ABSTRACT
Indoxyl sulfate (IS) induces fibrosis and inflammation in kidneys via oxidative stress through the induction of transforming growth factor-β1 (TGF-β1) and monocyte chemotactic protein-1 (MCP-1). Furthermore, IS is a potent endogenous agonist for aryl hydrocarbon receptor (AHR), which regulates the transcription of genes such as cytochrome P450 (CYP) 1A1. Indole-3-propionic acid (IPA) is an antioxidant and has been reported to be neuroprotective. We determined whether IPA suppresses IS-induced expression of AHR, CYP1A1, TGF-β1, and MCP-1 in proximal tubular cells. The effects of IS on the expression of AHR, CYP1A1, TGF-β1, and MCP-1 were studied using normotensive rats and hypertensive rats. The effects of IPA on IS-induced expression of AHR, CYP1A1, TGF-β1, and MCP-1 were studied using proximal tubular cells (HK-2). Furthermore, the effects of IPA on IS-induced expression and phosphorylation of signal transducer and activator of transcription 3 (Stat3) were studied in HK-2 cells. Administration of IS induced the expression of AHR, CYP1A1, TGF-β1, and MCP-1 in the tubular cells of rat kidneys. IPA significantly suppressed IS-induced mRNA and protein expression of AHR, CYP1A1, TGF-β1, and MCP-1 in HK-2 cells. IPA suppressed the IS-induced expression and phosphorylation of Stat3 in HK-2 cells. Furthermore, knockdown of Stat3 inhibited the IS-induced mRNA and protein expression of AHR, CYP1A1, TGF-β1, and MCP-1 in HK-2 cells. In conclusion, IPA suppressed the IS-induced expression of AHR, CYP1A1, TGF-β1, and MCP-1 through suppression of Stat3 in proximal tubular cells. Thus, IPA suppresses IS-induced expression of fibrotic and inflammatory genes in proximal tubular cells.
Key Words: indole-3-propionic acid, indoxyl sulfate, aryl hydrocarbon receptor, TGF-β1, proximal tubular cells
INTRODUCTION
Indoxyl sulfate (IS), a uremic toxin, is involved in the progression of not only chronic kidney disease (CKD)1-3) but also cardiovascular disease. 4) IS is a metabolite of tryptophan derived from dietary protein, and is synthesized in the liver from indole that is produced by intestinal flora including Escherichia coli. IS is normally excreted into urine. As renal function deteriorates, IS accumulates in serum due to its reduced renal clearance.1,2) IS induces dysfunction of the kidney, especially proximal tubular cells.5-8)
IS induces reactive oxygen species (ROS) and activates nuclear factor-κB (NF-κB), 6) p53, 5) and signal transducer and activator of transcription 3 (Stat3). 9) Then, IS stimulates renal expression of fibrotic genes such as transforming growth factor-β1 (TGF-β1),3,6) and α-smooth muscle actin (SMA),5,6) and inflammatory genes such as monocyte chemotactic protein-1 (MCP-1) and intercellular adhesion molecule 1 (ICAM-1) in proximal tubular cells.10,11) These pathophysiological changes lead to kidney dysfunction such as interstitial fibrosis and inflammation, accelerating the progression of CKD.1-4)
IS is an aryl hydrocarbon receptor (AHR) agonist in human hepatocytes, 12) endothelial cells, 13) and vascular smooth muscle cells. 14) Activation of AHR mediates IS-induced expression of MCP-1 and tissue factors in endothelial cells. 13) AHR regulates transcription of multiple genes including cytochrome P450 (CYP) 1A1, CYP1A2, and CYP1B1. 12) We have demonstrated that AHR and Stat3 are involved in IS-induced downregulation of the Mas receptor in proximal tubular cells. 15)
Indole-3-propionic acid (IPA) is a potent anti-oxidant devoid of pro-oxidant activity. 16) IPA is an inhibitor of β-amyloid fibril formation, and is a potent neuroprotectant against a variety of oxidative toxins.17,18) IPA protects neurons from ischemia-induced neuronal damage by reducing DNA damage and lipid peroxidation. 19) Potassium bromate induces tumors and pro-oxidative effects in kidneys, and IPA prevents potassium bromate-induced lipid peroxidation in rat kidneys. 20) Thus, IPA is an antioxidant and free radical scavenger, inhibits β-amyloid fibril formation, and protects neuron damage and lipid peroxidation.
