Abstract
A ‘Switch’ catalysis method is reviewed whereby a single catalyst is switched between ring-opening polymerization and ring-opening copolymerization cycles. It allows the efficient synthesis of block copolymers from mixtures of lactones, epoxides, anhydrides and carbon dioxide. In order to use and further develop such ‘Switch’ catalysis, it is important to understand how to monitor the catalysis and characterize the product block copolymers. Here, a step-by-step guide to both the catalysis and the identification of block copolymers is presented.
This article is part of a discussion meeting issue ‘Providing sustainable catalytic solutions for a rapidly changing world’.
Keywords: ‘Switch’ catalysis, block copolymers, ring-opening polymerization, ring-opening copolymerization
1. Introduction
Block copolymers are a particularly interesting class of macromolecules, as they feature covalently linked, chemically distinct, polymer blocks [1]. Many block copolymers phase-separate to form nanostructures either in the solid state or in solution—these domain structures are responsible for moderating the overall physical and chemical properties [2,3]. Current applications include thermoplastic elastomers and as compatibilizers for polymer blends; however, there is also significant interest in their use in fields as diverse as medicine, nanotechnology, lithography, photonics and electronics [4–9]. There are already a range of successful methods to synthesize block copolymers, and generally these apply either in situ approaches or post-polymerization modifications.
The in situ approach generally applies living polymerization methods where sequential monomer additions are used to build up the individual blocks [10–14]. The in situ method is generally preferable to post-polymerization coupling because it reduces intermediate purification steps and obviates problems of low reactivity of polymer chain ends in coupling reactions. The ring-opening polymerization (ROP) of lactones is such a living polymerization route to aliphatic polyesters, and a wide range of different catalysts have been reported for this process [15–18]. An alternative approach to polyesters and polycarbonates under living conditions is the ring-opening alternating copolymerization (ROCOP) from epoxides/CO2 and epoxides/anhydrides; so far, there are fewer catalysts reported for ROCOP than for ROP [19–22].
In terms of block copolymers, there are reports of post-polymerization modifications and tandem catalyses which apply both ROP and ROCOP to prepare block copolymers. In 2012, our group reported the preparation of α,ω-hydroxyl-terminated poly(cyclohexene carbonate) using a di-zinc catalyst [23,24]. This macro-initiator was subsequently used, with an yttrium catalyst, in lactide ROP. This method enabled the production of ABA triblock copolymers [25]. Darensbourg and co-workers reported a one-pot tandem catalysis method whereby a metal salen catalyst was used for epoxide/CO2 ROCOP and an organo-catalyst was applied for lactide ROP; the two processes were linked by the addition of water as a chain transfer agent [26]. This concept was extended to prepare block copoly(carbonate-b-lactide)s [25,26], poly(carbonate-b-ether)s and poly(carbonate-b-phosphoester)s [27,28]. It was also reported that heterogeneous double metal cyanide catalysts for epoxide/CO2 ROCOP could be used with tin octanoate catalysts for lactide ROP to produce copolymers with a proposed tapering multiblock structure [29]. It should be noted that studies using heterogeneous catalysts are necessarily more complex due to the limited understanding of the active sites and thereby of chain growth and exchange reactions.
In terms of mixing together monomers for ROCOP and ROP processes, Döring and co-workers reported in 2006 that zinc β-diiminate catalysts could be used to prepare copolymers from lactide, cyclohexene oxide and carbon dioxide, but the precise structures were not investigated in detail [30]. Furthermore, a number of zinc heterogeneous surfaces, including zinc glutarate and zinc adipate, were reported to copolymerize lactide, epoxide and carbon dioxide—nonetheless, understanding of copolymer structure was severely hampered by very broad molecular-weight distributions and a lack of insight into the active site structures [31].
In 2014, our group presented a new ‘Switch’ catalysis approach in which the ROP and ROCOP are combined in one pot using a well-defined homogeneous catalyst. It allows the rational incorporation of different monomers via two distinct catalytic cycles so as to produce selectively block copolyesters and copoly(ester-b-carbonate)s [32–35].
