: early-acting ESCRT proteins are first recruited to the site of membrane detachment and they recruit late-acting ESCRT proteins. These late-acting ESCRT proteins are also known as the charged MVB protein (CHMP) family and are involved in membrane deformity and isolation in cooperation with VPS4. There are seven CHMP genes in budding yeasts and eleven CHMP genes in humans, and they are classified into subgroups of CHMP1 to CHMP7. The relationship between ESCRT proteins and T-cell differentiation has been unclear, but Adoro et al. showed that CHMP5 is essential for T-cell differentiation. However, CHMP5 likely contributes to T-cell differentiation via functions other than that in the ESCRT pathway, because CHMP5 is dispensable in the ESCRT pathway in developing thymocytes.
CHMP5 has been reported to regulate osteoclast differentiation by suppressing nuclear factor kappa B (NFκB) signaling.3 In osteoclasts, CHMP5 promotes deubiquitination and stabilization of IκBα in collaboration with USP15, leading to dampening of NFκB. However, in developing thymocytes, CHMP5 is dispensable for NFκB regulation. Molecules involved in thymocyte differentiation are often regulators of TCR signaling or factors related to survival and death. It was revealed that CHMP5 did not affect TCR signaling, whereas CHMP5 positively regulated survival of thymocytes by binding to and suppressing sulfenylation of Bcl-2, which is one of the anti-apoptotic factors.
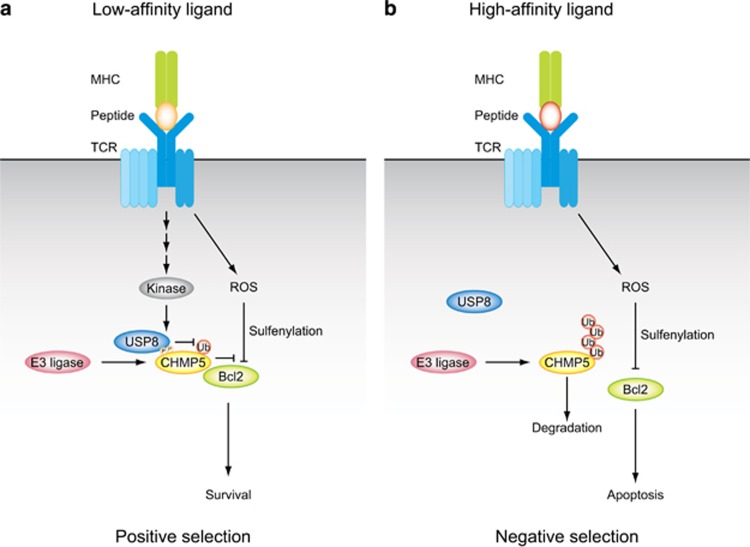
Although CHMP5 does not affect TCR signaling, CHMP5 is affected by TCR signaling and undergoes phosphorylation when TCR recognizes adequate peptide-MHC ligands. CHMP5 is constantly ubiquitinated in the absence of stimulation, leading to degradation by the proteasome. Phosphorylation of CHMP5 inhibits its ubiquitination, followed by the stabilization of CHMP5. Inhibition of CHMP5 ubiquitination is not accomplished through the regulation of E3 ubiquitin ligases, which conjugate ubiquitin molecules to substrates, but is carried out via recruitment of the deubiquitinating enzyme USP8, which removes ubiquitin molecules from target proteins (Figure 1).
Figure 1.
Models for post-translational control of CHMP5 during T-cell development. (a) When TCR is stimulated by low-affinity ligands, an unknown kinase activated by TCR signaling phosphorylates CHMP5 at Ser26 and Ser30, leading to recruitment of a deubiquitinating enzyme, USP8. USP8 stabilizes CHMP5 via its deubiquitination. Increased CHMP5 proteins directly bind to Bcl-2 and prevent reactive oxygen species (ROS)-mediated sulfenylation of Bcl-2 and subsequent degradation. These processes result in survival of thymocytes. (b) When TCR is stimulated by high-affinity ligands, CHMP5 is ubiquitinated and degraded via the proteasome pathway. CHMP5 proteins fail to prevent ROS-mediated sulfenylation and degradation of Bcl-2. These processes result in apoptosis of thymocytes. CHMP, charged MVB protein; TCR, T-cell receptor.