We hypothesized that IPA counteracts IS-induced toxic effects on proximal tubular cells. This study aimed to examine whether IPA suppresses the toxic effects induced by IS on the expression of AHR, and fibrotic and inflammatory genes in proximal tubular cells.
METHODS
Reagents
Reagents and antibodies were obtained from the following companies: HK-2 cells were purchased from American Type Culture Collection (ATCC) (Manassas, VA, USA). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin-streptomycin, and trypsin-EDTA solutions were purchased from Gibco (Invitrogen, Grand Island, NY, USA). IS and IPA were purchased from Sigma Chemical (St. Louis, MO, USA). Anti-CYP1A1 and anti-MCP-1 antibodies were purchased from Abcam (Cambridge, UK). Anti-TGF-β1 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-AHR antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-phospho-Stat3 (Tyr705) and anti-Stat3 antibodies were purchased from Cell Signaling (Boston, MA, USA). Anti-α-tubulin antibody was purchased from Calbiochem (La Jolla, CA, USA). Anti-rabbit IgG horseradish peroxidase (HRP)- and anti-mouse IgG HRP-linked antibodies were obtained from Cell Signaling Technology (Beverly, MA, USA).
Animal studies
Experimental rats were prepared as reported previously. 8) Briefly, the animal groups consisted of: (1) Dahl normotensive rats (DN, n=8), (2) Dahl normotensive IS-administered rats (DN+IS, n=8), (3) Dahl hypertensive rats (DH, n=8), and (4) Dahl hypertensive IS-administered rats (DH+IS, n=8). IS (200 mg/kg/day in drinking water) was administered to the rats. At 48 weeks of age (32nd week of the study), their kidneys were excised for immunohistochemical analysis.
The Animal Care Committee of Kureha Biomedical Research Laboratories approved these animal studies (IRB approval number: 21060016), which proceeded according to the Guiding Principles for the Care and Use of Laboratory Animals of the Japanese Pharmacological Society.
Immunohistochemistry
Immunohistochemistry was performed according to the streptavidin-biotinylated peroxidase complex (SABC) method. Kidney sections were deparaffinized with xylene, and dehydrated with ethanol. Endogenous peroxidase activity was inhibited with 0.3% H2O2 in methanol at room temperature for 10 min, followed by a rinse with phosphate buffered saline (PBS). All sections were incubated with 10% normal serum at room temperature for 30 min. The heat-mediated antigen retrieval method was performed twice by microwave treatment with 0.01 mol/L citrate buffer (pH 6.0) for 5 min. Then, the sections were treated at 4°C overnight with a primary antibody, anti-AHR antibody (1:100), anti-CYP1A1 antibody (1:100), anti-TGF-β1 antibody (1:100), anti-MCP-1 antibody (1:100), anti-Stat3 antibody (1:100), or anti-phospho-Stat3 antibody (Tyr705) (1:100). Sections were then incubated with a secondary antibody at room temperature for 30 min followed by a rinse with PBS, and treatment with peroxidase-conjugated streptavidin (Nichirei Co) at 37°C for 30 min. Finally, localization of AHR, CYP1A1, TGF-β1, and MCP-1 was visualized using 3,3-diaminobenzidine tetrahydrochloride (DAB tablet; Merck KGaA, Darmstadt, Germany) at a concentration of 30 mg/mL, containing 0.03% H2O2. Subsequently, the sections were counterstained with methylene green, and mounted in mounting media (Mount-quick, Daydo Sangyo Co., Saitama, Japan). All sections were photographed under light microscopy with a digital camera (DN100, E-600, Nikon; Tokyo, Japan). Immunostaining-positive areas were determined using Adobe Photoshop, and quantified in 10 random fields per section using NIH Image 1.62.
