ABSTRACT
Positron emission tomography/computed tomography (PET/CT) integrated with 2-[18F]fluoro-2-deoxy-D-glucose (FDG) is a useful tool for acquisition of both glucose metabolism and anatomic imaging data, as only a single device and one diagnostic session is required, thus opening a new field in clinical oncologic imaging. FDG-PET/CT has been successfully used for initial staging, restaging, assessment of early treatment response, evaluation of metastatic disease response, and prognostication of intestinal cancer as well as various malignant tumors. We reviewed the current status and role of FDG-PET/CT for management of patients with esophageal cancer, gastric cancer, and colorectal cancer, with focus on both its usefulness and limitations.
Key Words: fluorodeoxyglucose (FDG), positron emission tomography/computed tomography (PET/CT), esophageal cancer, gastric cancer, colorectal cancer
INTRODUCTION
Development of positron emission tomography (PET) with 2-[18F]fluoro-2-deoxy-D-glucose (FDG), which exploits increased utilization and high uptake of glucose by malignant cells, in the late 1990s opened a new field in clinical oncologic imaging. More recently, integrated positron emission tomography/computed tomography (PET/CT), which combines a full-ring-detector clinical PET scanner with a multi-detector-row helical CT scanner, has made it possible to acquire both metabolic and anatomic imaging data with a single device in one diagnostic session, and has been demonstrated to show precise anatomic localization of suspicious areas of increased FDG uptake.
FDG-PET/CT use in clinical settings results in significant improvement in diagnostic accuracy, with considerable impact on patient management, including diagnosis, initial staging, treatment optimization, restaging, monitoring of response to therapy, and prognostication of various malignant tumors. We present here a review of the current and future roles of FDG-PET/CT for management of patients with esophageal cancer, gastric cancer, and colorectal cancer (CRC), as well as its usefulness and limitations.
ESOPHAGEAL CANCER
In histology findings, more than 90% of esophageal cancers are either squamous cell carcinoma or adenocarcinoma. Chemoradiotherapy (CRT) followed by surgery is now considered to be standard care for patients with potentially curable esophageal or esophagogastric junction cancer,1) with the overall median survival reported to be 30 months in patients with localized disease, 13 months in those with regional disease, and 6 months in those with metastatic disease.2) These findings underscore the importance of early and accurate selection of patients who might benefit from therapy with curative intent.
Primary staging
Following diagnosis of esophageal cancer from endoscopic biopsy results, the disease is commonly staged according to TNM classification in order to determine a treatment plan and prognosis, and also distinguish potentially curable disease from incurable advanced local or metastatic disease. Tumors confined to the esophageal wall or with less extensive extension into the periesophageal adventitia are considered potentially resectable with or without neoadjuvant CRT (T1-3).3) On the other hand, patients with non-resectable disease (i.e., T4b, M1) may undergo primary CRT, brachytherapy, stent placement, or another less invasive form of palliative treatment. Due to the high rates of morbidity and mortality associated with CRT and esophagectomy, appropriate patient selection criteria are critical.
In clinical settings, endoscopic ultrasonography (EUS) and contrast-enhanced CT are the primary imaging modalities for esophageal cancer, while they have also been shown to be complementary. Of those modalities, it has been shown that contrast-enhanced CT can better determine tumor length and exclude invasion of adjacent structures, while EUS is better for determining primary tumor depth and identifying loco-regional lymph node (LN) metastasis, thus is considered superior to FDG-PET/CT in that context.4) Visible FDG avidity of tumors is dependent on sufficient tumor volume and metabolic activity that exceeds a certain detection threshold (currently, approximately 5 mm). The limited spatial resolution of PET in particular limits detection of early esophageal cancer with a small volume (i.e., Tis, T1).5)
In a meta-analysis of 245 patients, patient-based sensitivity of FDG-PET/CT for detecting loco-regional LN metastasis (N staging) was found to be poor [55%; 95% confidence interval (CI) 34-74%), while specificity was moderate (76%; 95% CI 66-83%) (Fig. 1). Nevertheless, it should be noted that loco-regional LN assessment may be less significant clinically, because those nodes are likely to be resected along with the primary tumor and may also be included in the field of radiation in patients undergoing that therapy. A more important advantage of FDG-PET/CT is its ability to detect remote nodal involvement and systemic metastatic disease (M staging). Furthermore, FDG-PET/CT provides incremental staging information and its findings result in changed management in approximately one-third more examined patients as compared with EUS and CT.6)
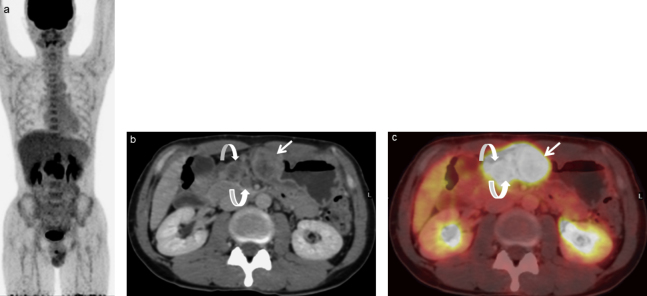
Fig 1.
A 65-year-old man with advanced esophageal cancer and multiple regional lymph node (LN) metastases (cT2N3M0).
(a) Maximum intensity projection (MIP) of positron emission tomography (PET) scan shows many areas of abnormal fluorodeoxyglucose (FDG) uptake in the esophagus and lymph node areas.
(b) Contrast-enhanced computed tomography (CT) and (c) FDG-PET/CT scans show focal uptake (standardized uptake value [SUVmax]:7.2) in the lower intrathoratic esophagus (arrow), suggesting esophageal cancer.
(d) Contrast-enhanced CT and (e) FDG-PET/CT scans show focal uptake (SUVmax: 6.8) in the left supraclavicular LN measuring 5×8 mm (arrow), confirming #104L node metastasis.
(f) Contrast-enhanced CT and (g) FDG-PET/CT scans show focal uptake in two LNs along the left gastric artery (SUVmax: 9.2) measuring 10×11 mm (arrow) and the celiac artery (SUVmax: 8.5) measuring 9×10 mm (curved arrow), confirming #7 and #9 node metastases, respectively.
(h) Contrast-enhanced CT and (i) FDG-PET/CT scans show focal uptake in 5×6 mm LN along the superior mesenteric artery (SUVmax: 4.5, arrow) and in para-aortic LN measuring 4×5 mm (SUVmax: 5.6, curved arrow), confirming #14a and #16 node metastases, respectively.
