Abstract
Both the anti- and pro-apoptotic members of the Bcl-2 family are regulated by a conserved Bcl-2 homology (BH3) domain. ABT-263 (Navitoclax), a novel BH3 mimetic and orally bioavailable Bcl-2 family inhibitor with high affinity for Bcl-xL, Bcl-2 and Bcl-w has entered clinical trials for cancer treatment. But the anticancer mechanisms of ABT-263 have not been fully elucidated. In this study we investigated the effects of ABT-263 on human esophageal cancer cells in vitro and to explore its anticancer mechanisms. Treatment with ABT-263 dose-dependently suppressed the viability of 3 human esophageal cancer cells with IC50 values of 10.7±1.4, 7.1±1.5 and 8.2±1.6 μmol/L, in EC109, HKESC-2 and CaES-17 cells, respectively. ABT-263 (5–20 μmol/L) dose-dependently induced G1/G0-phase arrest in the 3 cancer cell lines and induced apoptosis evidenced by increased the Annexin V-positive cell population and elevated levels of cleaved caspase 3, cleaved caspase 9 and PARP. We further demonstrated that ABT-263 treatment markedly increased the expression of p21Waf1/Cip1 and decreased the expression of cyclin D1 and phospho-Rb (retinoblastoma tumor suppressor protein) (Ser780) proteins that contributed to the G1/G0-phase arrest. Knockdown of p21Waf1/Cip1 attenuated ABT-263-induced G1/G0-phase arrest. Moreover, ABT-263 treatment enhanced pro-survival autophagy, shown as the increased LC3-II levels and decreased p62 levels, which counteracted its anticancer activity. Our results suggest that ABT-263 exerts cytostatic and cytotoxic effects on human esophageal cancer cells in vitro and enhances pro-survival autophagy, which counteracts its anticancer activity.
Keywords: human esophageal cancer cells, ABT-263, G1/G0 phase arrest, p21Waf1/Cip1, cyclin D1 and phospho-Rb (Ser780), apoptosis, autophagy, LC3-II, p62
Introduction
Esophageal cancer is the eighth most common cancer worldwide, with a projected incidence of 455 800 new cases and 400 200 deaths in 2012 and a higher incidence in China1,2,3. It is also one of the least studied and most deadly cancers, with an overall 5-year survival rate of less than 25%4,5. Depending on the accuracy of staging at diagnosis, patients with esophageal cancer are generally treated with a multimodal approach including surgery, chemotherapy, radiotherapy and endoscopic therapy5,6. In this regard, chemotherapy is indispensable in the management of metastatic esophageal malignances5,7.
The Bcl-2 family, a class of genes that consist of anti-apoptotic (eg, Bcl-2, Mcl-1, Bcl-xL and Bcl-w) and pro-apoptotic (eg, Bax and Bak) members, comprises key regulators of apoptosis8,9,10. Specifically, the dysregulation of Bcl-2 family members is a major criterion for evaluating differentiation in human esophageal cancer cells and plays an important role in the cellular sensitivity to apoptosis and life-death balance11,12,13. Consequently, the levels of Bcl-2 family members serve as critical determinants in the relative sensitivity or resistance of cancer cells to current therapies14. In addition to their oncogenic potential, anti-apoptotic members of the Bcl-2 family have also been found to suppress cell death induced by anticancer drugs. Thus, therapies that inhibit Bcl-2 family members may be a potential strategy to fight cancer15,16. Moreover, structural and functional studies have demonstrated that both the anti- and pro-apoptotic members are regulated by a conserved Bcl-2 homology (BH3) domain, thus resulting in the development of various BH3 mimetics for cancers treatment11,17.
ABT-263 (Navitoclax), a novel BH3 mimetic and orally bioavailable Bcl-2 family inhibitor with high affinity for Bcl-xL, Bcl-2 and Bcl-w, but not Mcl-1 and A1, has entered clinical trials for treatment of lymphoid malignancies and solid tumors18,19. The effects of BH3 mimetics have been more complex than anticipated; they not only induce apoptosis but also modify various cellular processes, including cell cycle progression, autophagy and stemness20. Because the anticancer mechanisms of ABT-263 have not been fully elucidated, we assessed the effect of ABT-263 on the viability of human esophageal cancer cells on the basis of cell cycle progression and autophagy. We anticipate that this study should provide new insight into the anti-cancer mechanism of this BH3 mimetic and their potential application in the treatment of patients with esophageal cancer.
Material and methods
Compounds
ABT-263 and Staurosporine were obtained from Selleck Chemicals (Houston, TX, USA) and first dissolved in DMSO (Sigma-Aldrich, St Louis, MO, USA) before being diluted with culture medium in the experiments. 3-(4,5-dimethylt-hiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), Chloroquine (CQ) and other chemical regents were obtained from Sigma-Aldrich (St Louis, MO, USA).
Cell culture
EC109 and CaES-17 cells were purchased from the Cancer Institute of the Chinese Academy of Medical Sciences (Beijing, China) and the China Center for Type Culture Collection (Wuhan, China), respectively. HKESC-2 cells were kindly provided by Prof Gopesh SRIVASTAVA at the Department of Pathology of the University of Hong Kong (Hong Kong, China). HKESC-2 cells were maintained in MEM (Corning Cellgro, Manassas, VA, USA), whereas EC109 and CaES-17 cells were grown in RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) at 37 °C in a humidified atmosphere of 5% CO2. Both media were supplemented with 10% fetal bovine serum (Invitrogen), 100 U/mL penicillin G, and 100 μg/mL streptomycin.
Cell viability assay
Cell viability was measured with MTT assays. In brief, cells were plated in 96-well plates and allowed to attach overnight before being incubated for 48 h with different concentrations of ABT-263 and CQ alone or both. In the next step, MTT was added, and the cells were further incubated for 3 h. The colored formazan product was photometrically measured at 570 nm in a multiwell plate reader (Bio-Rad Laboratories, USA).