Cell culture
HK-2 cells purchased from ATCC were maintained in DMEM/F12 supplemented with 10% fetal bovine serum, insulin-transferrin-selenium, 100-U/mL penicillin, and 100-mg/mL streptomycin, and were incubated at 37°C under a 5% CO2 humidified atmosphere. HK-2 is an immortalized proximal tubule epithelial cell line from normal adult human kidney tissue. The medium was replaced every three days until confluence. Only cells between passages 2 to 8 were used for experiments.
Transfection of small interfering RNAs specific to Stat3
Small interfering RNAs (siRNAs) specific to Stat3 (Stat3 siRNA) were obtained from Nippon EGT (Tokyo, Japan). Lipofectamine RNA iMAX (Invitrogen, Life Technologies, Carsbad, CA, USA) was used to transfect siRNA into HK-2 cells according to the manufacturer’s protocol. HK-2 cells were incubated with or without Stat3 siRNA (10 nmol/L) for 72 h. The sense sequences of the siRNA for Stat3 were 5′-GGA GCA GCA CCU UCA GGA UdTdT-3′.9,21)
Quantitative real-time polymerase chain reaction (RT-PCR)
Total RNA was extracted from HK-2 cell lysates using TRIzol Reagent (Life Technologies, Carlsbad, CA) and subjected to reverse transcription. The cDNA was subjected to a quantitative RT-PCR analysis with the use of a Bio-Rad CFX96™ RT-PCR Detection System and Power SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA). Serial dilutions of a control sample of cDNA were used for the standard curve for each reaction. All experiments were performed in triplicate. Changes in gene expression were calculated by the 2–ΔΔCt method and the values were normalized to the levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Primers (Sigma-Aldrich) used in this study are listed in Table 1.
Table 1.
Sequences of primers used for RT-PCR.
| Gene | Forward (5’-3’) | Reverse (5’-3’) | Size |
|---|---|---|---|
| AHR | TCAACAGCAACAGTCCTTGG | TCCAATTTTAAACATGCCA | 166 |
| CYP1A1 | TCACATTCCTCTGCCCTTCC | GAGAAGGCAGCCCTGTTTGT | 51 |
| TGF-β1 | CAATTCCTGGCGATACCTCAG | GCACAACTCCGGTGACATCAA | 86 |
| MCP-1 | ACTCTCGCCTCCAGCATGAA | TTGATTGCATCTGGCTGAGC | 101 |
| GAPDH | ATGGGGAAGGTGAAGGTCG | GGGGTCATTGATGGCAACAATA | 108 |
Western blot analysis
Serum-starved HK-2 cells were incubated with 250 μmol/L of IS for the indicated time periods. Cells were pretreated with 1000 μmol/L IPA for 30 min before IS stimulation for 24 h. Cells were lysed in lysis buffer (65 mmol/L Tris-HCl (pH 6.8), 3.3% sodium dodecyl sulfate (SDS), 10% glycerol, 2.2% bromophenol blue), and were fractionated by SDS-polyacrylamide gel electrophoresis (PAGE) on polyacrylamide gels. Then, proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Immobilon-P, Millipore Bedford, MA, USA). The membranes were blocked with 5% bovine serum albumin (BSA) in Tris-buffered saline tween-20 (TBS-T) at room temperature for 1 h. After washing with TBS-T, the membranes were treated with rabbit polyclonal anti-AHR antibody (1:1000), rabbit polyclonal anti-CYP1A1 antibody (1:1000), rabbit polyclonal anti-TGF-β1 antibody (1:1000), mouse monoclonal anti-MCP-1 antibody (1:1000), rabbit monoclonal anti-phospho-Stat3 (Tyr705) antibody (1:1000), or rabbit monoclonal anti-Stat3 antibody (1:1000). Then, the membranes were further incubated with HRP-linked secondary antibody (1:10000) at room temperature for 1 h. After washing with TBS-T three times, protein expression was visualized using the enhanced Chemi-Lumi one system (Nacalai Tesque, Kyoto, Japan). The intensity of protein bands was normalized to the amount of α-tubulin (an internal control, 1:1000) and is expressed as ratios (fold increase) of the control value.