Synchronous neoplasms can be detected using FDG-PET/CT in a considerable number of patients with esophageal cancer who undergo staging, with a significant impact on treatment planning. Malik et al. evaluated 591 patients with biopsy-proven esophageal cancer who underwent staging via FDG-PET/CT and suspicion of a synchronous neoplasm was noted in 9.3%, while malignancy was later confirmed in 18.6% of those who underwent further investigations.7)
Prognosis assessment
Tumor stage is currently considered to be the most important clinical prognostic factor in patients with esophageal cancer and several studies have suggested that FDG-PET/CT imaging parameters provide valuable prognostic information for such cases. A meta-analysis of 11 reports demonstrated that a high pretreatment maximum standardized uptake (SUVmax) value was a significant predictor of poor overall survival (OS) [hazard ratio (HR) 1.86, 95% CI 1.53-2.27) and disease-free survival (DFS) (HR 2.52, 95% CI 1.98-3.21).8) In contrast, another review article that synthesized the results of 15 individual studies found that the pretreatment SUVmax value for the primary tumor was not an unequivocal predictor of survival.9) While 12 of those studies showed that pretreatment SUVmax was a predictor of survival in univariate analysis findings, only 2 found that to be a predictor of survival in multivariate analysis.
In recent years, pretreatment metabolic tumor volume (MTV), including MTV and total lesion glycolysis (TLG), has been shown to be another parameter that may be a better predictor for survival than SUVmax.10-12) However, an important issue that must be considered prior to incorporating any FDG-PET-derived parameter other than TNM staging in a clinical prognostic model is standardization of the methods used for acquisition and image analysis.
Radiation therapy planning
Accurate delineation of gross tumor volume for subsequent irradiation is a prerequisite for successful radiation therapy.13) With the addition of PET to CT-based radiation treatment planning, target volume definition has been significantly improved because of more accurate localization of the primary tumor and any involved regional LNs, which in turn improves loco-regional disease control and reduces radiation-induced complications.14) However, there is yet no uniform method for tumor volume delineation, which is either performed by visual interpretation or (semi-) automatic contouring using an SUV threshold. Additional exploratory and validation studies are required prior to implementation of FDG-PET/CT for this purpose as a part of routine patient care.
Treatment response assessment
Neoadjuvant chemotherapy (NAC) and CRT have become important treatment options for patients with esophageal cancer, though toxicity and radiation-induced complications are often encountered. When patients show insufficient response, neoadjuvant treatment must be discontinued and surgery cannot be delayed. Thus, it is crucial to accurately identify patients who respond to neoadjuvant therapy as early as possible.
Several studies have demonstrated the usefulness of FDG-PET/CT scanning for monitoring of therapeutic response in patients with esophageal cancer (Fig. 2, 3). A meta-analysis of 20 studies investigated the diagnostic performance of FDG-PET/CT for assessing tumor response to NAC or CRT, with the results ranging widely for sensitivity and specificity with pooled estimates (95% CI) of 67% (62-72%) and 68% (64-73%), respectively.15) More recently, a meta-analysis of 30 studies estimated slightly better sensitivity (70%, 95% CI 64-76%) and specificity (70%, 95% CI 65-75%), and the authors suggested a 50% reduction in SUVmax and SUVmean values between pre-treatment PET and PET performed within the first 2 weeks of NAC or CRT as an optimal cut-off for predicting response to neoadjuvant treatment.16) Furthermore, they recommended that PET should not be used in routine clinical settings to guide neoadjuvant therapy decisions. CRT-induced esophagitis or ulceration of the esophagus can cause increased FDG accumulation, and persistently raises the SUV value, which leads to false-positive results in an FDG-PET examination, preventing accurate detection of residual cancer. In a well-designed prospective study by Malik et al., FDG-PET/CT examinations were performed 14 days after the start of neoadjuvant CRT, with no correlation seen for change in tumor SUVmax with either histopathologic response or survival.17)
Fig. 2.

A 66-year-old man with advanced esophageal cancer (cT3N2M0), showing a pathological complete response (pCR) at evaluation of response to neoadjuvant chemotherapy using FDG-PET/CT.
(a) Pretreatment MIP of PET scan shows two areas of strong FDG uptake in the upper intrathoratic esophagus and right supraclavicular node area.
(b) Pretreatment FDG-PET/CT scan shows that SUVmax in the primary tumor was 30.3.
(c) Preoperative MIP of PET scan obtained 3 months after starting of neoadjuvant chemotherapy (NAC) shows faint FDG uptake in the upper intrathoratic esophagus.
(d) Preoperative FDG-PET/CT scan obtained 3 months after starting of NAC shows that SUVmax in the primary tumor was 3.15, corresponding to a 90% decrease.
The surgical histological analysis revealed no residual cancer (treatment response was grade 3).
Fig. 3.

A 71-year-old man with advanced esophageal cancer (cT3N3M0), showing no pathological complete response during evaluation of response to NAC using FDG-PET/CT.
(a) Pretreatment MIP of PET scan shows three areas of strong FDG uptake in the lower intrathoratic esophagus and mediastinum and upper abdominal node areas.
(b) Pretreatment FDG-PET/CT scan shows that SUVmax in the primary tumor was 17.3.
(c) Preoperative MIP of PET scan obtained 3 months after starting of NAC shows strong FDG uptake in the lower intrathoratic esophagus.
(d) Preoperative FDG-PET/CT scan obtained 3 months after starting of NAC shows that SUVmax in the primary tumor was 16.3, corresponding to a 5.8% decrease.
Note: The surgical histological analysis revealed residual cancer and the treatment response was classified as grade 1.
