Abstract
The association between type 2 diabetes (T2DM) and bone metabolism has been discussed previously but is controversial. In this study we aimed to evaluate the association of bone turnover markers with glucose metabolism in Chinese population, in which 919 males and 4171 postmenopausal females in a region of Shanghai were recruited. Anthropometric and biochemical traits related to glucose and bone metabolism were analyzed. Participants were classified according to their glucose tolerance as normal glucose tolerance (NGT), impaired glucose regulation (IGR) or T2DM. Males and females were analyzed separately, and then associations between bone turnover markers (BTMs) and glucose metabolism were evaluated. The results showed that in females, the serum levels of N-terminal osteocalcin (N-MID), N-terminal procollagen of type I collagen (PINP) and β-cross-linked C-telopeptide of type I collagen (β-CTX) were significantly decreased in the T2DM group compared to the NGT group (P<0.01). When age, body mass index, serum lipids, fat percentage, visceral fat area, subcutaneous fat area, anti-diabetic medicines, PINP, N-MID and β-CTX were included in one logistic model, N-MID (OR [95% CI]: 0.954 [0.932; 0.976]; P=0.0001) was significantly associated with T2DM in females. In females, N-MID was associated with insulin sensitivity and HOMA-β. PINP was significantly associated with HOMA-β, GUTT-ISI, Stumvoll first-phase insulin secretion index (STU-1) and Stumvoll second-phase insulin secretion index (STU-2), but β-CTX was associated only with HOMA-β (β±SE: 0.1331±0.0311; P=1.95×10-5) and GUTT-ISI (β±SE: 0.0727±0.0229; P=0.0015). In males, N-MID was significantly correlated with HOMA-β (β±SE: 0.3439±0.0633; P=7.75×10-8), GUTT-ISI (β±SE: 0.1601±0.0531; P=0.0027) and STU-1 (β±SE: 0.2529±0.1033; P=0.0146). Significant associations were also detected between β-CTX and HOMA-β (β±SE: 0.2736±0.0812; P=0.0009). This study reveals that BTMs are highly associated with T2DM, insulin sensitivity and beta cell function in both Chinese males and postmenopausal females.
Keywords: bone turnover markers, N-MID, PINP, β-CTX, T2DM, insulin sensitivity, beta cell function, Chinese
Introduction
Osteoporosis and type 2 diabetes (T2DM), which are both important public health problems in an aging society, share many common risk factors, including abdominal obesity, smoking and glucocorticoid exposure. Due to the coincidence of osteoporosis and T2DM1,2, the association between T2DM and bone metabolism has been discussed previously but is controversial. Despite T2DM historically being considered a risk factor for osteoporosis3, recent evidence suggests a more complex interaction between bone metabolism and glucose metabolism. Therefore, bone has been recognized as an endocrine organ that plays a vital role in energy metabolism4.
Bone remodeling is a dynamic process that includes the formation of new bone by osteoblasts followed by the resorption of older bone by osteoclasts. This process generates many bone turnover markers (BTMs) from bone cells or the bone matrix5,6. Specifically, serum osteocalcin is a small noncollagenous protein secreted by osteoblasts during bone formation, bone alkaline phosphatase promotes bone calcification, and procollagen type I N-terminal propeptide (PINP) is released by osteoblasts during the synthesis of collagen type I; all of these molecules are key markers of bone formation. Regarding BTMs associated with the process of bone resorption, β-cross-linked C-telopeptide of type I collagen (β-CTX) is the C-terminal telopeptide of type I collagen that is released by the degradation of mature type I collagen7, and serum tartrate-resistant acid phosphatase is an enzyme expressed on osteoclasts that is involved in the degradation of bone matrix during bone resorption8. In addition to being used as indicators of bone formation, as indicators of bone loss, for the assessment of osteoporosis therapy, or for the diagnosis of secondary osteoporosis6, these BTMs have been shown to be associated with energy metabolism in recent studies9.