2. ‘Switch’ catalysis
The term ‘Switch’ catalysis is widely used to describe a change in the chemical reactivity by an external stimulus, such as thermal, redox, photochemical and mechanical stimuli [36,37]. In the context of this review, ‘Switch’ catalysis refers to a change in polymerization mechanism from ROP to ROCOP and vice versa (scheme 1). It is important to emphasize the difference between the ‘Switch’ method and terpolymerizations, the latter of which may apply monomer mixtures but only a single polymerization process, e.g. ROP or ROCOP, to form block copolymers [38–46]. Terpolymerizations do not contain any type of mechanistic switch and are therefore conceptually different from ‘Switch’ catalysis.
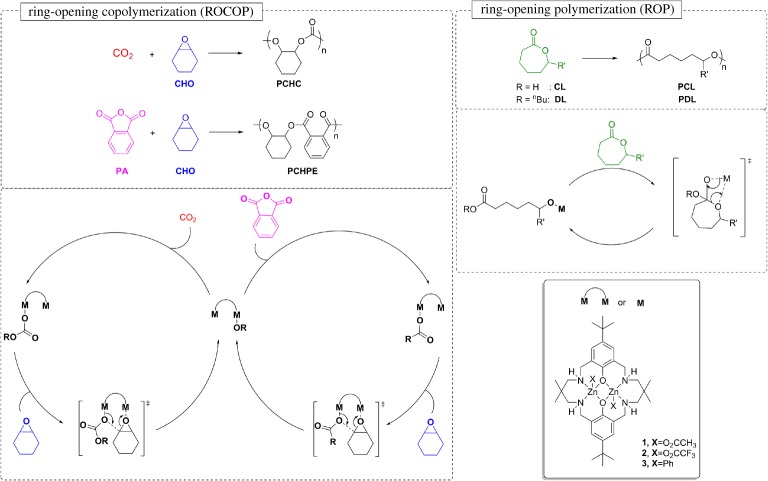
Scheme 1.
The key pathways for ring-opening copolymerization (ROCOP) and ring-opening polymerization (ROP). (Online version in colour.)
The two different polymerization processes comprising ‘Switch’ catalysis are illustrated in scheme 1 [20,47,48]. The ROCOP process is an alternating copolymerization whereby a metal alkoxide reacts with CO2 or an anhydride to generate a metal carbonate or carboxylate intermediate. These intermediates react with an epoxide to regenerate the metal alkoxide. The two-step process thus involves cycling between alkoxide-carbonate or alkoxide-carboxylate intermediates to build up the polycarbonate or polyester chains. In contrast, ROP involves only the ring-opening process and the sole intermediate is a metal alkoxide species. The metal alkoxide undergoes sequential reactions with lactones to generate the growing polymer chain. Thus, although the reactions are related, they are not mechanistically the same—there are differences in the number and the nature of the intermediates.
Our group reported ‘Switch’ catalysis using di-zinc catalyst 1 (scheme 1) [24]; the catalyst had already been extensively studied in CO2/epoxide ROCOP enabling polymerizations at 1 atm of CO2 [49,50]. Moreover, 1 was also studied in polymerizations using carbon dioxide captured from a coal-fired power station. It was tolerant to common impurities found in carbon dioxide and from any capture process [51]. Detailed mechanistic studies of 1 by nuclear magnetic resonance (NMR) spectroscopy, in situ attenuated total reflectance--infrared (ATR-IR) spectroscopy and computational analysis using density functional theory (DFT) enabled a ‘chain-shuttling mechanism’ to be proposed whereby the rate-determining step is the ring opening of the epoxide [49,50,52–54]. The nucleophilic ring opening of the epoxide is facilitated by a dinuclear mechanism in which one metal binds the epoxide and the other provides a labile carbonate. Following this, the activity could successfully be increased with heterodinuclear catalysts [22,55]. We have also reported that catalyst 1 is active for the epoxide/anhydride ROCOP and have very recently demonstrated that selective terpolymerizations using a range of different anhydrides/epoxide/carbon dioxide lead to block copoly(ester-carbonate)s [56,57].