Assessment of apoptosis
The cells were seeded in 6-well plates at 2×105 cells per well and exposed to various concentrations of ABT-263 and Staurosporine for 48 h. Cells were then harvested with 0.25% trypsin without EDTA (Gibco Laboratory), and apoptosis was evaluated by using an Annexin V-FITC Apoptosis Detection Kit (BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer's instructions.
Cell cycle analysis
After drug treatment, the cells were fixed with ice-cold 75% ethanol in phosphate-buffered saline (PBS) and incubated with 50 μg/mL propidium iodide and 0.5 μg/mL RNase A at 37 °C for 0.5 h before being analyzed by BD FACSCanto™ II flow cytometry (BD Biosciences). The resultant DNA histograms were generated and analyzed using FlowJo 7.6 software (Treestar, Inc, Ashland, OR, USA).
Western blotting
After treatment, cells were washed with PBS and lysed with RIPA buffer containing protease inhibitors (Merck, New York, USA) and phosphatase inhibitors (Merck). Equal amounts of protein (40 μg/lane) were resolved by SDS-PAGE and transferred to PVDF membranes (Roche, Indianapolis, IN, USA). All primary antibodies were incubated overnight at 4 °C, and the membranes were then incubated for 1 h with secondary peroxidase-conjugated antibodies. Chemiluminescent signals were then developed with Lumiglo reagent (Cell Signaling Technology) and exposed to X-ray film (FUJIFILM Europe GmbH, Düsseldorf, Germany).
Colony formation assay
Cells were treated with ABT-263 in the absence or presence of CQ for 48 h. The cells were then trypsinized and counted to assess viability. Subsequently, 3000 of these viable cells were plated in a six-well plate (in triplicate) and allowed to adhere and grow for 10 to 12 d. To visualize colonies, cells were fixed in 100% ethanol and stained with hematoxylin solution (Sigma-Aldrich). The colony numbers were calculated in ImageJ software.
RNA interference
The expression of p21Waf1/Cip1 was knocked down using target-specific siRNA molecules purchased from Sigma-Aldrich. Cells at 40%–60% confluence were transfected with gene-specific siRNA or control siRNA (Sigma-Aldrich) by using HiperFect Transfection Reagent (QIAgen) according to the manufacturer's instructions.
Antibodies
The antibodies against caspase 9, caspase 3, PARP, p21Waf1/Cip1, p27Kip1, cyclin D1, CDK2, phospho-Rb (Ser780), β-actin, LC3, and p62 as well as secondary antibodies were obtained from Cell Signaling Technology (Boston, MA, USA). The Rb antibody was obtained from BD Biosciences (Franklin Lakes, NJ, USA), and the antibodies against CDK4 and CDK6 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Statistical analysis
Statistical significance was assessed with one-way ANOVA in Graphpad Prism 5 software (GraphPad Software, Inc, San Diego, CA, USA). P values less than 0.05 were considered significant. All results were from three independent experiments, and all data reported are expressed as the mean±SEM.
Results
ABT-263 decreased the viability of human esophageal cancer cells
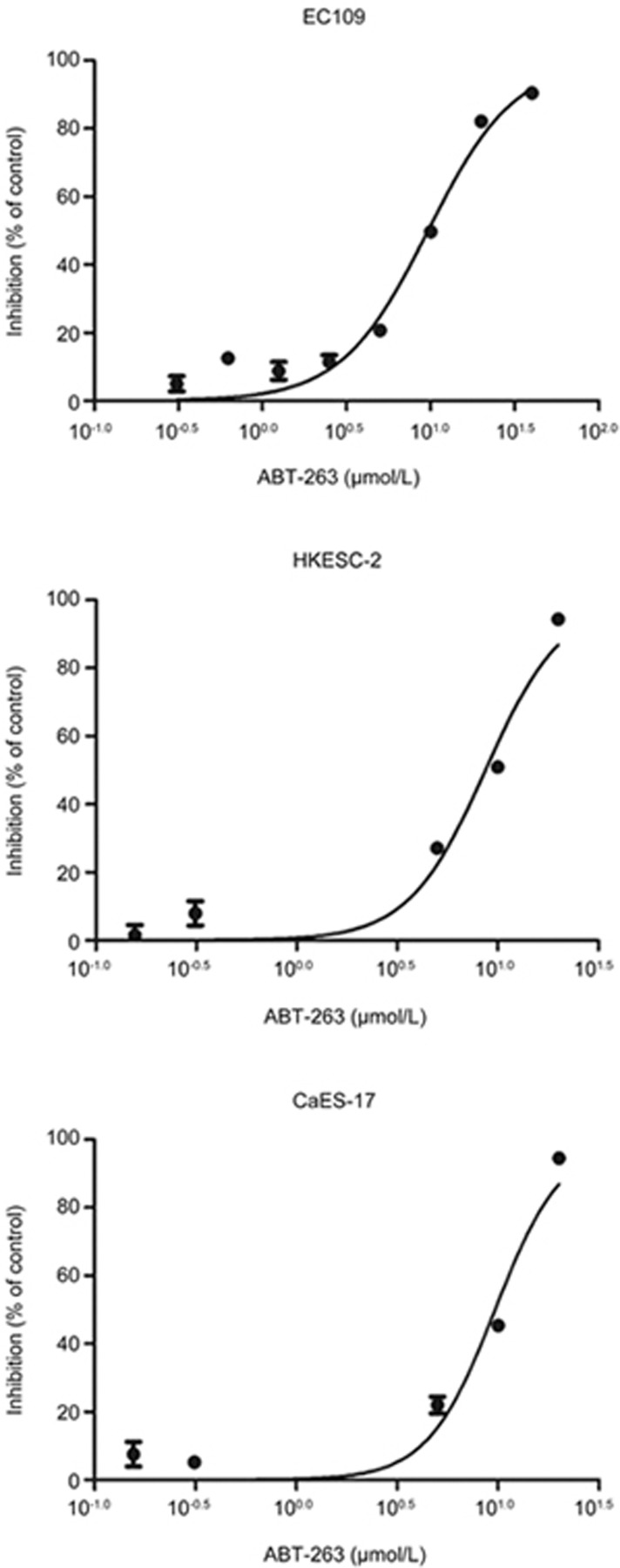
The effects of ABT-263 on cell viability were investigated in three human esophageal cancer cell lines, EC109, HKESC-2 and CaES-17, with MTT assays. As shown in Figure 1, ABT-263 decreased the viability of EC109, HKESC-2 and CaES-17 cells, with IC50 values of 10.7±1.4, 7.1±1.5, and 8.2±1.6 μmol/L, respectively. For the convenience of calculation, a concentration of 10 μmol/L was used for the following experiments.