Among these BTMs, osteocalcin is the main contributor to the effect of bone on energy metabolism10,11. Animal studies have shown that the osteocalcin receptor is expressed in both islet β cells12 and epithelial cells of the mouse small intestine, through which osteocalcin promotes β cell proliferation and glucagon-like peptide secretion, the latter of which can also promote insulin secretion13. Mice lacking osteocalcin develop metabolic abnormalities, including decreased insulin secretion, decreased β cell proliferation and insulin resistance14. Furthermore, the intermittent infusion of osteocalcin was shown to partially correct the glucose intolerance caused by a high-fat diet in mice15. Although animal studies have provided evidence supporting a key role for bone in energy metabolism via osteocalcin, the association between osteocalcin and glucose metabolism remains controversial based on previous observational studies in humans16,17,18,19.
In addition to osteocalcin, there are numerous studies of other BTMs associated with glucose metabolism. As the processes of bone resorption and bone formation are coupled, there might be interactions between these BTMs. Therefore, studies of the association of BTMs such as PINP and β-CTX with glucose metabolism in humans might offer more insight into the effect of bone on energy metabolism. In this study, we investigated the association of BTMs such as osteocalcin, β-CTX, and PINP with T2DM, insulin sensitivity and beta cell function in males and postmenopausal females.
Materials and methods
Ethics statement
This study was conducted in accordance with the principles of the Second Revision of the Declaration of Helsinki and was approved by the institutional review board of the Shanghai Jiao Tong University Affiliated Sixth People's Hospital. Written informed consent was obtained from all participants.
Participants
We recruited 5204 participants from the Nicheng community in Shanghai. Based on the 2010 criteria of the American Diabetes Association (ADA)20 and the 1999 World Health Organization definition21, participants with fasting plasma glucose (FPG) ≥7.0 mmol/L or 2-h plasma glucose (2-h PG) ≥11.1 mmol/L during the 75-g oral glucose tolerance test (OGTT) or hemoglobin A1c (HbA1c)≥6.5% were diagnosed with type 2 diabetes, and participants with FPG from 6.1 to 7.0 mmol/L or 2-h PG during the 75-g OGTT from 7.8 to 11.1 mmol/L were diagnosed with impaired glucose regulation (IGR). Participants with cancer, hepatic disease or kidney disease and those using medications that might influence bone metabolism were excluded. Participants using insulin treatment were also excluded. Finally, a total of 5090 participants (919 males and 4171 postmenopausal females) were retained for further analysis.
Clinical measurements
Anthropometric and biochemical traits were evaluated for all participants. Body mass index (BMI) was calculated as weight/height2. Blood pressure (in mmHg) was measured three times at 3-min intervals, and the average of the three measurements was calculated. Waist circumference was measured midway between the lowest rib and the iliac crest with the person in the standing position. HbA1c values were determined by high-performance liquid chromatography using a Bio-Rad Variant II hemoglobin testing system (Bio-Rad Laboratories, Hercules, CA, USA). Serum triglyceride, total cholesterol, low-density lipoprotein cholesterol (LDL-c), and high-density lipoprotein cholesterol (HDL-c) levels were measured with a type 7600-020 Automated Analyzer (Hitachi, Tokyo, Japan). Insulin sensitivity was assessed by HOMA-IR and GUTT-ISI22. Beta cell function was assessed by HOMA-β, the Stumvoll first-phase insulin secretion index (STU-1) and the Stumvoll second-phase insulin secretion index (STU-2)23. Factors associated with bone metabolism, including N-terminal osteocalcin (N-MID), PINP, β-CTX, 25-hydroxy vitamin D3 [25(OH)D3], and parathyroid hormone were measured with an automated Roche electrochemiluminescence system (Roche Diagnostics Gmbh, Germany).
Body fat percentage was measured with a BC-420 Tanita Body Composition Analyzer (Tanita, Tokyo, Japan). Images of all participants were obtained at the abdominal level between the fourth and fifth lumbar vertebrae with the person in the supine position via 3.0 T magnetic resonance imaging (Achieva; Philips, Best, the Netherlands). Then, abdominal visceral fat area (VFA), subcutaneous fat area (SFA) and lean mass were calculated by two trained observers who were unaware of the experimental design using image analysis software (SLICEOMATIC, version 4.2; TomoVision Inc, Montreal, QC, Canada).
Anti-diabetic medicines influence glucose metabolism, so we recorded the details of anti-diabetic medicines; 114 participants using insulin were excluded. We ignored 14 patients who were using α glucosidase inhibitors due to the small number of patients involved and the limited effect of α glucosidase inhibitors on insulin sensitivity and beta cell function. We divided other anti-diabetic medicines into insulin-secreting agents (sulfonylureas and glinides) and insulin-sensitizing agents (metformin and thiazolidinedione) for further adjustment in the regression model.