Tumor response can also be assessed after completion of neoadjuvant therapy. A separate recent meta-analysis of 26 reports that included 1544 patients who received neoadjuvant therapy showed that post-treatment FDG-PET effectively predicted long-term outcome.18) In that analysis, the pooled HR for complete metabolic response as compared to no response was 0.51 for OS (95% CI 0.40-0.64) and 0.47 for DFS (95% CI 0.38-0.57). Even though complete clinical response shown by PET findings may reflect a true pathological complete response, it is important to note that such findings should be cautiously interpreted when considering omission of surgical resection. In PET-proven complete responders who did not undergo an esophagectomy, the subsequent loco-regional recurrence rate was as high as 42%.19) Furthermore, in the largest series presented to date (n=284), the specificity of combined PET and endoscopic biopsy-proven clinical complete response showed that true pathological complete response was actually quite low (30%).20)
Prior to application in daily practice, inter-institutional harmonization projects regarding study designs and therapeutic regimens, as well as FDG-PET/CT acquisition protocols and response criteria are urgently needed. Yanagawa et al. compared 2 current and widely used response evaluation criteria (PET response criteria in solid tumors [PERCIST] vs. response evaluation criteria in solid tumors [RECIST]) in patients with locally advanced esophageal cancer following NAC and concluded that PERCIST is more suitable for evaluating chemotherapeutic response to esophageal cancer.21)
Restaging
Findings obtained in the CROSS I and II trials revealed that 35% of the enrolled patients developed recurrent disease after a minimum follow-up of 24 months after treatment. Distant recurrence occurred in 59% and distant plus loco-regional recurrence was found in 31% of those patients, while loco-regional recurrence was seen in only 9%.22)
FDG-PET/CT is a useful modality to detect recurrent and distant metastatic lesions, excluding those in the brain. A recent meta-analysis of 8 studies with a total of 486 patients who underwent FDG-PET or PET/CT as part of routine follow-up examinations, or because of clinical suspicion showed pooled estimates of sensitivity and specificity for FDG-PET/CT or PET in diagnosing recurrent esophageal cancer of 96% (95% CI 93-97%) and 78% (95% CI 66-86%), respectively.23) However, false-positive findings are often caused by radiation therapy- and/or surgically-induced inflammatory changes, or chronic inflammation of mediastinal lymph nodes, thus specificity is moderate and histopathological confirmation of suspected lesions based on FDG-PET findings remains a requirement.
GASTRIC CANCER
Gastric adenocarcinoma comprises approximately 95% of all types of gastric cancer and is divided into 2 major subtypes, intestinal type, which predominantly involves the distal stomach and is found most commonly in Asians (often associated with chronic infection by Helicobacter pylori), and diffuse or signet ring type, which commonly involves the proximal stomach and is often found in Western patients in association with chronic reflux and obesity. Contrast-enhanced CT and EUS are typically used to determine the depth of involvement, and presence of local and distant disease. In addition, a staging laparoscopy procedure is performed when the tumors are considered to be resectable or imaging findings are indeterminate for possible resection, so as to avoid surgery in patients with non-resectable tumors.24) The only potentially curative therapeutic modality for gastric adenocarcinoma is complete resection, which involves removal of at last part of the stomach as well as LN dissection. Accurate staging and characterization of disease burden is of vital importance for determining treatment strategy.
Detection
FDG-PET/CT has been reported to have a low detection rate for diagnosis of primary gastric cancer (approximately 55%), especially in the early stage, as well as signet-ring cell, mucinous, and poorly differentiated adenocarcinoma, which are typically less metabolically active.25) Moreover, it is not uncommon for variable and occasionally intense physiological uptake to exist within the gastric wall, which can mask FDG uptake by the primary tumor. Increased FDG uptake may also be caused by the presence of gastritis. Therefore, FDG-PET plays only a minor role in gastric cancer diagnosis. Indeed, Minamimoto et al. demonstrated that gastric endoscopy should be included in an FDG-PET cancer screening program to screen for gastric cancer.26)
Staging
For gastric cancer staging, FDG-PET/CT has a limited role and is not particularly helpful for evaluation of T stage. In view of its low level of spatial resolution, FDG-PET/CT provides very limited information about the involved layer of the gastric wall or invasion of adjacent organs.
Although FDG-PET/CT appears to be more specific for detection of loco-regional LNs and peritoneal lesions as compared to CT alone, it is actually less sensitive.25,27,28) FDG-PET/CT sensitivity, specificity, and accuracy for detection of regional LN metastasis (N stage) from gastric cancer range from 41-74%, 75-100%, and 51-76%, respectively, (Fig 4), while those for contrast-enhanced CT are from 70-83%, 62-92%, and 67-80%, respectively. The addition of PET/CT to contrast-enhanced CT, EUS, and laparoscopy has been shown to improve TNM staging, such as detection of distant lymph node and bone metastases, thus has significantly influenced decision making.27,28) After a complete workup, a gastrectomy procedure has been shown to be unnecessary in 6-10% of examined patients.29,30)
Fig. 4.
A 41-year-old man with gastric cancer and regional LN metastases.
(a) MIP of PET scan shows several areas of abnormal FDG uptake in the upper abdomen.
(b) Contrast-enhanced CT scan shows irregular mass-like wall thickening and enhancement in the lower part of the stomach (arrow) and two swollen LNs in the left gastric area (curved arrows).
(c) FDG-PET/CT scan shows focal FDG uptake in the gastric mass (SUVmax: 18.3) and regional LNs (SUVmax: 12.7), reflecting gastric cancer (arrow) and regional LN metastases (#3 and #5, curved arrows).
Treatment response assessment
Use of neoadjuvant therapy for treatment of gastroesophageal adenocarcinoma has been increasing since presentation of the results of the MAGIC trial showing improved outcomes of perioperative chemotherapy when combined with surgery.31) Ott et al. reported that a 35% decrease in FDG uptake between the period prior to chemotherapy administration and PET scan examination performed 2 weeks after initiation of therapy predicted response with an accuracy of 85%.32) With use of this criterion, their 2-year survival rate was 90% in responders and 25% in nonresponders, with a significance of p=0.002. Additional study is needed to clarify the usefulness of FDG-PET/CT for evaluation of response to NAC in gastric cancer patients.