Statistical analysis
Results are expressed as the mean ± SE. The quantitative data among different groups were analyzed by Fisher’s protected least significant difference (PLSD) test of one-way analysis of variance (ANOVA). Results were considered statistically significant when the P value was < 0.05.
RESULTS
IS induces the expression of AHR, CYP1A1, TGF-β1, and MCP-1 in rat kidneys
Figure 1 shows immunostaining of AHR, CYP1A1, TGF-β1, and MCP-1 in rat kidneys. AHR, CYP1A1, TGF-β1, and MCP-1 were positively stained in the renal tubular cells of DN+IS, DH, and DH+IS rats. Quantitative data revealed that positive areas for AHR, CYP1A1, TGF-β1, and MCP-1 were significantly increased in DN+IS rats compared to that in DN rats. Furthermore, positive areas for AHR, CYP1A1, TGF-β1, and MCP-1 were significantly increased in DH+IS rats compared with that in DH rats. Thus, administration of IS induced the expression of AHR, CYP1A1, TGF-β1, and MCP-1 in the kidneys of normotensive rats and hypertensive rats.
Fig. 1.

Immunostaining of AHR, CYP1A1, TGF-β1, and MCP-1 in rat kidney
Immunostaining of AHR (a), CYP1A1 (b), TGF-β1 (c), and MCP-1 (d) (× 200), and quantitative data of AHR (e), CYP1A1 (f), TGF-β1 (g), and MCP-1 (h)-positive areas in the kidneys of DN, DN+IS, DH, and DH+IS rats (mean ± SE, n=8).
*P<0.01 vs DN, **P<0.001 vs DN, ***P<0.0001 vs DN, #P<0.001 vs DH.
Previously, we reported that DH+IS and DH rats showed significantly increased Masson trichrome-positive fibrosis areas 22) and increased infiltration of ED-1-positive macrophages 10) in the kidneys compared with that in DH and DN, respectively.
IPA suppresses the IS-induced expression of AHR, CYP1A1, TGF-β1, and MCP-1
We determined if IS induces the expression of AHR, CYP1A1, TGF-β1, and MCP-1 in cultured human proximal tubular cells (HK-2 cells). Figure 2 shows the effects of IS on mRNA and protein expression of AHR, CYP1A1, TGF-β1, and MCP-1 in HK-2 cells. IS significantly induced the expression of AHR, CYP1A1, TGF-β1, and MCP-1 in HK-2 cells. Then, we determined if IPA affects IS-induced mRNA and protein expression of AHR, CYP1A1, TGF-β1, and MCP-1 in HK-2 cells. Figure 2 shows the effects of IPA on IS-induced mRNA and protein expression of AHR, CYP1A1, TGF-β1, and MCP-1 in HK-2 cells. IPA significantly suppressed the IS-induced mRNA and protein expression of AHR, CYP1A1, TGF-β1, and MCP-1 in HK-2 cells.
Fig. 2.
IPA suppresses the IS-induced expression of AHR, CYP1A1, TGF-β1, and MCP-1 in HK-2 cells.