Statistical analysis
The data were analyzed using SAS 9.2 (SAS Institute, Cary, NC, USA). Normality testing was performed, and variables with skewed distributions were log transformed. The mean±SD or the median (interquartile range) was used to describe continuous variables, and proportions were used for categorical variables. Differences between groups were determined with the Wilcoxon test or Kruskal Wallis test for continuous variables and the χ2 test for categorical variables. Multivariable logistic regression analysis was performed to determine the effects of BTMs on T2DM susceptibility. Multiple linear regression analysis was performed to evaluate the association of BTMs with insulin sensitivity and beta cell function.
Results
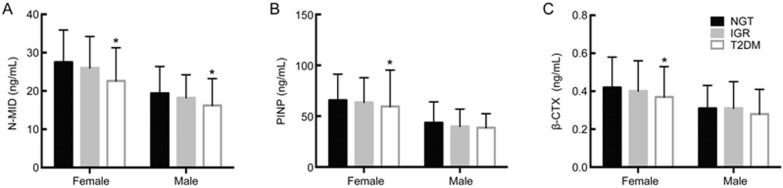
The basic characteristics of the participants are shown in Table 1. Participants were classified according to their glucose tolerance as follows: normal glucose tolerance (NGT), IGR and T2DM. Males and females were analyzed separately. As shown in Figure 1, in females, serum N-MID, PINP and β-CTX were significantly decreased in the IGR group compared to the NGT group, and they were further decreased in the T2DM group (P<0.01), while in males, the same finding was obtained only with serum N-MID (P<0.01).
Table 1. Clinical characteristics of the study cohort.
| Total | Male | Female | P value | |
|---|---|---|---|---|
| Sample (n) | 5090 | 919 | 4171 | |
| NGT | 1861 (36.56%) | 336 (36.56%) | 1525 (36.56%) | 0.7840 |
| IGR | 1897 (37.27%) | 335 (36.45%) | 1562 (37.45%) | |
| T2DM | 1332 (26.17%) | 248 (26.99%) | 1084 (25.99%) | |
| Osteoporosis | 2495 (49.02%) | 133 (14.47%) | 2362 (56.63%) | <0.0001 |
| Insulin-secreting agents (n) | 287 (5.6%) | 55 (6.0%) | 232 (5.6%) | – |
| Insulin-sensitizing agents (n) | 176 (3.5%) | 34 (3.7%) | 142 (3.4%) | – |
| Age (year) | 62.76±4.40 | 67.21±2.15 | 61.78±4.16 | <0.0001 |
| BMI (kg/m2) | 24.95±3.38 | 24.70±3.28 | 24.99±3.40 | 0.0390 |
| WC (cm) | 84 (78, 90) | 87 (80, 93) | 84 (77, 90) | <0.0001 |
| HbA1c (%) | 5.7 (5.4, 6.1) | 5.7 (5.4, 6.0) | 5.7 (5.5, 6.1) | 0.0011 |
| HbA1c (mmol/mol) | 39 (36, 43) | 39 (36, 42) | 39 (37, 43) | 0.0011 |
| SBP (mmHg) | 133 (124, 144) | 133 (125, 145) | 133 (124, 144) | 0.1528 |
| DBP (mmHg) | 82 (79, 88) | 82 (79, 89) | 82 (79, 88) | 0.2237 |
| Fat percentage (%) | 34.0 (28.4, 38.4) | 23.1 (19.4, 26.3) | 35.5 (31.6, 39.1) | <0.0001 |
| Fat mass (kg) | 20.5 (16.2, 25.0) | 15.9 (11.9, 19.5) | 21.4 (17.4, 25.8) | <0.0001 |
| VFA (cm2) | 113.7 (85.8, 143.1) | 118.8 (84.9, 156.3) | 112.7 (86.0, 140.5) | 0.0018 |
| SFA (cm2) | 151.4 (115.4, 193.9) | 119.6 (90.4, 150.8) | 158.5 (123.7, 201.4) | <0.0001 |
| HOMA-IR | 2.0 (1.3, 2.9) | 1.6 (1.1, 2.5) | 2.0 (1.4, 3.0) | <0.0001 |
| HOMA-β | 58.8 (40.6, 83.7) | 47.4 (31.9, 67.5) | 61.3 (42.9, 86.3) | <0.0001 |