Restaging
A recent meta-analysis of 14 reports that studied 828 patients who underwent FDG-PET or PET/CT after a surgical resection procedure noted that the pooled estimates of sensitivity and specificity for FDG-PET/CT and PET alone for diagnosing recurrent gastric cancer were 85% (95% CI 75-92%) and 78% (95% CI 72-84%), respectively (Fig. 5).33) More recently, a multicenter retrospective study investigated 1754 patients with T1-4N0-3M0 gastric cancer who had received D2 gastrectomy and lymphadenectomy procedures with a median follow-up period of 31 months. Of those, 814 (46%) developed recurrent disease, and CT and FDG-PET detected 94% and 91%, respectively, of those cases of recurrences.34)
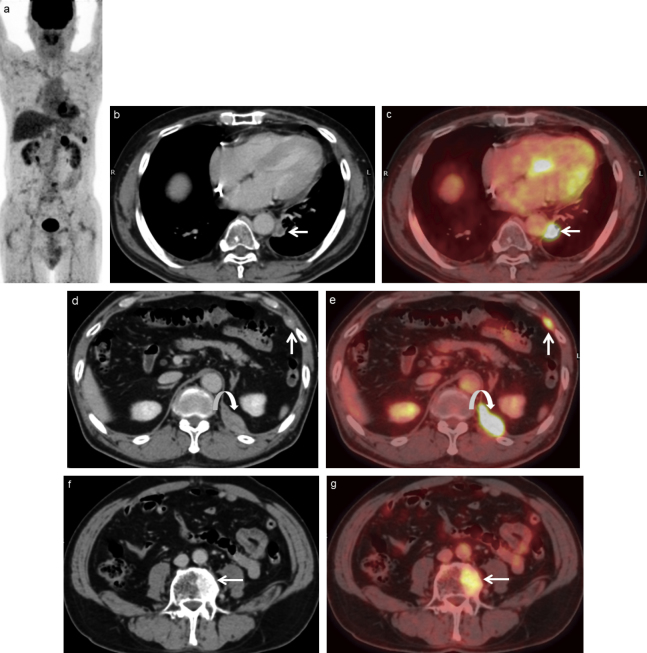
Fig. 5.
A 65-year-old man with recurrence who underwent gastrectomy for gastric cancer 15 months ago.
(a) MIP of PET scan shows several areas of abnormal FDG uptake in the abdomen.
(b) Contrast-enhanced CT and (c) FDG-PET/CT scans show 9×11 mm LN swelling with FDG uptake (arrow), confirming nodal recurrence.
(d) Contrast-enhanced CT and (e) FDG-PET/CT scans show two soft tissue masses in the left chest wall (arrow) and left diaphragm (curved arrow) with abnormal FDG uptake, confirming metastases.
(f) Contrast-enhanced CT and (g) FDG-PET/CT scans show abnormal FDG uptake in the vertebra (arrow), confirming bone metastasis.
COLORECTAL CANCER
The most important treatment strategy for CRC in the early stage is potentially curative surgery. However, for locally advanced rectal cancer (pT3–4 N0 M0 or any T N1 M0), a multimodality strategy has been shown to be the best option to improve local control. Multimodality treatment includes preoperative concomitant chemotherapy and radiotherapy, followed by surgery. Particularly, neoadjuvant CRT helps to decrease tumor volume and stage, thus increasing the chance for potential resectability and sphincter conservation.
Diagnosis
Physiological uptake is frequently observed in the gastrointestinal tract and may complicate CRC detection. Generally, though diffuse uptake can be considered to be a normal variant, it can also occur secondary to inflammation or administration of certain drugs such as metformin that significantly increase FDG uptake in the colon and, to a lesser extent, the small intestine.35) In contrast, a finding of focal uptake frequently indicates that a malignant or premalignant colonic lesion is present. Approximately 75-88% of cases of incidental colonic focal uptake are related to malignant or premalignant lesions shown by endoscopy, of which 28-67% are premalignant (dysplastic adenomas), 20-46% are adenocarcinomas, and 13-30% are benign.25)
Staging
T-staging, which is based on lesion size, mural invasion, and infiltration of adjacent structures, is typically assessed by CT and/or magnetic resonance imaging (MRI) results, with the addition of endorectal ultrasonography for CRC. However, the consensus is that FDG-PET/CT provides little added value for initial staging in patients with CRC without metastasis. In a meta-analysis of studies of a total of 409 patients, patient-based sensitivity of FDG-PET or PET/CT for detecting loco-regional LN metastasis (N staging) was poor (43%; 95% CI 36-50%), while specificity was moderate (88%; 95% CI 83-92%).36) In a retrospective study by Lee et al. of 266 patients with colon cancer who were assessed with both FDG-PET/CT and conventional studies for colon cancer staging, FDG-PET/CT results led to a change in management for 1 of 40 (2.5%) with clinical stage I, 0 of 25 (0%) with stage II, 9 of 138 (6.5%) with stage III, and 8 of 63 (12.7%) with stage IV disease.37) Based on their systematic review of 30 reports, Brush et al. concluded that there was insufficient evidence to support routine use of PET/CT for staging of primary CRC.38)
Liver metastasis is very common in patients with CRC, with an incidence of approximately 50-60%, and one-third of those are diagnosed at the time of detection of the primary tumor.39) FDG-PET/CT is known to be valuable for imaging of hepatic and extrahepatic metastasis in patients with primary CRC, and can also be useful for preoperative assessment of resectable liver metastasis from CRC prior to surgical resection. A recent meta-analysis of 18 studies comprised of 1059 patients conducted by Margherita et al. evaluated both the diagnostic accuracy and impact of FDG-PET and PET/CT for management of colorectal liver metastasis, and reported the following. 1) FDG-PET and PET/CT have high accuracy for detection and staging of liver lesions in CRC patients (pooled sensitivity and specificity of 93% in patient-based analysis, and 60% and 79%, respectively, in lesion-based analysis). However, both modalities had lower sensitivity than MRI and CT in patient-based analysis (93%, 100%, and 98%, respectively) and lesion-based analysis (66%, 89%, and 79%, respectively). In contrast, PET appeared to be more specific than MRI and CT in both patient-based and lesion-based analysis (81%, 70%, and 70%, respectively, and 86%, 81%, and 67%, respectively). 2) FDG-PET and PET/CT results led to change in management for an average 24% of the patients, including both exclusion from curative surgery and modification of the surgical approach. 3) The mean incidence of extrahepatic disease shown by FDG-PET or PET/CT, but not detected by conventional imaging, was 32%.40) In a recent report, MRI was shown to have become the best modality for detection and characterization of small lesions, and for liver evaluation, especially with development of techniques such as diffusion-weighted and gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced imaging.41)
Radiation therapy planning
Most institutions employ CT to plan target volume prior to performing radiotherapy. However, the National Comprehensive Cancer Network (NCCN) is increasingly recognizing the usefulness of FDG-PET/CT in radiation therapy planning.42) CT use alone is correlated with wide variations in target volume among different operators, a variability that interferes with high-precision radiation therapy.43) Patel et al. investigated 6 consecutive patients with locally advanced rectal cancer and showed that FDG-PET/CT had a higher similarity index, reflecting decreased inter-observer variability among several radiation oncologists, as compared to CT alone for primary gross tumor volume (GTV) (similarity index for FDG-PET/CT vs. CT, 0.81 vs. 0.77; p=0.013) and nodal GTV (0.70 vs. 0.22; p<0.0001).44) More accurate tumor volume delineation has potential to reduce geographic mistakes and minimize exposure of non-tumor areas to radiation, and should allow multidimensional radiotherapy and dose variation in different areas of the tumor (so-called dose painting), thereby improving patient care. On the other hand, decreased inter-observer variability resulting in greater standardization of tumor volume delineation has not been shown to impact patient outcomes.