Serum starved HK-2 cells were pretreated with 1000 μmol/L of IPA for 30 min before incubation with IS (250 μmol/L) for 24 h. The mRNA and protein expressions of AHR (a, e), CYP1A1 (b, f) , TGF-β1 (c, g), and MCP-1 (d, h) in cultured HK-2 cells (mean ± SE, n=4). The size of TGF-β1 was 20 kDa, an active (mature) form.
*P<0.01, **P<0.001 vs control, #P<0.01 vs IS-treated cells.
IPA suppresses the IS-induced expression and phosphorylation of Stat3
Stat3 is involved in the IS-induced expression of TGF-β1, and MCP-1 in HK-2 cells. 9) Immunohistochemical analysis demonstrated that DN+IS, DH, and DH+IS rats showed significantly elevated expressions of phospho-Stat3 (Tyr705) and Stat3 in the kidneys compared to that of DN rats (Fig. 3a, b). IS induced the expression and phosphorylation of Stat3 in HK-2 cells. 9) We found that IS induced the phosphorylation of Stat3 from 1 to 10 min in HK-2 cells (Fig. 3c). More notably, IPA suppressed the IS-induced phosphorylation of Stat3 in HK-2 cells (Fig. 3d, e). Figure 3f demonstrates that IS induced the expression of total Stat3, and that IPA suppressed the IS-induced expression of total Stat3 in HK-2 cells.
Fig. 3.
IPA suppresses the IS-induced expression of Stat3 in HK-2 cells.
Immunostaining of phospho-Stat3 (Tyr705) and Stat3 in the kidneys of DN, DN+IS, DH, and DH+IS rats (× 200) (a, b). Serum-starved HK-2 cells were incubated with or without 1000 μmol/L of IPA for 30 min, followed by incubation with IS (250 μmol/L) for the indicated times (min). (c) Phosphorylated Stat3 (Tyr705) and total Stat3 protein expression in IS-treated HK-2 cells (mean ± SE, n=4). *P<0.01 vs 0 min. (d) phosphorylated Stat3 (Tyr705) and total Stat3 protein expressions in (IPA+IS) -treated HK-2 cells (mean ± SE, n=4). *P<0.01 vs 0 min. (e) Comparison of the ratio of phosphorylated Stat3 (Tyr705) and total Stat3 protein expressions at 3 and 5 min between IS-treated HK-2 cells and (IPA+IS)-treated HK-2 cells (mean ± SE, n=4). †P<0.001 vs IS at 3 min, ‡P<0.001 vs IS at 5 min. (f) Protein expression of Stat3 in cultured HK-2 cells. Serum starved HK-2 cells were pretreated with 1000 μmol/L IPA for 30 min before incubation with IS for 24 h (mean ± SE, n=4). *P<0.01, **P<0.001 vs control, #P<0.01 vs IS-treated cells.
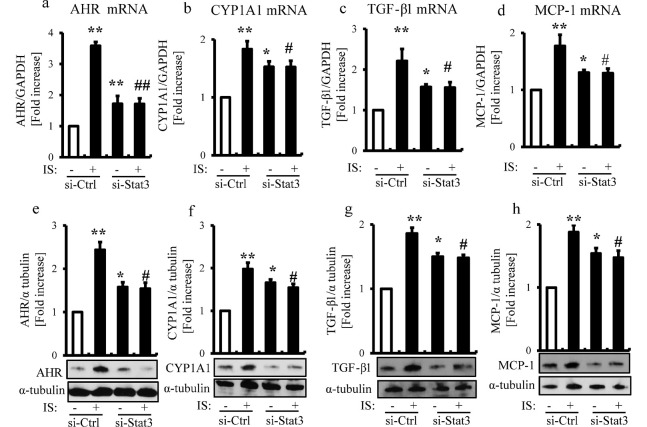
Stat3 siRNA suppresses the IS-induced expression of AHR, CYP1A1, TGF-β1, and MCP-1
We reported in previous studies that IS-induced Stat3 activation was involved in the expression of MCP-1, TGF-β1, α-SMA, and p65, and cellular senescence in proximal tubular cells.9,21) IS significantly upregulated the mRNA and protein expression levels of AHR, CYP1A1, TGF-β1, and MCP-1 in HK-2 cells. Stat3 siRNA suppressed the IS-induced mRNA and protein expressions of AHR, CYP1A1, TGF-β1, and MCP-1 in HK-2 cells (Fig. 4a-h).