| GUTT-ISI | 57.4 (41.3, 76.2) | 63.0 (44.5, 86.2) | 56.4 (40.6, 74.3) | <0.0001 |
| STU-1 | 1426.0 (868.1, 1826.0) | 1448.4 (793.8, 1925.2) | 1419.8 (882.7, 1806.9) | 0.1393 |
| STU-2 | 363.9 (237.5, 450.0) | 369.1 (221.2, 468.0) | 363.3 (241.4, 445.6) | 0.2761 |
| N-MID (ng/mL) | 23.1 (18.2, 29.0) | 16.7 (13.6, 21.3) | 24.5 (19.6, 30.1) | <0.0001 |
| PINP (ng/mL) | 57.1 (41.9, 74.0) | 38.0 (28.8, 48.3) | 60.0 (45.4, 77.0) | <0.0001 |
| β-CTX (ng/mL) | 0.36 (0.27, 0.48) | 0.28 (0.22, 0.36) | 0.37 (0.28, 0.49) | <0.0001 |
Data are shown as the mean±SD or as the median (interquartile range). NGT: normal glucose tolerance; IGR: impaired glucose regulation; BMI: body mass index; WC: waist circumstance; SBP: systolic blood pressure; DBP: diastolic blood pressure; VFA: visceral fat area; SFA: subcutaneous fat area; HOMA: homeostasis model analysis; STU-1: Stumvoll first-phase insulin secretion index; STU-2: Stumvoll second-phase insulin secretion index; N-MID: N-terminal osteocalcin; PINP: N-terminal procollagen of type I collagen; β-CTX: β-cross-linked C-telopeptide of type I collagen. The variables with a skewed distribution were compared using the Wilcoxon test. Proportions of the categorical variables were compared using the χ2 test.
Figure 1.
Levels of BTMs in the different glucose tolerance groups. Participants were classified according to their glucose tolerance as normal glucose tolerance (NGT), impaired glucose regulation (IGR) and type 2 diabetes (T2DM). Differences in the levels of serum N-MID, PINP and β-CTX between the groups were determined with the Kruskal-Wallis test. In females, serum N-MID, PINP and β-CTX were all significantly decreased in the IGR group compared to the NGT group and were further decreased in the T2DM group; the same finding was obtained only with N-MID for males. N-MID: N-terminal osteocalcin; PINP: N-terminal procollagen of type I collagen; β-CTX: β-cross-linked C-telopeptide of type I collagen. *P<0.01.
After adjusting for age and BMI in the multivariable logistic regression analysis, serum N-MID, PINP and β-CTX were all revealed to be protective against T2DM in females, while only N-MID was significantly associated with T2DM in males (OR [95% CI]: 0.946 [0.920; 0.974]; P=0.0002). We obtained similar associations when the multivariable logistic regression analysis was further adjusted for age, BMI, serum lipids, fat percentage, VFA, SFA and anti-diabetic medicines in both genders (Table 2). When N-MID, PINP and β-CTX were included in one multivariable logistic regression model adjusted for age, BMI, serum lipids, fat percentage, VFA, SFA and anti-diabetic medicines, there was a significant association between N-MID (OR [95% CI]: 0.954 [0.932; 0.976]; P=0.0001) and T2DM only in females, while neither β-CTX nor PINP was significantly associated with T2DM in either males or females.
Table 2. Effect of BTMs on type 2 diabetes in Chinese people.