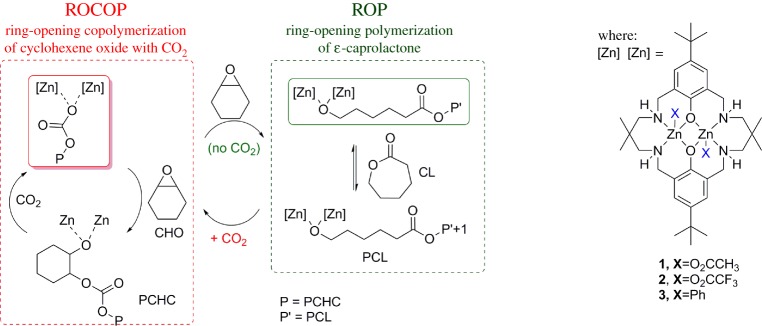
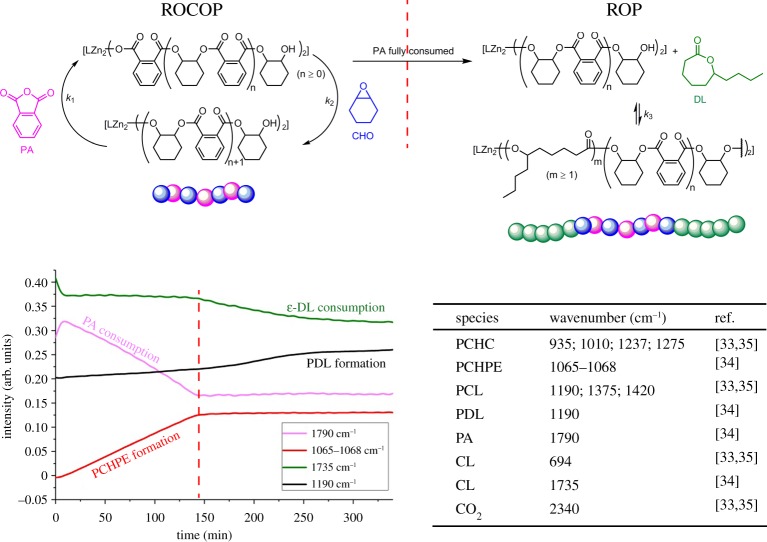
Nonetheless, 1 was inactive for the ROP of lactones, as exemplified by reactions between 1 and ε-caprolactone, which failed to yield any polyester. However, ROP was ‘switched on’ upon the addition of either sub-stoichiometric or excess epoxide (CHO). Furthermore, the epoxide was only present as a chain end-group and there was no evidence of any ether linkages in the polyester (which would form from competitive epoxide ROP). The switch-on and the selectivity was rationalized by the reaction between 1 and an equivalent of epoxide to form an alkoxide, which could act as an initiator for ROP. The reaction of 1 with a mixture of CO2, CL and CHO resulted in only the formation of polycarbonate. The production of block copolymers was possible if the polycarbonate block was produced and then the CO2 was removed (by rapid cycles of vacuum--nitrogen), the ROP was then ‘switched’ on and a polyester block was formed (figure 1, right). Considering the proposed mechanisms for the ROP and ROCOP, it is clear that there is a common zinc alkoxide intermediate in both processes. The selectivity could be interpreted to arise due to a higher rate of carbon dioxide insertion compared to lactone ring opening at the zinc alkoxide intermediate. Further analysis revealed that the zinc carbonate, which was formed from the insertion of CO2, was unreactive in lactone ROP, even under forcing conditions, and indeed ROP could be ‘switched off’ by addition of carbon dioxide into polymerizations [33]. Thus, the chemistry of the zinc--oxygen bond of the growing polymer chain is responsible for the selectivity for ROCOP or ROP processes during the polymerization [35].
Figure 1.
Illustration of ‘Switch’ catalysis (polymer structures are given in scheme 1). (Online version in colour.)
The ‘Switch’ catalysis was applied to prepare ABA triblock copoly(ester-b-carbonate-b-ester)s from mixtures of epoxide/carbon dioxide/lactone using catalyst 2 (X = trifluoroacetate) [33]. Earlier studies of ROCOP using catalyst 2 demonstrated that it produced α,ω-dihydroxyl-terminated polycarbonates and, in separate work, we investigated the influence of 1,2-cyclohexane diol groups as initiators for ROP [25,57]. The related catalytic phenomenon was also substantiated by research by Darensbourg and co-workers, using metal salen catalysts with trifluoroacetate initiating groups, who also observed the selective formation of α,ω-dihydroxyl-terminated polycarbonates [27]. It was proposed that the expected trifluoroacetate end-group is easily hydrolysed, either during or after polymerization, to produce the dihydroxyl-terminated chains. The investigation of ‘Switch’ catalysis using catalyst 2 led to the selective production of ABA block copolymers and demonstrated a range of different compositions and block lengths.