Fig. 4.
Stat3 siRNA suppresses the IS-induced expression of AHR, CYP1A1, TGF-β1, and MCP-1 in HK-2 cells.
HK-2 cells were transfected with or without Stat3 siRNA (10 nmol/L) and then serum starved for 24 h, followed by incubation with IS (250 μmol/L) for 24 h. The mRNA and protein expressions of AHR (a, e), CYP1A1 (b, f) , TGF-β1 (c, g), and MCP-1 (d, h) in cultured HK-2 cells (mean ± SE, n=4).
*P<0.01, **P<0.001 vs control, #P<0.01, ##P<0.001 vs IS-treated cells.
Ctrl: control.
DISCUSSION
The present study first demonstrates that IPA suppressed the IS-induced expression of AHR, CYP1A1, TGF-β1, and MCP-1 in proximal tubular cells. Furthermore, IPA suppressed the IS-induced expression and phosphorylation of Stat3 in proximal tubular cells. Knockdown of Stat3 suppressed the IS-induced expression of AHR, CYP1A1, TGF-β1, and MCP-1 in proximal tubular cells. Thus, IPA suppressed the IS-induced fibrotic and inflammatory genes through suppression of Stat3 in proximal tubular cells, and might be protective against IS-induced tubular damage in the kidney.
Both IS and IPA are produced from tryptophan in the intestine by intestinal bacteria.23,24) Indole is produced from tryptophan by tryptophanase of intestinal bacteria. Indole is transported to the liver, where it is oxidized by cytochrome P-450 (CYP2E1), and then sulfated by sulfotransferase to form IS. Conversely, a different set of intestinal bacteria has been implicated in the metabolic transformation of indole to IPA. 23) Indole is transformed to IPA by the bacterium Clostridium sporogenes. 23) IPA is a powerful antioxidant, 25) and might be a possible drug for Alzheimer’s disease.17,18)
IS is a potent endogenous ligand that selectively activates the human AHR in primary human hepatocytes, regulating transcription of multiple genes, including CYP1A1, CYP1A2, CYP1B1, uridine diphosphate glucuronosyltransferase (UGT) (UGT1A1, UGT1A6), interleukin-6 (IL-6), and serum amyloid A 1 (SAA1). 12) Thus, IS stimulates AHR activation and altered drug metabolism. Prolonged activation of AHR by IS might contribute to toxicity observed in kidney dialysis patients. In the present study, IPA suppressed the IS-induced expression of AHR and CYP1A1 in proximal tubular cells.
IS is taken up by proximal tubular cells through OAT1 and OAT3 at the basolateral membrane. IS generates ROS, reduces superoxide scavenging activity, and consequently causes tubular cell injury. The damaged tubular cells produce TGF-β1 as well as chemokines such as MCP-1, ICAM-1, and osteopontin. These chemokines promote the infiltration of macrophages which produce TGF-β1. The secreted TGF-β1 stimulates the production of tissue inhibitor of metalloproteinase-1 (TIMP-1) and collagen. These changes facilitate interstitial fibrosis. Thus, IS accelerates tubular cell injury and subsequent interstitial fibrosis. In the present study, IPA suppressed the IS-induced expression of TGF-β1 and MCP-1 in the proximal tubular cells.