| Male |
Female |
||||
|---|---|---|---|---|---|
| OR [95% CI] | P value | OR [95% CI] | P value | ||
| Model I | N-MID (ng/mL) | 0.946 [0.920; 0.974] | 0.0002 | 0.942 [0.932; 0.952] | 1.51×10−30 |
| PINP (ng/mL) | 0.991 [0.975; 1.007] | 0.2655 | 0.993 [0.989; 0.997] | 0.0019 | |
| β-CTX (ng/mL) | 0.150 [0.017; 1.361] | 0.0918 | 0.277 [0.139; 0.552] | 0.0003 | |
| Model II | N-MID (ng/mL) | 0.926 [0.889; 0.964] | 0.0002 | 0.957 [0.945; 0.969] | 7.94×10−12 |
| PINP (ng/mL) | 0.994 [0.976; 1.013] | 0.5464 | 0.999 [0.994; 1.003] | 0.5513 | |
| β-CTX (ng/mL) | 0.554 [0.093; 7.925] | 0.6637 | 0.601 [0.264; 1.371] | 0.2264 | |
N-MID: N-terminal osteocalcin; PINP: N-terminal procollagen of type I collagen; β-CTX: β-cross-linked C-telopeptide of type I collagen. Model I: Multivariable logistic regression adjusted for age and BMI. Model II: Multivariable logistic regression adjusted for age, BMI, fat percentage, VFA, SFA, serum lipids and anti-diabetic medicines.
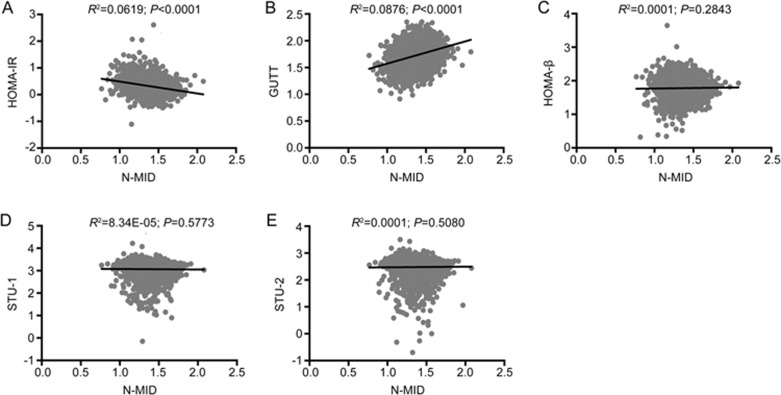
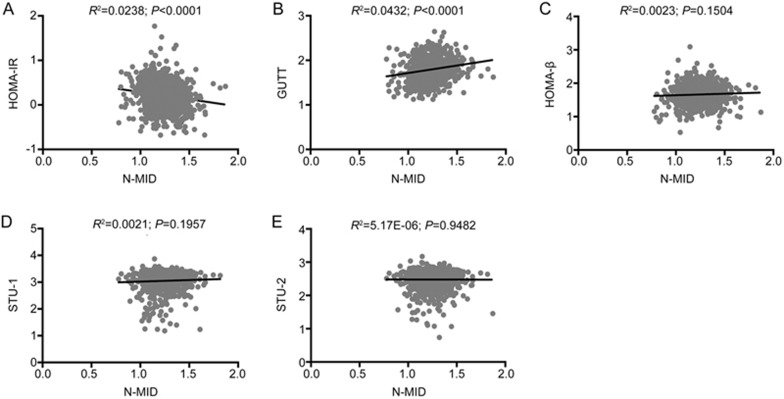
The associations of N-MID with insulin sensitivity and beta cell function in females are shown in Figure 2. After further adjusting for age, BMI, fat percentage, VFA, SFA and anti-diabetic medicines, N-MID was negatively correlated with HOMA-IR (β±SE: −0.1642±0.0276; P=2.93×10-9) and positively correlated with GUTT-ISI (β±SE: 0.2043±0.0214; P=2.46×10-8) and HOMA-β (β±SE: 0.1124±0.0280; P=0.0001). PINP was significantly associated with HOMA-β (β±SE: 0.0794±0.0276; P=0.0040), GUTT-ISI (β±SE: 0.0510±0.0203; P=0.0121), STU-1 (β±SE: 0.2386±0.0455; P=1.85×10-7) and STU-2 (β±SE: 0.1296±0.0393; P=0.0010). β-CTX was associated only with HOMA-β and GUTT-ISI (Supplementary Table S1). The correlations of N-MID with insulin sensitivity and beta cell function in males are shown in Figure 3. N-MID was significantly correlated with GUTT-ISI (β±SE: 0.1601±0.0531; P=0.0027), HOMA-β (β±SE: 0.3439±0.0633; P=7.75×10-8) and STU-1 (β±SE: 0.2529±0.1033; P=0.0146) when further controlled for age, BMI, fat percentage, VFA, SFA and anti-diabetic medicines. Significant associations were also observed between β-CTX and HOMA-β (β±SE: 0.2736±0.0812; P=0.0009) (Supplementary Table S1).