Figure 1.
ABT-263 decreased the viability of human esophageal cancer cells. Cells (EC109, HKESC-2, and CaES-17) were exposed to increasing concentrations of ABT-263 for 48 h. Cell viability was assessed with MTT assays. Data are presented as the mean±SEM of three independent experiments.
ABT-263 induced G1/G0-phase arrest and apoptosis
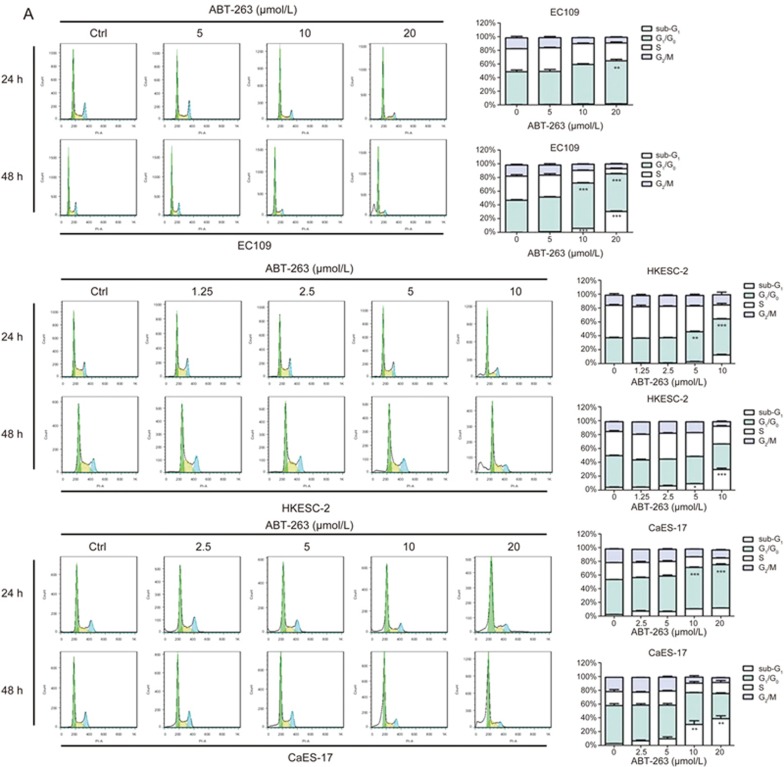
A flow cytometry-based cell cycle analysis showed that ABT-263 exposure for 24 h resulted in substantial accumulation of cells at the G1/G0-phase in all three tested cell lines. When the treatment time was prolonged to 48 h, the sub-G1 cell population markedly increased, thus suggesting enhanced apoptotic cell death (Figure 2A).
Figure 2A.
ABT-263 induced G1/G0-phase arrest and apoptosis in human esophageal cancer cells, in a dose-dependent manner. (A) Cells (EC109, HKESC-2, and CaES-17) were exposed to the indicated concentrations of ABT-263 for 24 h and 48 h, and their DNA contents were measured by PI staining.
Cellular apoptosis was further evaluated by measurement of the exposure of phosphatidylserine on the cell membrane by using Annexin V-fluorescein isothiocyanate (Annexin V-FITC) and propidium iodide (PI) staining. The results showed that the number of Annexin V-positive cells significantly increased after ABT-263 treatment for 48 h (Figure 2B). Concordantly, the protein levels of cleaved caspase 3, cleaved caspase 9 and PARP dramatically increased in response to ABT-263 treatment (Figure 2C).
Figure 2B 2C.
ABT-263 induced G1/G0-phase arrest and apoptosis in human esophageal cancer cells, in a dose-dependent manner. (B) EC109, HKESC-2 and CaES-17 cells were exposed to ABT-263 (5, 10 and 20 μmol/L) or staurosporine (positive control, 0.15 μmol/L) for 48 h, and apoptosis was then evaluated by flow cytometry after Annexin V-FITC and PI staining. Data are presented as the mean±SEM of three independent experiments. NS indicates “no significant difference” (P>0.05). *P<0.05, **P<0.01, ***P<0.001 compared with the control. (C) ABT-263 increased the cleavage of caspase 9, caspase 3 and PARP in EC109, HKESC-2 and CaES-17 cells. Cells were exposed to ABT-263 (5, 10 and 20 μmol/L) or staurosporine (positive control, 0.15 μmol/L) for 48 h, and the protein expression was assessed by Western blotting. β-Actin was used to evaluate protein loading.