Treatment response assessment
Overall tumor response to neoadjuvant CRT is quite heterogeneous, with 15-27% of patients achieving a pathological complete response (pCR) and 54-75% a partial response, while others show no response.45) Patients who achieve a pCR have favorable long-term outcomes with excellent local control and DFS regardless of the initial T- and N-stage. Adoption of a non-operative strategy for clinical complete responders can avoid risks related to surgical morbidity and mortality, and remove the need for a stoma.
Metabolic response shown by FDG-PET typically occurs before a decline in volume and is considered more useful for assessment of therapy response than findings obtained with CT. A meta-analysis of 10 studies with 302 patients who underwent FDG-PET/CT to assess early response prior to and during neoadjuvant CRT in cases of locally advanced rectal cancer demonstrated that FDG-PET/CT results had good early predictive value for the entire cohort (sensitivity 79%, specificity 78%) and greater accuracy for percentage decrease in SUVmax (sensitivity 82%, specificity 85%), with a mean cut-off of 42%.46) A more recent meta-analysis that included 34 studies and a total of 1526 patients showed the following. 1) FDG-PET/CT had good pooled accuracy for the entire cohort (pooled sensitivity 73%, pooled specificity 77%). 2) There was a high level of pooled accuracy for early PET restaging performed between 1 and 2 weeks after beginning CRT (pooled sensitivity 84%, pooled specificity 81%). 3) Patients with major response tended to show better sensitivity as compared to those with complete response (74% and 71%, respectively, not significant). Furthermore, choosing the target of major response (complete absence of tumor cells or presence of rare residual cancer cells only) seems to be more reasonable, because PET/CT is unable to detect residual disease at the cellular level. 4) SUVmax and visual response analysis were the most frequent parameters, and showed a pooled accuracy similar to that of the entire cohort, with post-operative pooled cut-off values for response index and SUVmax of 63% and 4.4, respectively. 5) The pooled time points to perform PET after CRT and during therapy were shown to be 1.5 and 6.5 weeks, respectively.47) PET/CT has also been reported valuable for assessment of targeted therapies, including selective internal radiation therapy, radiofrequency ablation, and transcatheter arterial chemoembolization, in patients with non-resectable colorectal liver metastasis.48) However, not all results support use of FDG-PET in patients with locally advanced rectal cancer after NAC. For example, FDG-PET/CT was not able to distinguish between pCR and incomplete response in a prospective study of 121 patients conducted by Ruby and colleagues.49) Despite multiple presented reports, scant information exists regarding universal PET assessment of response, or the optimal timing of PET during and after CRT for locally advanced rectal cancer. Notably, international guidelines still state that PET/CT should not be used to monitor preoperative therapy progress.
Restaging
Whole-body FDG-PET/CT is a valuable tool for detection of recurrent CRC (Fig. 6). In a meta-analysis of studies published up to 2011, Mass et al. attempted to determine which whole-body imaging modality had the highest accuracy for detecting local and distant CRC recurrence in patients with clinically and biochemically suspected recurrence. They concluded that both FDG-PET and PET/CT were more accurate than CT, with an area under the curve value for PET of 94% (95% CI 87-98%), for PET/CT of 94% (95% CI 87-98%), and for CT of 83% (95% CI 72-90%).50) Another meta-analysis of 11 reports that included 510 patients with increased carcinoembryonic antigen (CEA) levels and suspected recurrent disease who underwent FDG-PET or PET/CT found that the pooled estimates of sensitivity and specificity for FDG-PET/CT and PET in regard to diagnosis of recurrent CRC were 90% (95% CI, 86-94%) and 80% (95% CI, 67-90%), respectively, for FDG-PET, and 94% (95% CI, 90-97%) and 77% (95% CI, 66-86%), respectively, for FDG-PET/CT.51) Also, several more recent studies have indicated the usefulness of FDG-PET/CT for detecting recurrence in patients with an increased CEA level.52)
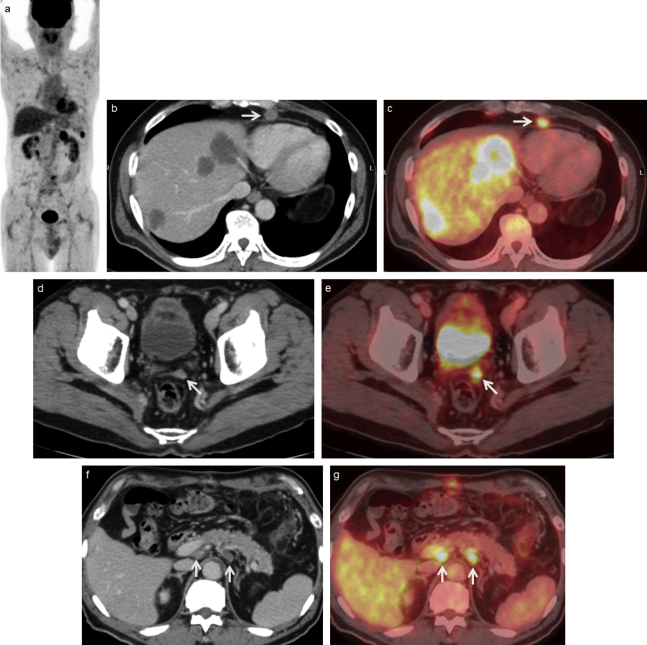
Fig. 6.
A 67-year-old man with recurrence who underwent surgery for descending colon cancer 12 months ago.
(a) MIP of PET scan shows several areas of abnormal FDG uptake in the abdomen. (b) Contrast-enhanced CT and (c) FDG-PET/CT scans show three hepatic hypodense masses with strong FDG uptake, confirming hepatic metastases and 10×10 mm pericardium surrounding LN swelling with FDG uptake (arrow), confirming nodal recurrence.