Figure 2.
Associations of BTMs with insulin sensitivity and beta cell function in females. N-MID: N-terminal osteocalcin; HOMA: homeostasis model analysis; STU-1: Stumvoll first-phase insulin secretion index; STU-2: Stumvoll second-phase insulin secretion index.
Figure 3.
Associations of BTMs with insulin sensitivity and beta cell function in males. N-MID was found to be significantly associated with HOMA-β, GUTT-ISI and STU-1. N-MID: N-terminal osteocalcin; HOMA: homeostasis model analysis; STU-1: Stumvoll first-phase insulin secretion index; STU-2: Stumvoll second-phase insulin secretion index.
Discussion
The interaction between bone and energy metabolism has long been investigated24, and bone has been identified as an endocrine organ that modulates glucose homeostasis4. Furthermore, previous studies have suggested that BTMs might be the link between bone and glucose metabolism. As a traditional marker of bone formation, osteocalcin was shown to stimulate insulin secretion directly and via glucagon-like peptide-1 through its receptor that is expressed in both islet β cells and epithelial cells of the mouse small intestine13. Additionally, intermittent injections of osteocalcin were shown to be protective against T2DM in mice15. The role of osteocalcin was further verified in cultured human islets by the augmentation of insulin content and the enhancement of β-cell proliferation in response to decarboxylated osteocalcin25. The current study found that, for both genders, serum N-MID was significantly lower in the T2DM group, and N-MID was found to be significantly associated with T2DM in the multivariable logistic regression analysis. In addition, N-MID was associated with insulin sensitivity and beta cell function (HOMA-β). These findings are in accordance with previous results showing that N-MID was protective against T2DM by improving insulin sensitivity and secretion. Furthermore, the same role of osteocalcin in glucose metabolism was also demonstrated in previous population studies19,26,27. Therefore, osteocalcin is a critical molecule that favorably modulates glucose homeostasis.
As shown in the current study, PINP, another marker of bone formation, was significantly associated with insulin sensitivity and beta cell function. This association was not reported in a previous study9 in which PINP had a positive correlation with HOMA-IR and HbA1c, and such difference is likely due to the limited sample size. Considering the high correlation between PINP and osteocalcin, the protective role of PINP against T2DM was most likely due to the function of osteocalcin. This hypothesis was further confirmed when the association between PINP and T2DM was attenuated after further adjustment for N-MID.
Some studies have investigated the association of bone resorption markers such as β-CTX with glucose metabolism28. In our study, β-CTX was associated with T2DM susceptibility in females, but adjusting for N-MID eliminated this association. Considering that previous studies have shown that it is the undercarboxylated osteocalcin in the serum that mainly affects glucose metabolism and that osteocalcin decarboxylation is promoted by osteoclast activation, the association between β-CTX and T2DM may be mediated by N-MID. This hypothesis is supported by both our results and previous studies9,29.
As the major hormonal regulator of bone metabolism in both genders30, estrogen helps maintain bone homeostasis, and BTMs are elevated in postmenopausal women due to estrogen deficiency31,32. Considering the decreased estrogen levels in postmenopausal women and the possible effect of androgen in males, the association of BTMs with T2DM, insulin sensitivity and beta cell function differed to some extent between the genders.
There are some limitations to our study. First, we could observe only associations, not causal relationships, between BTMs and glucose metabolism in this cross-sectional study. Further prospective and mechanistic studies are required to validate these associations. Second, we did not evaluate the associations of BTMs with glucose metabolism in premenopausal women, which might be different from those in the populations included in the present study. Third, as both osteocalcin and undercarboxylated osteocalcin exert beneficial effects on glucose metabolism and N-MID is the most stable form of osteocalcin in serum, we evaluated only N-MID, not undercarboxylated osteocalcin, in this study. Fourth, we did not record the anti-osteoporotic therapies of the participants; therefore, we could not take such medication treatments, which might influence the BTMs levels, into consideration.
In conclusion, these results suggested that BTMs were significantly associated with T2DM susceptibility, insulin sensitivity and beta cell function in both males and postmenopausal females and that serum N-MID in particular might play a key role in the association of bone with glucose metabolism.