ABT-263 regulated the expression of proteins related to G1-S transition
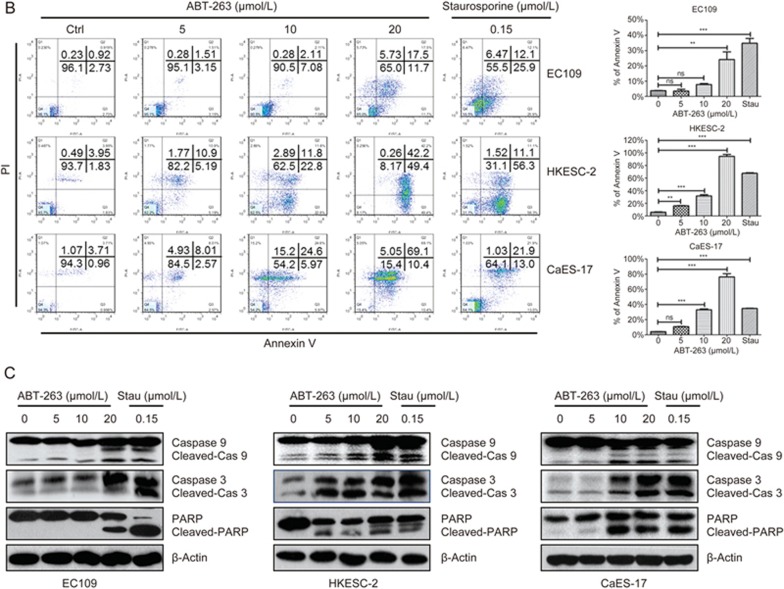
Because ABT-263 induces G1/G0 cell cycle arrest, we investigated its effect on the expression of regulatory proteins mediating the G1-to-S transition. As shown in Figure 3A, ABT-263 increased the protein expression of the CDK inhibitor p21Waf1/Cip1, whereas it did not alter the expression of p27Kip1. Moreover, ABT-263 markedly decreased the expression of cyclin D1, whereas the expression levels of CDK2, CDK4 and CDK6 were not affected (Figure 3B).
Figure 3.
Effects of ABT-263 on the expression of proteins related to the G1-S phase transition in human esophageal cancer cells. (A) EC109, HKESC-2 and CaES-17 cells were treated with ABT-263 (10 μmol/L) for the indicated times (0, 0.25, 0.5, 1, 3, 6, 12, 24, or 48 h). The protein expression levels of p21Waf1/Cip1 and p27Kip1 were assessed by Western blotting. β-Actin was used to evaluate protein loading. (B) The protein expression levels of cyclin D1, CDK2, CDK4, and CDK6 were assessed by Western blotting. (C) The protein expression levels of Rb and phospho-Rb (Ser780) were assessed by Western blotting.
D-type cyclins activate CDK4 and CDK6, which consequently form a CDK4/6-cyclin D complex that phosphorylates Rb (retinoblastoma tumor suppressor protein) and then activates the E2F transcription factors21. Accordingly, western blot analysis revealed that the expression of phospho-Rb (Ser780) was markedly decreased after ABT-263 treatment (Figure 3C).
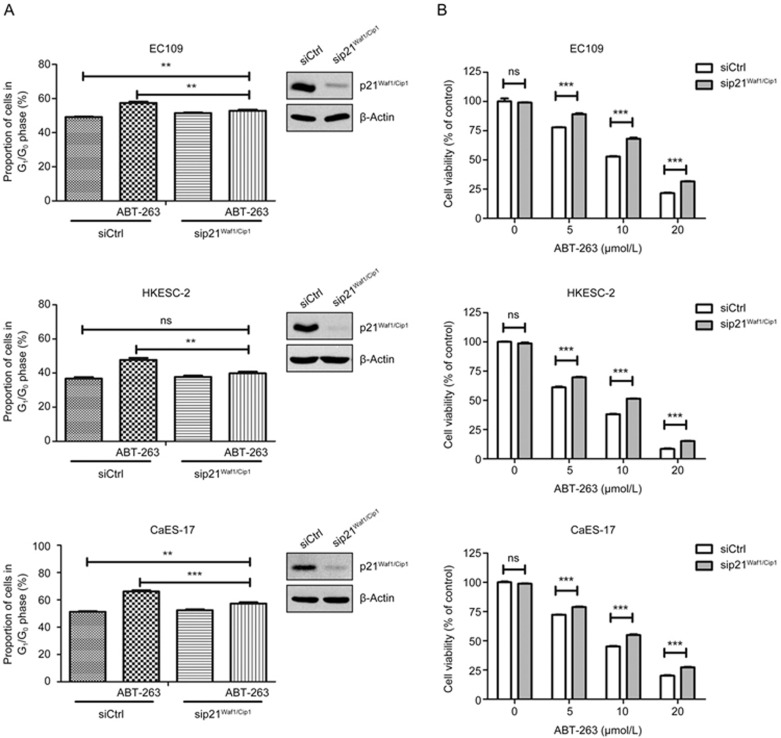
Knockdown of p21Waf1/Cip1 attenuated ABT-263-induced G1/G0-phase arrest
To assess the participation of p21Waf1/Cip1 in ABT-263-induced G1/G0 arrest, RNA interference was used to knock down p21Waf1/Cip1 in EC109, HKESC-2 and CaES-17 cells. The downregulation efficacy of p21Waf1/Cip1 was verified by western blotting in the tested three cell lines. Figure 4A shows that ABT-263 significantly increased the proportion of cells in the G1/G0-phase in the three tested cell lines. Moreover, the downregulation of p21Waf1/Cip1 significantly attenuated ABT-263-induced G1/G0-phase arrest in EC109 and CaES-17 cells, whereas it completely blocked ABT-263-induced G1/G0-phase arrest in HKESC-2 cells. These findings suggested that p21Waf1/Cip1 mediates ABT-263-mediated cell cycle arrest. Moreover, the knockdown of p21Waf1/Cip1 significantly attenuated ABT-263-mediated decreases in cell viability (Figure 4B). These findings suggested that p21Waf1/Cip1 at least in part mediates the ABT-263-induced inhibition of cell cycle progression and cell viability.
Figure 4.