(d) Contrast-enhanced CT and (e) FDG-PET/CT scans show a peritoneal mass with abnormal FDG uptake (arrow), confirming peritoneal dissemination.
(f) Contrast-enhanced CT and (g) FDG-PET/CT scans show two swollen LNs in the para-aortic region with abnormal FDG uptake (arrows), confirming nodal recurrence.
Differentiating recurrence from post-treatment scarring and radiation fibrosis is challenging, particularly when occurring in the presacral region. Conventional imaging requires serial examinations to assess interval anatomic changes, though that technique has low accuracy and often results in delayed fibrosis. On the other hand, FDG-PET has been shown superior to both CT and MRI in this regard (Fig. 7, 8). In an evaluation of 30 patients with indeterminate presacral lesions following surgical resection of CRC, Evan-Sapir et al. showed that FDG-PET/CT identified all 7 cases of local recurrence (sensitivity 100%, specificity 96%).53) FDG-PET is also useful for detection of intrahepatic tumor recurrence after various treatments, while CT and MRI findings are hampered by a typical posttreatment enhancement pattern that mimics residual/recurrent disease.54)
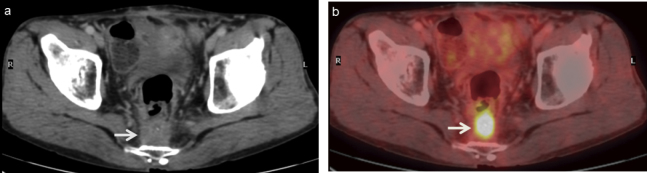
Fig. 7.
A 58-year-old man with local recurrence after the surgery of rectal cancer.
(a) Contrast-enhanced CT and (b) FDG-PET/CT scans show presacral tumor-like lesion with strong FDG uptake (arrow), suggesting local recurrence. The patient was referred for surgical removal of the pelvic recurrence.
Fig. 8.
A 59-year-old man with suspected local recurrence after the surgery of rectal cancer.
(a) Contrast-enhanced CT and (b) FDG-PET/CT scans show presacral tumor-like lesion with faint FDG uptake (arrow), suggesting no local recurrence. Biopsy findings of the mass were negative.
CONCLUSION
Use of FDG-PET/CT allows for combined metabolic and morphological assessment of tumors, with significant diagnostic accuracy improvement as well as considerable impact on management, therapy planning, treatment response assessment, re-staging, and prognosis for esophageal, gastric, and colorectal cancer patients. As for staging, FDG-PET/CT results are excellent for evaluation beyond local lymphadenopathy and metastatic disease. In the future, establishment of universal guidelines for PET assessment of response, as well as optimal timing for PET during and after chemoradiotherapy will be needed.
CONFLICT OF INTEREST
We declare no financial support or relationship that may pose a conflict of interest.
Abbreviations:
- PET/CT
positron emission tomography/computed tomography
- FDG
2-[18F]fluoro-2-deoxyd-D-glucose
- PET
positron emission tomography
- CRC
colorectal cancer
- CRT
chemoradiotherapy
- EUS
endoscopic ultrasonography
- CT
computed tomography
- LN
lymph node
- CI
confidence interval
- SUVmax
maximum standardized uptake value
- OS
overall survival
- HR
hazard ratio
- DFS
disease-free survival
- MTV
metabolic tumor volume
- TLG
total lesion glycolysis
- NAC
neoadjuvant chemotherapy
- MRI
magnetic resonance imaging
- NCCN
National Comprehensive Cancer Network
- GTV
gross tumor volume
- pCR
pathological complete response
- CEA
carcinoembryonic antigen
REFERENCES
- 1).Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol, 2011; 12: 681-692. [DOI] [PubMed]
- 2).Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: A SEER database analysis. J Gastroenterol Hepatol, 2016; 31: 1141-1146. [DOI] [PMC free article] [PubMed]
- 3).van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med, 2012; 366: 2074-2084. [DOI] [PubMed]
- 4).Walker AJ, Spier BJ, Perlman SB, Stangl JR, Frick TJ, Gopal DV, et al. Integrated PET/CT fusion imaging and endoscopic ultrasound in the pre-operative staging and evaluation of esophageal cancer. Mol Imaging Biol, 2011; 13: 166-171. [DOI] [PubMed]
- 5).Kato H, Miyazaki T, Nakajima M, Takita J, Kimura H, Faried A, et al. The incremental effect of positron emission tomography on diagnostic accuracy in the initial staging of esophageal carcinoma. Cancer, 2005; 103: 148-156. [DOI] [PubMed]
- 6).Barber TW, Duong CP, Leong T, Bressel M, Drummond EG, Hicks RJ. 18F-FDG PET/CT has a high impact on patient management and provides powerful prognostic stratification in the primary staging of esophageal cancer: a prospective study with mature survival data. J Nucl Med, 2012; 53: 864-871. [DOI] [PubMed]
- 7).Malik V, Johnston C, Donohoe C, Claxton Z, Lucey J, Ravi N, et al. 18F-FDG PET-detected synchronous primary neoplasms in the staging of esophageal cancer: incidence, cost, and impact on management. Clin Nucl Med, 2012; 37: 1152-1158. [DOI] [PubMed]
- 8).Pan L, Gu P, Huang G, Xue H, Wu S. Prognostic significance of SUV on PET/CT in patients with esophageal cancer: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol, 2009; 21: 1008-1015. [DOI] [PubMed]
- 9).Omloo JM, van Heijl M, Hoekstra OS, van Berge Henegouwen MI, van Lanschot JJ, Sloof GW. FDG-PET parameters as prognostic factor in esophageal cancer patients: a review. Ann Surg Oncol, 2011; 18: 3338-3352. [DOI] [PMC free article] [PubMed]
- 10).Hyun SH, Choi JY, Shim YM, Kim K, Lee SJ, Cho YS, et al. Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol, 2010; 17: 115-122. [DOI] [PubMed]