‘Switch’ catalysis using mixtures of epoxide/anhydride/lactone was also explored using catalyst 3 (X = Ph) with a diol, once again exploiting the potential to produce telechelic polymers [34,58]. Well-defined ABA block copolyesters were formed from mixtures of CHO, PA and ε-decalactone (DL). Multiblock copolyesters with up to heptablocks could be synthesized by a second monomer mixture addition—such a method may offer advantages over alternative living polymerization methods which would require seven additions to access related heptablocks.
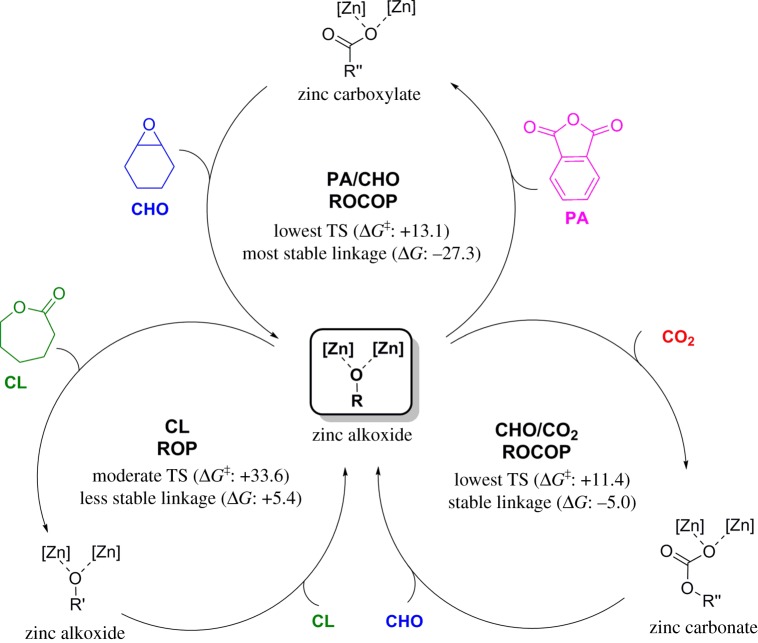
The fundamental basis for ‘Switch’ catalysis was further investigated using DFT [32]. The entire cycle of monomer additions was explored using catalyst 1 and the differences between the relative barriers and stabilities of intermediates compared. In particular, the different insertions into the metal alkoxide intermediate enabled comparisons between the different pathways. It was found that a combination of the thermodynamic stability of the formed linkages and the kinetic barriers for the different reaction pathways could rationalize the experimentally observed selectivity in ‘Switch’ catalysis (scheme 2). Starting from the zinc alkoxide intermediate and investigating the insertions of epoxide (CHO), CO2, anhydride (PA) and lactone (CL), three pathways were possible: (i) the reaction with anhydride leads to the most stable linkage and has a low-energy transition state; (ii) the reaction with CO2, which also forms a stable linkage and has the lowest-energy transition state; and (iii) the reaction with CL, which forms the least stable linkage and has a moderate-energy transition state (TS). Accordingly, the ROP of CL has the least favourable thermodynamics and kinetics and indeed is not observed experimentally until the consumption/removal of the other monomers. When comparing the insertion of anhydride versus CO2, the insertion of PA leads to more stable linkages while both have similar energy barriers. Thus, experimentally, the ROCOP of PA/CHO occurs selectively over the ROCOP of CHO/CO2. It was also interesting to observe that the barrier for epoxide ROP is very high and cannot be accessed under the experimental conditions, thereby explaining the lack of ether linkages in any of the polymer blocks.
Scheme 2.
Possible mechanisms and their theoretical evaluation of a zinc alkoxide in a mixed monomer feedstock of CHO, CO2, PA and CL (kcal mol−1) [32]. (Online version in colour.)