IS induces the expression and phosphorylation of Stat3 in proximal tubular cells. Stat3 is involved in the IS-induced expression of MCP-1, TGF-β1, α-smooth muscle actin, and NF-ĸB p65 in proximal tubular cells. 9) Therefore, we focused on the Stat3-dependent pathway in the effects of IS. In the present study, IPA suppressed the expression of Stat3, and also suppressed its phosphorylation in proximal tubular cells. However, it is possible that the Stat3-independent pathway is involved in the effects of IS.
Taken together, IPA might be protective against IS-induced tubular damage by suppressing IS-induced expression of inflammatory and fibrotic genes in proximal tubular cells. Further study is required to demonstrate in vivo evidence for the therapeutic effects of IPA.
ACKNOWLEDGMENTS
The authors acknowledge the gift of rat kidney samples from Kureha Co., Tokyo, Japan. This study was supported in part by JSPS research grant (JSPS KAKENHI Grant Number 15J00397). MY is a JSPS doctoral fellow overseas researcher.
CONFLICTS OF INTEREST
All the authors have declared no competing interest.
REFERENCES
- 1).Niwa T, Ise M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J Lab Clin Med, 1994; 124: 96-104. [PubMed]
- 2).Niwa T, Ise M, Miyazaki T. Progression of glomerular sclerosis in experimental uremic rats by administration of indole, a precursor of indoxyl sulfate. Am J Nephrol, 1994; 14: 207-212. [DOI] [PubMed]
- 3).Miyazaki T, Ise M, Seo H, Niwa T. Indoxyl sulfate increases the gene expressions of TGF-beta1, TIMP-1 and pro-alpha 1(I) collagen in uremic rat kidneys. Kidney Int, 1997; S62: S15-22. [PubMed]
- 4).Niwa T, Indoxyl sulfate is a nephro-vascular toxin. J Ren Nutr, 2010; 20(Suppl 1): S2-S6. [DOI] [PubMed]
- 5).Shimizu H, Bolati D, Adijiang A, Enomoto A, Nishijima F, Dateki M, et al. Senescence and dysfunction of proximal tubular cells are associated with activated p53 expression by indoxyl sulfate. Am J Physiol Cell Physiol, 2010; 299: C1110-C1117. [DOI] [PubMed]
- 6).Shimizu H, Bolati D, Adijiang A, Muteliefu G, Enomoto A, Nishijima F, et al. NF-κB plays an important role in indoxyl sulfate-induced cellular senescence, fibrotic gene expression, and inhibition of proliferation in proximal tubular cells. Am J Physiol Cell Physiol, 2011; 301: C1201-C1212. [DOI] [PubMed]
- 7).Shimizu H, Saito S, Higashiyama Y, Nishijima F, Niwa T. CREB, NF-κB, and NADPH oxidase coordinately upregulate indoxyl sulfate-induced angiotensinogen expression in proximal tubular cells. Am J Physiol Cell Physiol, 2013; 304: C685-C692. [DOI] [PubMed]
- 8).Adijiang A, Shimizu H, Higuchi Y, Nishijima F, Niwa T. Indoxyl sulfate reduces klotho expression and promotes senescence in kidneys of hypertensive rats. J Ren Nutr, 2011; 21: 105-109. [DOI] [PubMed]
- 9).Shimizu H, Yisireyili M, Nishijima F, Niwa T. Stat3 contributes to indoxyl sulfate-induced inflammatory and fibrotic gene expression and cellular senescence. Am J Nephrol, 2012; 36: 184-189. [DOI] [PubMed]
- 10).Shimizu H, Bolati D, Higashiyama Y, Nishijima F, Shimizu K, Niwa T. Indoxyl sulfate upregulates renal expression of MCP-1 via production of ROS and activation of NF-κB, p53, ERK, and JNK in proximal tubular cells. Life Sci, 2012; 90: 525-530. [DOI] [PubMed]