Ubiquitination modifications are involved in various aspects of thymocyte differentiation. It has been reported that there are several ubiquitin ligases and deubiquitinating enzymes that activate thymocytes and promote proliferation, but there are also ubiquitin ligases and deubiquitinating enzymes that negatively regulate thymocyte activation. The E3 ubiquitin ligase STUB1 is required for activation of T cells, and it conjugates K27-linked polyubiquitin chains to CARMA1.4 The deubiquitinating enzymes Otud7b and USP9X deubiquitinate ZAP70 and promote TCR signaling.5, 6 One of the E3 ubiquitin ligases, Casitas B-lineage lymphoma proto-oncogene (c-Cbl), has a phosphotyrosine binding (PTB) domain in its N-terminus and negatively regulates TCR signaling by ubiquitination and degradation of TCRζ and Lck.7, 8 Another ubiquitin ligase, gene related to anergy in lymphocytes (GRAIL), also negatively regulates T-cell differentiation and proliferation by ubiquitination and degradation of TCRζ, CD40L or STAT6.9, 10 Deubiquitinating enzymes OTUB1 and USP8 cooperatively deubiquitinate GRAIL and regulate its stability.11 Many ubiquitinating and/or deubiquitinating enzymes have been reported to be involved in thymocyte differentiation and activation; however, most of them control thymocyte differentiation via modulation of TCR signaling, downstream MAPK signaling, or NFκB signaling. However, ubiquitin-related enzymes that control other pathways have not been identified so far. CHMP5 ubiquitination is decreased by undergoing phosphorylation only when TCR is stimulated by low-affinity ligands, leading to survival of thymocytes by stabilization of Bcl-2. Since this pathway does not act when TCR is stimulated by high-affinity ligands, CHMP5-mediated signaling is a new mechanism that regulates positive selection, in which thymocytes receive intermediate stimuli by ligands with suitable affinity and consequently thymocytes survive and proliferate. This discovery shows that the ubiquitination system also plays an important role in the process of positive selection independently of the already known signaling pathway (Figure 1).
Several issues remain to be elucidated in this model shown as a molecular mechanism in positive selection. What is the E3 ubiquitin ligase that ubiquitinates CHMP5? What is the kinase that phosphorylates CHMP5 by TCR stimulation by a low-affinity ligand? Why is the status of phosphorylation with stimulation by a low-affinity ligand different from that with stimulation by a high-affinity ligand? How does stabilized CHMP5 inhibit sulfenylation by binding to Bcl-2?
CHMP5 also forms a complex with USP15, another deubiquitinating enzyme, during osteoclast differentiation and inhibits ubiquitination of IκBα. As CHMP5 binds to USP8 and its own ubiquitination is suppressed by deubiquitination in thymocytes, CHMP5 may regulate cell type-specific differentiation via functioning as a hub that changes the deubiquitinating enzyme depending on the cell type. CHMP5 also has an anti-apoptotic function in acute myeloid leukemia cells and may be involved in malignant tumor development and proliferation by dysregulation of a CHMP5-mediated mechanism. Further investigation of CHMP5 in malignancy may be important for finding a therapeutic target for several diseases including cancers, leukemia and autoimmune diseases.
Acknowledgments
This work was supported in part by KAKENHI (15H04690, 17H05784 and 17K19506 to SH; 16H06221 and 17H05989 to MW), Takeda Science Foundation (to SH), Japan Foundation for Applied Enzymology (to SH), Grant-in-Aid from Tokyo Biochemical Research Foundation (to SH) and Nakatani Foundation for advancement of measuring technologies in biomedical engineering (to MW).
Footnotes
The authors declare no conflict of interest.
References
- Teixeiro E, Daniels MA. ERK and cell death: ERK location and T cell selection. FEBS J 2010; 277: 30–38. [DOI] [PubMed] [Google Scholar]
- Adoro S, Park KH, Bettigole SE, Lis R, Shin HR, Seo H et al. Post-translational control of T cell development by the ESCRT protein CHMP5. Nat Immunol 2017; 18: 780–790. [DOI] [PubMed] [Google Scholar]
- Greenblatt MB, Park KH, Oh H, Kim JM, Shin DY, Lee JM et al. CHMP5 controls bone turnover rates by dampening NF-kappaB activity in osteoclasts. J Exp Med 2015; 212: 1283–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Li Y, Hu YH, Song R, Gao Y, Liu HY et al. STUB1 is essential for T-cell activation by ubiquitinating CARMA1. Eur J Immunol 2013; 43: 1034–1041. [DOI] [PubMed] [Google Scholar]
- Hu H, Wang H, Xiao Y, Jin J, Chang JH, Zou Q et al. Otud7b facilitates T cell activation and inflammatory responses by regulating Zap70 ubiquitination. J Exp Med 2016; 213: 399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik E, Dixit VM. Usp9X is required for lymphocyte activation and homeostasis through its control of ZAP70 ubiquitination and PKCbeta kinase activity. J Immunol 2016; 196: 3438–3451. [DOI] [PubMed] [Google Scholar]
- Rao N, Miyake S, Reddi AL, Douillard P, Ghosh AK, Dodge IL et al. Negative regulation of Lck by Cbl ubiquitin ligase. Proc Natl Acad Sci USA 2002; 99: 3794–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Altman Y, Fang D, Elly C, Dai Y, Shao Y et al. Cbl promotes ubiquitination of the T cell receptor zeta through an adaptor function of Zap-70. J Biol Chem 2001; 276: 26004–26011. [DOI] [PubMed] [Google Scholar]
- Lineberry NB, Su LL, Lin JT, Coffey GP, Seroogy CM, Fathman CG. Cutting edge: The transmembrane E3 ligase GRAIL ubiquitinates the costimulatory molecule CD40 ligand during the induction of T cell anergy. J Immunol 2008; 181: 1622–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo A, Alekseev A, Obertas L, Nurieva R. Grail controls Th2 cell development by targeting STAT6 for degradation. Nat Commun 2014; 5: 4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares L, Seroogy C, Skrenta H, Anandasabapathy N, Lovelace P, Chung CD et al. Two isoforms of otubain 1 regulate T cell anergy via GRAIL. Nat Immunol 2004; 5: 45–54. [DOI] [PubMed] [Google Scholar]