Very recently, Rieger et al. applied the ‘Switch’ catalysis concept with a different di-zinc catalyst supported by a β-diiminate ligand, and using a mixture of CHO, CO2 and β-butyrolactone (BBL) [59]. At 40 atm pressure of CO2, selective ROCOP of epoxide/CO2 occurred first and was followed by the ROP of BBL to build up block copolymers. However, at 3 atm CO2 there was no selectivity and random copolymers were formed. In this case, the carbon dioxide pressure was important to achieve good selectivity and switchability.
3. ‘Switch’ catalysis: a guide to block copolymer identification
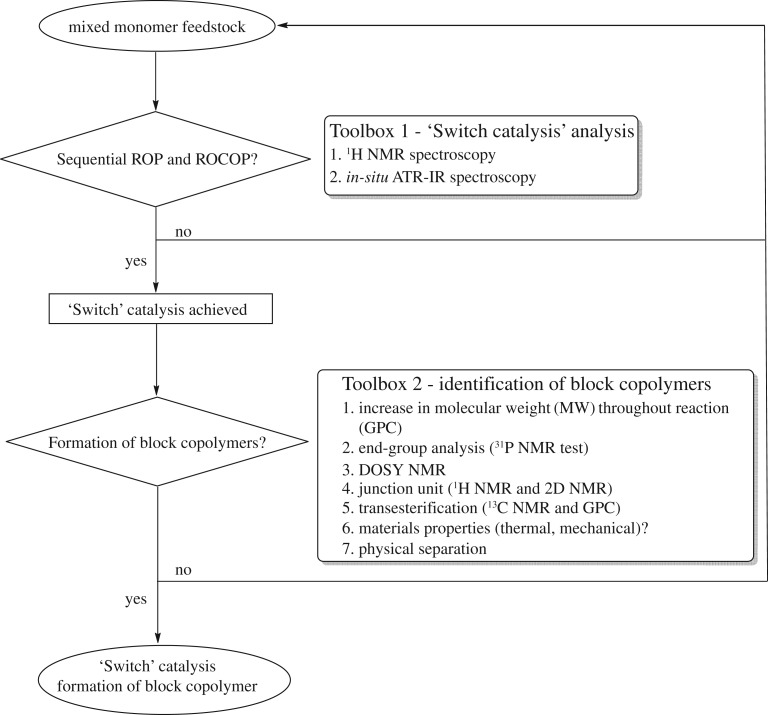
‘Switch’ catalysis enables the production of multiblock copolymers and has the potential to allow production of a particular block sequence. When implementing and testing catalysts for this process, it is important to fully characterize the products and processes. The main challenges in working with monomer mixtures are: (i) monomer discrimination and (ii) formation of block copolymers over alternative structures, such as random copolymers or even homopolymers. To address these challenges, a series of experiments are proposed to distinguish between block copolymers and statistical copolymers (from the random insertion of monomers) or mixtures of homopolymers (figure 2). The following guide is meant to illustrate this ‘decision tree’. However, it should be noted that suitable proof for block copolymers and ‘Switch’ catalysis is not limited to the suggested experiments and should be extended based on the specific research questions and findings. In the flow diagram, a ‘no’ outcome suggests the system cannot be considered for ‘Switch’ catalysis, and a change to the catalytic system in terms of conditions, choice of monomers or catalyst (and/or co-catalyst, if applicable) would be necessary as a next step.
Figure 2.
Guide to the formation of block copolymers using ‘Switch’ catalysis.
Starting from a mixed monomer feedstock, the first area to investigate is whether there is selectivity between ROCOP and ROP (or vice versa) over a random incorporation of monomers. The investigation of monomer selectivity can be achieved by in situ monitoring or aliquot analysis using spectroscopy, with 1H NMR or in situ ATR-IR spectroscopy being particularly useful (figure 3). Figure 3 illustrates the in situ monitoring using ATR-IR spectroscopy of ‘Switch’ catalysis using catalyst 3, cyclohexane diol and mixtures of CHO, PA and DL. During the first 150 min, the only absorptions that change correspond to the formation of polyester by ROCOP—i.e. the anhydride is consumed and the polyester band increases in intensity. At approximately 150 min, the anhydride is fully consumed (as confirmed by 1H NMR spectroscopy analysis of an aliquot) and, after this point, the ROP process occurs as observed by the decrease in resonance assigned to the lactone and the increase in the aliphatic polyester resonance. Figure 3 also includes the characteristic wavenumbers which are used to monitor some of the ‘Switch’ catalysis reactions.