- 11).Shimizu H, Yisireyili M, Higashiyama Y, Nishijima F, Niwa T. Indoxyl sulfate upregulates renal expression of ICAM-1 via production of ROS and activation of NF-κB and p53 in proximal tubular cells. Life Sci, 2013; 92: 143-148. [DOI] [PubMed]
- 12).Schroeder JC, Dinatale BC, Murray IA, Flaveny CA, Liu Q, Laurenzana EM, et al. The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry, 2010; 49: 393-400. [DOI] [PMC free article] [PubMed]
- 13).Watanabe I, Tatebe J, Namba S, Koizumi M, Yamazaki J, Morita T. Activation of aryl hydrocarbon receptor mediates indoxyl sulfate-induced monocyte chemoattractant protein-1 expression in human umbilical vein endothelial cells. Circ J, 2013; 77: 224-230. [DOI] [PubMed]
- 14).Gondouin B, Cerini C, Dou L, Sallée M, Duval-Sabatier A, Pletinck A, et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int, 2013; 84: 733-744. [DOI] [PubMed]
- 15).Ng HY, Yisireyili M, Saito S, Lee CT, Adelibieke Y, Nishijima F, et al. Indoxyl sulfate downregulates expression of Mas receptor via OAT3/AHR/Stat3 pathway in proximal tubular cells. PLoS One, 2014; 9: e91517.
- 16).Poeggeler B, Pappolla MA, Hardeland R, Rassoulpour A, Hodgkins PS, Guidetti P, et al. Indole-3-propionate: a potent hydroxyl radical scavenger in rat brain. Brain Res, 1999; 815: 382-388. [DOI] [PubMed]
- 17).Chyan YJ, Poeggeler B, Omar RA, Chain DG, Frangione B, Ghiso J, et al. Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid. J Biol Chem, 1999; 274: 21937-21942. [DOI] [PubMed]
- 18).Bendheim PE, Poeggeler B, Neria E, Ziv V, Pappolla MA, Chain DG. Development of indole-3-propionic acid (OXIGON) for Alzheimer’s disease. J Mol Neurosci, 2002; 19: 213-217. [DOI] [PubMed]
- 19).Hwang IK, Yoo KY, Li H, Park OK, Lee CH, Choi JH, et al. Indole-3-propionic acid attenuates neuronal damage and oxidative stress in the ischemic hippocampus. J Neurosci Res, 2009; 87: 2126-2137. [DOI] [PubMed]
- 20).Karbownik M, Stasiak M, Zygmunt A, Zasada K, Lewin´ski A. Protective effects of melatonin and indole-3-propionic acid against lipid peroxidation, caused by potassium bromate in the rat kidney. Cell Biochem Funct, 2006; 24: 483-489. [DOI] [PubMed]
- 21).Saito S, Shimizu H, Yisireyili M, Nishijima F, Enomoto A, Niwa T. Indoxyl sulfate-induced activation of (pro)renin receptor is involved in expression of TGF-β1 and α-smooth muscle actin in proximal tubular cells. Endocrinology, 2014; 155: 1899-1907. [DOI] [PubMed]
- 22).Bolati D, Shimizu H, Higashiyama Y, Nishijima F, Niwa T. Indoxyl sulfate induces epithelial-to-mesenchymal transition in rat kidneys and human proximal tubular cells. Am J Nephrol, 2011; 34: 318-323. [DOI] [PubMed]
- 23).Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A, 2009; 106: 3698-3703. [DOI] [PMC free article] [PubMed]
- 24).Smith EA, Macfarlane GT. Formation of phenolic and indolic compounds by anaerobic bacteria in the human large intestine. Microb Ecol, 1997; 33: 180–188. [DOI] [PubMed]
- 25).Karbownik M, Reiter RJ, Garcia JJ, Cabrera J, Burkhardt S, Osuna C, et al. Indole-3-propionic acid, a melatonin-related molecule, protects hepatic microsomal membranes from iron-induced oxidative damage: Relevance to cancer reduction. J Cell Biochem, 2001; 81: 507–513. [PubMed]