Effects of the siRNA-mediated knockdown of p21Waf1/Cip1 on ABT-263-induced G1/G0-phase arrest and the loss of viability in EC109, HKESC-2 and CaES-17 cells. (A) After transfection with control siRNA or p21Waf1/Cip1 siRNA, EC109, HKESC-2 and CaES-17 cells were treated with ABT-263 (EC109, 15 μmol/L, 24 h, HKESC-2, 10 μmol/L, 20 h and CaES-17, 10 μmol/L, 24 h) before being subjected to flow cytometry analysis for DNA contents. (B) After transfection with control siRNA or p21Waf1/Cip1 siRNA, cells were exposed to the indicated concentrations (5, 10 and 20 μmol/L) of ABT-263 for 48 h, and cell viability was assessed with MTT assays. Data are presented as the mean±SEM of three independent experiments. NS indicates “no significant difference” (P>0.05). *P<0.05, **P<0.01, ***P<0.001 compared with the control.
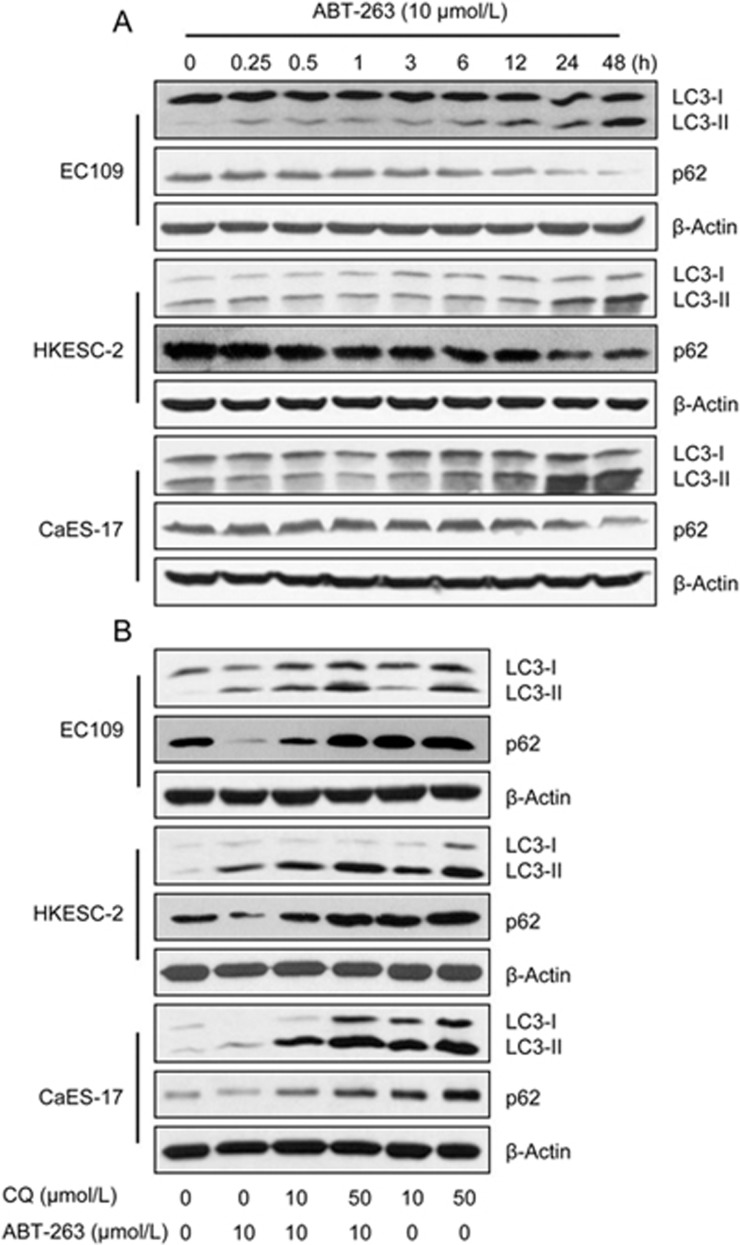
ABT-263 promoted pro-survival autophagy
Multiple studies have demonstrated that BH3 mimetics modify autophagic activity in a variety of cancer cell types22. Therefore, we studied the effect of ABT-263 on autophagy by measuring LC3-II and p62 protein expression. Western blots showed that ABT-263 treatment increased the LC3-II level, and the expression of p62, a protein degraded by autophagy, was concordantly decreased in all tested cell lines (Figure 5A). Because LC3-II is ultimately degraded by acidic hydrolases after the formation of autolysosomes, the lysosomal inhibitor chloroquine (CQ) was used to inhibit LC3-II degradation to measure autophagic flux. The addition of chloroquine stabilized ABT-263-induced LC3-II expression and suppressed p62 degradation, thereby indicating that autophagy induction by ABT-263 results in lysosomal degradation and is consistent with an increase in autophagic flux (Figure 5B). The inhibitory effect of CQ on autophagic flux in human esophageal cancer cells was confirmed by large increases in the levels of LC3-II and p62 (Figure 5B) and by the results of our previous study23.
Figure 5.
ABT-263 induced autophagy in EC109, HKESC-2 and CaES-17 cells. (A) EC109, HKESC-2 and CaES-17 cells were treated with ABT-263 at 10 μmol/L for the indicated times (0, 0.25, 0.5, 1, 3, 6, 12, 24 and 48 h). The protein expression levels of LC3-I, LC3-II and p62 were assessed by Western blotting. (B) CQ treatment inhibited the autophagy flux induced by ABT-263. Western blotting was used to measure the protein levels of LC3-I, LC3-II and p62 in cells that were treated with ABT-263 (10 μmol/L), CQ (10, 50 μmol/L) or both for 24 h, β-Actin was used to evaluate protein loading.