Author contribution
Jie WANG, Zhen-lin ZHANG, and Cheng HU conceived of and designed the experiments. Jie WANG and Dan-dan YAN performed the experiments; Jie WANG and Cheng HU analyzed the data; Xu-hong HOU and Yu-qian BAO contributed reagents/materials/analysis tools; Dan-dan YAN, Jie WANG, Yu-qian BAO, Cheng HU, and Wei-ping JIA wrote the paper; Jie WANG and Dan-dan YAN drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We would like to acknowledge the nursing and medical staff at the Shanghai Clinical Center for Diabetes for their help. We would also like to extend our gratitude to all of the study participants. This work was supported by grants from the National 863 Program (No 2015AA020110), the Drug Innovation Program of the National Science and Technology Project (No 2011ZX09307-001-02), the Shanghai Young Doctor Training and Funding Program, the Shanghai Jiao Tong Medical/Engineering Foundation (No YG2014MS18), the Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (No 20152527), the National Program for Support of Top-notch Young Professionals, the Shanghai Health and Family Planning Commission (No 2013ZYJB1001), the Shanghai Hospital Development Center (No SHDC12013115), the innovation foundation of translational medicine of Shanghai Jiao Tong University School of Medicine (No 15ZH4006) and Shanghai SJTUSM Biobank.
Footnotes
Supplementary information is available on the website of Acta Pharmacologica Sinica.
Supplementary Information
Effect of BTMs on insulin sensitivity and beta cell function in Chinese people.
References
- Piepkorn B, Kann P, Forst T, Andreas J, Pfutzner A, Beyer J. Bone mineral density and bone metabolism in diabetes mellitus. Horm Metab Res 1997; 29: 584–91. [DOI] [PubMed] [Google Scholar]
- Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 2007; 166: 495–505. [DOI] [PubMed] [Google Scholar]
- Leidig-Bruckner G, Ziegler R. Diabetes mellitus a risk for osteoporosis? Exp Clin Endocrinol Diabetes 2001; 109 Suppl 2: S493–514. [DOI] [PubMed] [Google Scholar]
- DiGirolamo DJ, Clemens TL, Kousteni S. The skeleton as an endocrine organ. Nat Rev Rheumatol 2012; 8: 674–83. [DOI] [PubMed] [Google Scholar]
- Garnero P. Bone markers in osteoporosis. Curr Osteoporos Rep 2009; 7: 84–90. [DOI] [PubMed] [Google Scholar]
- Cabral HW, Andolphi BF, Ferreira BV, Alves DC, Morelato RL, Chambo AF, et al. The use of biomarkers in clinical osteoporosis. Rev Assoc Med Bras 2016; 62: 368–76. [DOI] [PubMed] [Google Scholar]
- Souberbielle JC, Cormier C, Kindermans C. Bone markers in clinical practice. Curr Opin Rheumatol 1999; 11: 312–9. [DOI] [PubMed] [Google Scholar]
- Halleen JM, Tiitinen SL, Ylipahkala H, Fagerlund KM, Vaananen HK. Tartrate-resistant acid phosphatase 5b (TRACP 5b) as a marker of bone resorption. Clin Lab 2006; 52: 499–509. [PubMed] [Google Scholar]
- Iglesias P, Arrieta F, Pinera M, Botella-Carretero JI, Balsa JA, Zamarron I, et al. Serum concentrations of osteocalcin, procollagen type 1 N-terminal propeptide and beta-CrossLaps in obese subjects with varying degrees of glucose tolerance. Clin Endocrinol 2011; 75: 184–8. [DOI] [PubMed] [Google Scholar]
- Zhou M, Ma X, Li H, Pan X, Tang J, Gao Y, et al. Serum osteocalcin concentrations in relation to glucose and lipid metabolism in Chinese individuals. Eur J Endocrinol/Eur Federation Endocr Soc 2009; 161: 723–9. [DOI] [PubMed] [Google Scholar]
- Schwetz V, Pieber T, Obermayer-Pietsch B. The endocrine role of the skeleton: background and clinical evidence. Eur J Endocrinol/Eur Federation Endocr Soc 2012; 166: 959–67. [DOI] [PubMed] [Google Scholar]