- 11).Foley KG, Fielding P, Lewis WG, Karran A, Chan D, Blake P, et al. Prognostic significance of novel 18F-FDG PET/CT defined tumour variables in patients with oesophageal cancer. Eur J Radiol, 2014; 83: 1069-1073. [DOI] [PubMed]
- 12).Chen SW, Hsieh TC, Ding HJ, Yen KY, Lin CY, Liang JA, et al. Pretreatment metabolic tumor volumes to predict the short-term outcome of unresectable locally advanced squamous cell carcinoma of the esophagus treated with definitive chemoradiotherapy. Nucl Med Commun, 2014; 35: 291-297. [DOI] [PubMed]
- 13).Muijs CT, Beukema JC, Pruim J, Mul VE, Groen H, Plukker JT, et al. A systematic review on the role of FDG-PET/CT in tumour delineation and radiotherapy planning in patients with esophageal cancer. Radiother Oncol, 2010; 97: 165-171. [DOI] [PubMed]
- 14).Muijs CT, Pruim J, Beukema JC, Berveling MJ, Plukker JT, Langendijk JA. Oesophageal tumour progression between the diagnostic 18F-FDG-PET and the 18F-FDG-PET for radiotherapy treatment planning. Radiother Oncol, 2013; 106: 283-287. [DOI] [PubMed]
- 15).Kwee RM. Prediction of tumor response to neoadjuvant therapy in patients with esophageal cancer with use of 18F FDG PET: a systematic review. Radiology, 2010; 254: 707-717. [DOI] [PubMed]
- 16).Chen YM, Pan XF, Tong LJ, Shi YP, Chen T. Can 18F-fluorodeoxyglucose positron emission tomography predict responses to neoadjuvant therapy in oesophageal cancer patients? A meta-analysis. Nucl Med Commun, 2011; 32: 1005-1010. [DOI] [PubMed]
- 17).Malik V, Lucey JA, Duffy GJ, Wilson L, McNamara L, Keogan M, et al. Early repeated 18F-FDG PET scans during neoadjuvant chemoradiation fail to predict histopathologic response or survival benefit in adenocarcinoma of the esophagus. J Nucl Med, 2010; 51: 1863-1869. [DOI] [PubMed]
- 18).Schollaert P, Crott R, Bertrand C, D’Hondt L, Borght TV, Krug B. A systematic review of the predictive value of 18FDG-PET in esophageal and esophagogastric junction cancer after neoadjuvant chemoradiation on the survival outcome stratification. J Gastrointest Surg, 2014; 18: 894-905. [DOI] [PubMed]
- 19).Nakamura R, Obara T, Katsuragawa S, Tamakawa Y, Koeda K, Ikeda K, et al. Failure in presumption of residual disease by quantification of FDG uptake in esophageal squamous cell carcinoma immediately after radiotherapy. Radiat Med, 2002; 20: 181-186. [PubMed]
- 20).Cheedella NK, Suzuki A, Xiao L, Hofstetter WL, Maru DM, Taketa T, et al. Association between clinical complete response and pathological complete response after preoperative chemoradiation in patients with gastroesophageal cancer: analysis in a large cohort. Ann Oncol, 2013; 24: 1262-1266. [DOI] [PMC free article] [PubMed]
- 21).Yanagawa M, Tatsumi M, Miyata H, Morii E, Tomiyama N, Watabe T, et al. Evaluation of response to neoadjuvant chemotherapy for esophageal cancer: PET response criteria in solid tumors versus response evaluation criteria in solid tumors. J Nucl Med, 2012; 53: 872-880. [DOI] [PubMed]
- 22).Oppedijk V, van der Gaast A, van Lanschot JJ, van Hagen P, van Os R, van Rij CM, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol, 2014; 32: 385-391. [DOI] [PubMed]
- 23).Goense L, van Rossum PS, Reitsma JB, Lam MG, Meijer GJ, van Vulpen M, et al. Diagnostic performance of 18F-FDG PET and PET/CT for the detection of recurrent esophageal cancer after treatment with curative intent: A systematic review and meta-Analysis. J Nucl Med, 2015; 56: 995-1002. [DOI] [PubMed]
- 24).Abdalla EK, Pisters PW. Staging and preoperative evaluation of upper gastrointestinal malignancies. Semin Oncol, 2004; 31: 513-529. [DOI] [PubMed]
- 25).Gauthé M, Richard-Molard M, Cacheux W, Michel P, Jouve JL, Mitry E, et al. Role of fluorine 18 fluorodeoxyglucose positron emission tomography/computed tomography in gastrointestinal cancers. Dig Liver Dis, 2015; 47: 443-454. [DOI] [PubMed]
- 26).Minamimoto R, Senda M, Jinnouchi S, Terauchi T, Yoshida T, Inoue T. Performance profile of a FDG-PET cancer screening program for detecting gastric cancer: results from a nationwide Japanese survey. Jpn J Radiol, 2014; 32: 253-259. [DOI] [PubMed]
- 27).Malibari N, Hickeson M, Lisbona R. PET/Computed Tomography in the Diagnosis and Staging of Gastric Cancers. PET Clin, 2015; 10: 311-326. [DOI] [PubMed]
- 28).Kawanaka Y, Kitajima K, Fukushima K, Mouri M, Doi H, Oshima T, et al. Added value of pretreatment 18F-FDG PET/CT for staging of advanced gastric cancer: Comparison with contrast-enhanced MDCT. Eur J Radiol, 2016; 85: 989-995. [DOI] [PubMed]
- 29).Chen J, Cheong JH, Yun MJ, Kim J, Lim JS, Hyung WJ, et al. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer, 2005; 103: 2383-2390. [DOI] [PubMed]
- 30).Smyth E, Schöder H, Strong VE, Capanu M, Kelsen DP, Coit DG, Shah MA, et al. A prospective evaluation of the utility of 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography and computed tomography in staging locally advanced gastric cancer. Cancer, 2012; 118: 5481-5488. [DOI] [PubMed]
- 31).Jain VK, Cunningham D, Chau I. Preoperative and postoperative chemotherapy for gastric cancer. Surg Oncol Clin N Am, 2012; 21: 99-112. [DOI] [PubMed]
- 32).Ott K, Herrmann K, Krause BJ, Lordick F. The Value of PET Imaging in Patients with Localized Gastroesophageal Cancer. Gastrointest Cancer Res, 2008; 2: 287-294. [PMC free article] [PubMed]