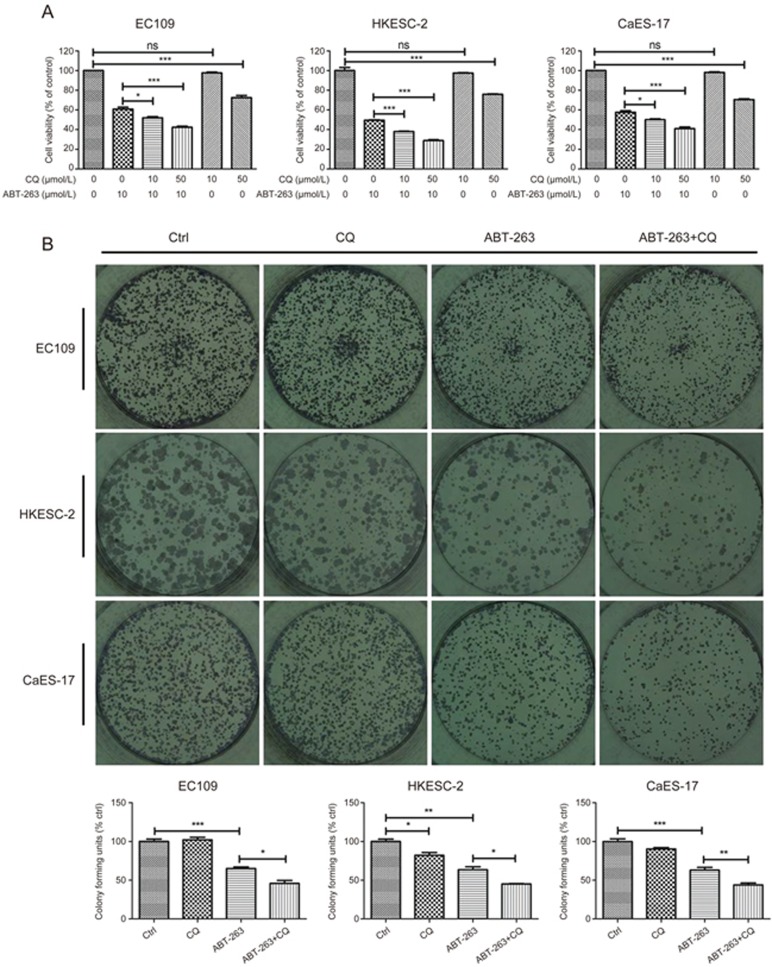
To further investigate the effect of ABT-263-induced autophagy on cell fate, CQ was used to block autophagy. Low concentrations (10 μmol/L) of CQ alone did not affect cell viability but significantly enhanced ABT-263-mediated decreases in viability (Figure 6A). In addition, the long-term effect of ABT-263 in combination with CQ was also tested. As shown in Figure 6B, CQ alone exerted no or marginal effects on the colony-forming ability of EC109, HKESC-2 and CaES-17 cells. However, when combined with ABT-263, CQ significantly further decreased colony formation, as compared with that in cells treated with ABT-263 alone.
Figure 6.
ABT-263-induced autophagy counteracted its inhibitory activity on cell viability and colony formation. (A) The autophagy inhibitor CQ attenuated the ABT-263-induced loss of cell viability in human esophageal cancer cell lines. Cells were treated with ABT-263 (10 μmol/L), CQ (10, 50 μmol/L) or both for 24 h. Cell viability was assessed with an MTT assay. (B) The autophagy inhibitor CQ sensitizes human esophageal cancer cells to ABT-263. EC109, HKESC-2 and CaES-17 cells were allowed to grow for 10 d after the withdrawal of ABT-263 (EC109, 10 μmol/L, HKESC-2, 6 μmol/L, CaES-17, 10 μmol/L) and CQ (EC109, 15 μmol/L, HKESC-2, 10 μmol/L, CaES-17, 10 μmol/L) alone or both at 48 h. The colonies were stained with hematoxylin, and their numbers are displayed in the charts. Data are presented as the mean±SEM of three independent experiments. NS indicates “no significant difference” (P>0.05). *P<0.05, **P<0.01, ***P<0.001 compared with the control.
Discussion
The BH3 mimetic ABT-263 is an orally bioavailable Bcl-2 family protein inhibitor that binds to multiple anti-apoptotic Bcl-2 family proteins, including Bcl-xL, Bcl-2 and Bcl-w24. This binding disrupts Bcl-2/Bcl-xL interactions with pro-death proteins (eg, Bim), thus leading to the initiation of apoptosis19. This compound has been tested in a variety of cell lines in vitro and in xenograft models25 and has significant activity against hematological malignancies, such as acute lymphoblastic leukemia, leukemia, non-Hodgkin's lymphoma, and myeloma, as well as solid tumors, including small-cell lung cancer (SCLC)26,27,28,29. Specifically, treating mice bearing SCLC xenograft tumors with single-agent ABT-263 results in dramatic tumor responses29,30. Although BH3 mimetics are designed to trigger apoptosis, accumulating evidence indicates that their anticancer activity is not limited to the initiation of apoptosis. In addition to their pro-apototic effect, BH3 mimetic agents have been shown to exert anti-proliferative effects by blocking cell cycle progression31,32 and to induce autophagy in tumor cells, mainly because of the release of Beclin 1 from Bcl-2 and Bcl-xL20,33,34,35. However, the mechanism underlying ABT-263-induced cell cycle arrest and autophagy induction had been unclear. Here, we show that ABT-263 inhibits cell viability by inducing G1/G0 phase arrest and apoptosis, whereas it induces pro-survival autophagy in human esophageal cancer cells. In our view, the most significant finding of the present study is that the treatment of cells with ABT-263 not only induced cellular apoptosis, a response commonly associated with Bcl-2 inhibition, but also induced G1/G0 phase arrest and pro-survival autophagy. In this respect, our experimental findings reveal a hitherto unreported effect of ABT-263 on cell cycle regulation and autophagy induction in human esophageal cancer cells. Although ABT-263 has impressive activity against xenograft models of various cancer types19,26,29, the in vivo effect of ABT-263 on tumor growth and autophagic activity in esophageal cancer xenografts remains unclear and warrants further exploration.