- Pi M, Kapoor K, Ye R, Nishimoto SK, Smith JC, Baudry J, et al. Evidence for osteocalcin binding and activation of GPRC6A in beta-cells. Endocrinology 2016; 157: 1866–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizokami A, Yasutake Y, Gao J, Matsuda M, Takahashi I, Takeuchi H, et al. Osteocalcin induces release of glucagon-like peptide-1 and thereby stimulates insulin secretion in mice. PLoS One 2013; 8: e57375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007; 130: 456–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone 2012; 50: 568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WW, Ke YH, He JW, Fu WZ, Liu YJ, Chen D, et al. Serum osteocalcin levels are inversely associated with plasma glucose and body mass index in healthy Chinese women. Acta Pharmacol Sin 2014; 35: 1521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan Y, Sun LH, Liu DM, Zhao L, Tao B, Wang WQ, et al. Positive association between serum levels of bone resorption marker CTX and HbA1c in women with normal glucose tolerance. J Clin Endocrinol Metab 2015; 100: 274–81. [DOI] [PubMed] [Google Scholar]
- Ma XY, Chen FQ, Hong H, Lv XJ, Dong M, Wang QY. The relationship between serum osteocalcin concentration and glucose and lipid metabolism in patients with type 2 diabetes mellitus—the role of osteocalcin in energy metabolism. Ann Nutr Metab 2015; 66: 110–6. [DOI] [PubMed] [Google Scholar]
- Shu H, Pei Y, Chen K, Lu J. Significant inverse association between serum osteocalcin and incident type 2 diabetes in a middle-aged cohort. Diabetes Metab Res Rev 2016; 32: 867–74. [DOI] [PubMed] [Google Scholar]
- American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 33: S62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetes Med 1998; 15: 539–53. [DOI] [PubMed] [Google Scholar]
- Gutt M, Davis CL, Spitzer SB, Llabre MM, Kumar M, Czarnecki EM, et al. Validation of the insulin sensitivity index (ISI(0,120)): comparison with other measures. Diabetes Res Clin Pract 2000; 47: 177–84. [DOI] [PubMed] [Google Scholar]
- Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki-Jarvinen H, Van Haeften T, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 2000; 23: 295–301. [DOI] [PubMed] [Google Scholar]
- Lieben L, Callewaert F, Bouillon R. Bone and metabolism: a complex crosstalk. Horm Res 2009; 71: 134–8. [DOI] [PubMed] [Google Scholar]
- Sabek OM, Nishimoto SK, Fraga D, Tejpal N, Ricordi C, Gaber AO. Osteocalcin effect on human beta-cells mass and function. Endocrinology 2015; 156: 3137–46. [DOI] [PubMed] [Google Scholar]
- Iki M, Tamaki J, Fujita Y, Kouda K, Yura A, Kadowaki E, et al. Serum undercarboxylated osteocalcin levels are inversely associated with glycemic status and insulin resistance in an elderly Japanese male population: fujiwara-kyo osteoporosis risk in men (FORMEN) study. Osteoporos Int 2012; 23: 761–70. [DOI] [PubMed] [Google Scholar]
- Yano S, Nabika T, Nagai A, Hamano T, Yamasaki M, Isomura M, et al. Interrelationship between glucose metabolism and undercarboxylated osteocalcin: a cross-sectional study in a community-dwelling population. Asia Pac J Clin Nutr 2015; 24: 489–95. [DOI] [PubMed] [Google Scholar]
- Lacombe J, Karsenty G, Ferron M. In vivo analysis of the contribution of bone resorption to the control of glucose metabolism in mice. Mol Metab 2013; 2: 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhofer Y, Kiefer FW, Handisurya A, Tura A, Klein K, Schneider B, et al. CTX (crosslaps) rather than osteopontin is associated with disturbed glucose metabolism in gestational diabetes. PLoS One 2012; 7: e40947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends Endocrinol Metab 2012; 23: 576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res 1996; 11: 337–49. [DOI] [PubMed] [Google Scholar]
- Ma L, Song Y, Li C, Wang E, Zheng D, Qu F, et al. Bone turnover alterations across the menopausal transition in south-eastern Chinese women. Climacteric 2016; 19: 400–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of BTMs on insulin sensitivity and beta cell function in Chinese people.