- 33).Li P, Liu Q, Wang C, Wang T, Liu J, Huang G, et al. Fluorine-18-fluorodeoxyglucose positron emission tomography to evaluate recurrent gastric cancer after surgical resection: a systematic review and meta-analysis. Ann Nucl Med, 2016; 30: 179-187. [DOI] [PubMed]
- 34).Baiocchi GL, Marrelli D, Verlato G, Morgagni P, Giacopuzzi S, Coniglio A, et al. Follow-up after gastrectomy for cancer: an appraisal of the Italian research group for gastric cancer. Ann Surg Oncol, 2014; 21: 2005-2011. [DOI] [PubMed]
- 35).Gontier E, Fourme E, Wartski M, Blondet C, Bonardel G, Le Stanc E, et al. High and typical 18F-FDG bowel uptake in patients treated with metformin. Eur J Nucl Med Mol Imaging, 2008; 35: 95-99. [DOI] [PubMed]
- 36).Lu YY, Chen JH, Ding HJ, Chien CR, Lin WY, Kao CH. A systematic review and meta-analysis of pretherapeutic lymph node staging of colorectal cancer by 18F-FDG PET or PET/CT. Nucl Med Commun, 2012; 33: 1127-1133. [DOI] [PubMed]
- 37).Lee JH, Lee MR. Positron emission tomography/computed tomography in the staging of colon cancer. Ann Coloprotocol, 2014; 30: 23-27. [DOI] [PMC free article] [PubMed]
- 38).Brush J, Boyd K, Chappell F, Crawford F, Dozier M, Fenwick E, et al. The value of FDG positron emission tomography/computerized tomography (PET/CT) in pre-operative staging of colorectal cancer: a systematic review and economic evaluation. Health Technol Assess, 2011;15:1–192, iii–iv. [DOI] [PMC free article] [PubMed]
- 39).Ismaili N. Treatment of colorectal liver metastases. World J Surg Oncol, 2011; 9: 154. [DOI] [PMC free article] [PubMed]
- 40).Maffione AM, Lopci E, Bluemel C, Giammarile F, Herrmann K, Rubello D. Diagnostic accuracy and impact on management of 18F-FDG PET and PET/CT in colorectal liver metastasis: a meta-analysis and systematic review. Eur J Nucl Med Mol Imaging, 2015; 42: 152-163. [DOI] [PubMed]
- 41).Tsurusaki M, Sofue K, Murakami T. Current evidence for the diagnostic value of gadoxetic acid-enhanced magnetic resonance imaging for liver metastasis. Hepatol Res, 2016; 46: 853-861. [DOI] [PubMed]
- 42).Frankel TL, Gian RK, Jarnagin WR. Preoperative imaging for hepatic resection of colorectal cancer metastasis. J Gastrointest Oncol, 2012; 3: 11–18. [DOI] [PMC free article] [PubMed]
- 43).Davis JB, Reiner B, Dusserre A, Giraud JY, Bolla M; EORTC. Quality assurance of the EORTC trial 22911. A phase III study of post-operative external radiotherapy in pathological stage T3N0 prostatic carcinoma: the dummy run. Radiother Oncol, 2002; 64: 65-73. [DOI] [PubMed]
- 44).Patel DA, Chang ST, Goodman KA, Quon A, Thorndyke B, Gambhir SS, et al. Impact of integrated PET/CT on variability of target volume delineation in rectal cancer. Technol Cancer Res Treat, 2007; 6: 31-36. [DOI] [PubMed]
- 45).Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol, 2010; 11: 835-844. [DOI] [PubMed]
- 46).Maffione AM, Chondrogiannis S, Capirci C, Galeotti F, Fornasiero A, Crepaldi G, et al. Early prediction of response by 18F-FDG PET/CT during preoperative therapy in locally advanced rectal cancer: a systematic review. Eur J Surg Oncol, 2014; 40: 1186-1194. [DOI] [PubMed]
- 47).Maffione AM, Marzola MC, Capirci C, Colletti PM, Rubello D. Value of 18F-FDG PET for Predicting Response to Neoadjuvant Therapy in Rectal Cancer: Systematic Review and Meta-Analysis. AJR Am J Roentgenol, 2015; 204: 1261-1268. [DOI] [PubMed]
- 48).Fendler WP, Philippe Tiega DB, Ilhan H, Paprottka PM, Heinemann V, Jakobs TF, et al. Validation of several SUV-based parameters derived from 18F-FDG PET for prediction of survival after SIRT of hepatic metastases from colorectal cancer. J Nucl Med, 2013; 54: 1202-1208. [DOI] [PubMed]
- 49).Guillem JG, Ruby JA, Leibold T, Akhurst TJ, Yeung HW, Gollub MJ, et al. Neither FDG-PET Nor CT can distinguish between a pathological complete response and an incomplete response after neoadjuvant chemoradiation in locally advanced rectal cancer: a prospective study. Ann Surg, 2013; 258: 289-295. [DOI] [PubMed]
- 50).Maas M, Rutten IJ, Nelemans PJ, Lambregts DM, Cappendijk VC, Beets GL, et al. What is the most accurate whole-body imaging modality for assessment of local and distant recurrent disease in colorectal cancer? A meta-analysis: imaging for recurrent colorectal cancer. Eur J Nucl Med Mol Imaging, 2011; 38: 1560-1571. [DOI] [PMC free article] [PubMed]
- 51).Lu YY, Chen JH, Chien CR, Chen WT, Tsai SC, Lin WY, et al. Use of FDG-PET or PET/CT to detect recurrent colorectal cancer in patients with elevated CEA: a systematic review and meta-analysis. Int J Colorectal Dis, 2013; 28: 1039-1047. [DOI] [PubMed]
- 52).Sanli Y, Kuyumcu S, Ozkan ZG, Kilic L, Balik E, Turkmen C, et al. The utility of FDG-PET/CT as an effective tool for detecting recurrent colorectal cancer regardless of serum CEA levels. Ann Nucl Med, 2012; 26: 551-558. [DOI] [PubMed]
- 53).Even-Sapir E, Parag Y, Lerman H, Gutman M, Levine C, Rabau M, et al. Detection of recurrence in patients with rectal cancer: PET/CT after abdominoperineal or anterior resection. Radiology, 2004; 232: 815-822. [DOI] [PubMed]
- 54).Langenhoff BS, Oyen WJ, Jager GJ, Strijk SP, Wobbes T, Corstens FH, et al. Efficacy of fluorine-18-deoxyglucose positron emission tomography in detecting tumor recurrence after local ablative therapy for liver metastases: a prospective study. J Clin Oncol, 2002; 20: 4453-4458. [DOI] [PubMed]