Our present study demonstrated that ABT-263 induced apoptosis in the three tested human esophageal cancer cell lines in a dose-dependent manner, as evidenced by the increase in the Annexin V-positive cell population and the elevated protein levels of cleaved caspase 3, cleaved caspase 9 and PARP. Notably, ABT-263-induced G1/G0 phase arrest occurred before apoptosis. In this scenario, ABT-263 treatment for 24 h substantially induced G1/G0 phase arrest, but the G1/G0 phase population reverted to basal levels when the treatment time was prolonged to 48 h in HKESC-2 and CaES-17 cells, and there was a concomitant increase in the sub-G1 population. Although cell cycle arrest can permit the repair of cellular damage, cell cycle arrest may also result in the activation of pathways leading to apoptosis if cellular damage cannot be properly repaired36. On the basis of our data, we propose that G1/G0-phase arrest induced by ABT-263 might contribute to subsequent apoptosis, but the mechanism underlying this correlation requires further exploration.
Cyclin-dependent kinases (CDKs) are important master regulators of the cell cycle, and the activities of CDK-cyclin complexes are negatively regulated by the endogenous CDK inhibitors p21Waf1/Cip1 and p27Kip1 or the degradation of cyclin37. p21Waf1/Cip1 and p27Kip1 bind to and inhibit Cdk4/cyclin D and Cdk2/cyclin E complexes, respectively, thus resulting in de-phosphorylation of retinoblastoma (RB) proteins and inhibition of the release of E2F transcription factors into the nucleus; consequently, the transcription of cell cycle-related genes is activated38,39. We measured the expression of proteins involved in the regulation of G1-to-S progression and found that ABT-263 substantially increased the protein level of p21Waf1/Cip1 in the three tested human esophageal cancer cell lines. In addition to p21Waf1/Cip1, p27Kip1 also belongs to the Cip and Kip family of CKIs, which have been implicated in G1/S transition checkpoint regulation40. However, treatment with ABT-263 did not alter the protein level of p27Kip1, thus suggesting that p27Kip1 does not contribute to ABT-263-induced cell cycle arrest. The upregulation of p21Waf1/Cip1, a negative regulator of cell cycle progression, may result in cell cycle arrest after ABT-263 treatment, and our data showed that the knockdown of p21Waf1/Cip1attenuated the ABT-263-induced accumulation of cells in the G1/G0 phase. Furthermore, ABT-263 treatment rarely affected the CDK2/4/6 proteins but clearly decreased the level of cyclin D1. In parallel with the decrease in cyclin D1, the expression of phospho-Rb (Ser780) was decreased after ABT-263 treatment, whereas the protein level of total Rb remained unchanged. These data suggested that an increase in p21Waf1/Cip1 and concomitant decreases in cyclin D1 and phospho-Rb (Ser780) mediate ABT-263-induced G1/G0 phase arrest in human esophageal cancer cells.
Autophagy may serve as a pro-survival or pro-death mechanism that counteracts or mediates the cytotoxicity of BH3 mimetics, including Obatoclax, Gossypol, ABT-737 and ABT-26313. In the present study, ABT-263 treatment was found to induce autophagy in the three tested human esophageal cancer cell lines, as evidenced by the increase in LC3-II protein and decrease in p62 protein. To assess the role of autophagy in the cellular response to ABT-263 exposure, the autophagy inhibitor chloroquine was used to block autophagic influx. In this scenario, chloroquine significantly enhanced the ABT-263-mediated decreases in cell viability and colony formation, thus suggesting ABT-263 induces pro-survival autophagy, which counteracts its anticancer activity.
In conclusion, our studies show that ABT-263 induces G1/G0 phase arrest and apoptosis as well as pro-survival autophagy in human esophageal cancer cells. Moreover, the inhibition of autophagy sensitizes human esophageal cancer cells to ABT-263 treatment. Our study may shed new light on the anti-cancer activity of ABT-263 and its potential application with autophagy inhibitors in the clinic.
Author contribution
Shu-wen LIU and Le YU designed the research; Qing-huan LIN, Fu-chang QUE, Chun-ping GU and De-sheng ZHONG performed the experiments and analyzed the data; Qing-huan LIN drafted the manuscript; Shu-wen LIU and Le YU revised the manuscript; Dan ZHOU and Yi KONG assisted with portions of the research.
Acknowledgments
This work was supported by research grants from the National Natural Science Foundation of China (81273551), the Program for Pearl River New Stars of Science and Technology in Guangzhou, China (2012J2200035), the Program for Outstanding Young Teacher in Guangdong Province, and Guangdong Pearl River Scholar Funded Scheme.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–86. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003; 349: 2241–52. [DOI] [PubMed] [Google Scholar]
- Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet 2013; 381: 400–12. [DOI] [PubMed] [Google Scholar]
- Sun L, Zhang H, Wu K. Esophageal cancer: current options for therapeutic management. Gastrointest Tumors 2014; 1: 105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyers C. Targeted cancer therapy. Nature 2004; 432: 294–7. [DOI] [PubMed] [Google Scholar]
- Craig RW. The Bcl-2 gene family. Semin Cancer Biol 1995; 6: 35–43. [DOI] [PubMed] [Google Scholar]
- Bonnefoy-Berard N, Aouacheria A, Verschelde C, Quemeneur L, Marcais A, Marvel J. Control of proliferation by Bcl-2 family members. Biochim Biophys Acta 2004; 1644: 159–68. [DOI] [PubMed] [Google Scholar]
- Petros AM, Olejniczak ET, Fesik SW. Structural biology of the Bcl-2 family of proteins. Biochim Biophys Acta 2004; 1644: 83–94. [DOI] [PubMed] [Google Scholar]
- Llambi F, Green DR. Apoptosis and oncogenesis: give and take in the BCL-2 family. Curr Opin Genet Dev 2011; 21: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HL, Dong YB, Elliott MJ, Liu TJ, Mcmasters KM. Caspase activation and changes in Bcl-2 family member protein expression associated with E2F-1-mediated apoptosis in human esophageal cancer cells. Clin Cancer Res 2000; 6: 1579–89. [PubMed] [Google Scholar]
- Yu L, Liu S. Autophagy contributes to modulating the cytotoxicities of Bcl-2 homology domain-3 mimetics. Semin Cancer Biol 2013; 23: 553–60. [DOI] [PubMed] [Google Scholar]
- Reed JC. Bcl-2 family proteins: regulators of chemoresistance in cancer. Toxicol Lett 1995; 82–83: 155–8. [DOI] [PubMed] [Google Scholar]
- Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene 2008; 27: 6398–406. [DOI] [PubMed] [Google Scholar]
- Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007; 26: 1324–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyhenmeyer B, Murphy AC, Prehn JH, Murphy BM. Targeting the anti-apoptotic Bcl-2 family members for the treatment of cancer. Exp Oncol 2012; 34: 192–9. [PubMed] [Google Scholar]
- Vogler M. Targeting Bcl2-proteins for the treatment of solid tumours. Adv Med 2014; 2014: 9436–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 2008; 68: 3421–8. [DOI] [PubMed] [Google Scholar]
- Malik SA, Orhon I, Morselli E, Criollo A, Shen S, Marino G, et al. BH3 mimetics activate multiple pro-autophagic pathways. Oncogene 2011; 30: 3918–29. [DOI] [PubMed] [Google Scholar]
- Ortega S, Malumbres M, Barbacid M. Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim Biophys Acta 2002; 1602: 73–87. [DOI] [PubMed] [Google Scholar]
- Reljic B, Conos S, Lee EF, Garnier JM, Dong L, Lessene G, et al. BAX-BAK1-independent LC3B lipidation by BH3 mimetics is unrelated to BH3 mimetic activity and has only minimal effects on autophagic flux. Autophagy 2016; 12: 1083–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Wu WK, Gu C, Zhong D, Zhao X, Kong Y, et al. Obatoclax impairs lysosomal function to block autophagy in cisplatin-sensitive and -resistant esophageal cancer cells. Oncotarget 2016; 7: 14693–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S. 466 POSTER ABT-263: an orally bioavailable small molecule inhibitor of Bcl2 family proteins. Ejc Supplements 2006; 4: 143. [Google Scholar]
- Lock R, Carol H, Houghton PJ, Morton CL, Kolb EA, Gorlick R, et al. Initial testing (stage 1) of the BH3 mimetic ABT-263 by the pediatric preclinical testing program. Pediatr Blood Cancer 2008; 50: 1181–9. [DOI] [PubMed] [Google Scholar]
- Suryani S, Carol H, Chonghaile TN, Frismantas V, Sarmah C, High L, et al. Cell and molecular determinants of in vivo efficacy of the BH3 mimetic ABT-263 against pediatric acute lymphoblastic leukemia xenografts. Clin Cancer Res 2014; 20: 4520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackler S, Mitten MJ, Chen J, Clarin J, Foster K, Jin S, et al. Navitoclax (ABT-263) and bendamustine+/- rituximab induce enhanced killing of non-Hodgkin's lymphoma tumours in vivo. Br J Pharmacol 2012; 167: 881–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackler S, Mitten MJ, Foster K, Oleksijew A, Refici M, Tahir SK, et al. The Bcl-2 inhibitor ABT-263 enhances the response of multiple chemotherapeutic regimens in hematologic tumors in vivo. Cancer Chemother Pharmacol 2010; 66: 869–80. [DOI] [PubMed] [Google Scholar]
- Shoemaker AR, Mitten MJ, Adickes J, Ackler S, Refici M, Ferguson D, et al. Activity of the Bcl-2 family inhibitor ABT-263 in a panel of small cell lung cancer xenograft models. Clin Cancer Res 2008; 14: 3268–77. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005; 435: 677–81. [DOI] [PubMed] [Google Scholar]
- Konopleva M, Watt J, Contractor R, Tsao T, Harris D, Estrov Z, et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax). Cancer Res 2008; 68: 3413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong D, Gu C, Shi L, Xun T, Li X, Liu S, et al. Obatoclax induces G1/G0-phase arrest via p38/p21waf1/Cip1 signaling pathway in human esophageal cancer cells. J Cell Biochem 2014; 115: 1624–35. [DOI] [PubMed] [Google Scholar]
- Sinha S, Levine B. The autophagy effector Beclin 1: a novel BH3-only protein. Oncogene 2008; 27: S137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999; 402: 672–76. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Criollo A, Tasdemir E, Vicencio JM, Tajeddine N, Hickman JA, et al. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-XL. Autophagy 2007; 3: 374–6. [DOI] [PubMed] [Google Scholar]
- Pietenpol JA, Stewart ZA. Cell cycle checkpoint signaling: cell cycle arrest versus apoptosis. Toxicology 2002; 181–182: 475–81. [DOI] [PubMed] [Google Scholar]
- Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif 2003; 36: 131–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacinti C, Giordano A. RB and cell cycle progression. Oncogene 2006; 25: 5220–7. [DOI] [PubMed] [Google Scholar]
- Munoz-Alonso MJ, Acosta JC, Richard C, Delgado MD, Sedivy J, Leon J. p21Cip1 and p27Kip1 induce distinct cell cycle effects and differentiation programs in myeloid leukemia cells. J Biol Chem 2005; 280: 18120–9. [DOI] [PubMed] [Google Scholar]
- Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 2009; 9: 400–14. [DOI] [PMC free article] [PubMed] [Google Scholar]