Figure 3.
In situ ATR-IR spectrum for the formation of copoly(PDL-b-PCHPE-b-PDL) (left) and indicative wavenumbers for different species for monitoring by in situ ATR-IR spectroscopy (wavenumbers obtained from neat compounds, right). Monomer and polymer structures are given in scheme 1 [34]. (Online version in colour.)
Using in situ spectroscopy, the sequential occurrence of ROCOP and ROP can be identified but the composition of the product is not yet known—the same results would be observed if a mixture of homopolymers or a block copolymer were formed. To understand the polymer composition, a range of further experiments are presented to characterize the block copolymers (‘Toolbox 2’ in figure 2):
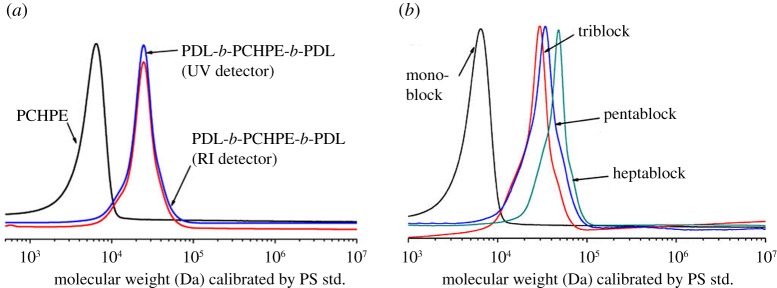
(1) Monitoring by gel permeation chromatography. A continuous increase in molecular weight (MW) is expected if copolymers are formed. One common method to assess the polymer molecular weight as the reaction progresses is by aliquot analysis using gel permeation chromatography (GPC). Typically, this involves calibration by narrow molecular-weight standards, such as polystyrene, to convert elution time to molecular weight. It should be noted that other methods to determine molecular weight (intrinsic viscosity) should also show the same phenomenon in the case of copolymer formation (figure 4). Where the polymer backbone contains a chromophore, detection based on ultraviolet-visible absorption is recommended in addition to the usual detection based on refractive index (RI)—clearly additional detection methods are expected to correspond to related number and positions of the observed peaks (figure 4). It is important to note that increases in molecular weight alone do not unambiguously confirm block copolymer formation; for example, this technique is not suitable to detect low levels of transesterification in the backbone.
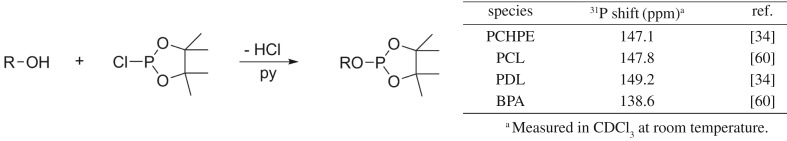
(2) End-group analysis. The formation of block copolymers corresponds to a chain extension reaction and, as such, the end-groups of inner block(s) should not be present after the addition of outer block(s). Thus, titrations to determine the nature and quantity of end-groups can be used to confirm block formation. One useful method of end-group analysis in the context of hydroxyl-terminated polymers is via a quantitative reaction with a phosphorus-containing reagent and the use of 31P{1H} NMR spectroscopy as an analytical method. In particular, the reaction of hydroxyl end-groups with 2-chloro-4,4,5,5-tetramethyl dioxaphospholane is used to form phosphites which show differing chemical shifts depending on the end-group environment [60]. It is important to use an internal standard, and in our work bisphenol-A (BPA) is applied. One limitation of this technique is the stability of the polymer (in particular polyesters) towards pyridine, which is used as a base to capture hydrochloric acid, which is released during the reaction (figure 5).
(3) DOSY NMR. Diffusion-ordered NMR spectroscopy (DOSY) enables an estimation of polymer diffusion rates. In the case of a block copolymer, a single diffusion coefficient is expected, with all blocks expected to diffuse at the same rate, whereas two coefficients are expected for a mixture of polymers. Figure 6 illustrates the single diffusion coefficient observed for the block copolyester formed by ‘Switch’ catalysis and compares against the two diffusion coefficients observed in the spectrum of a blend of the constituent polyesters.
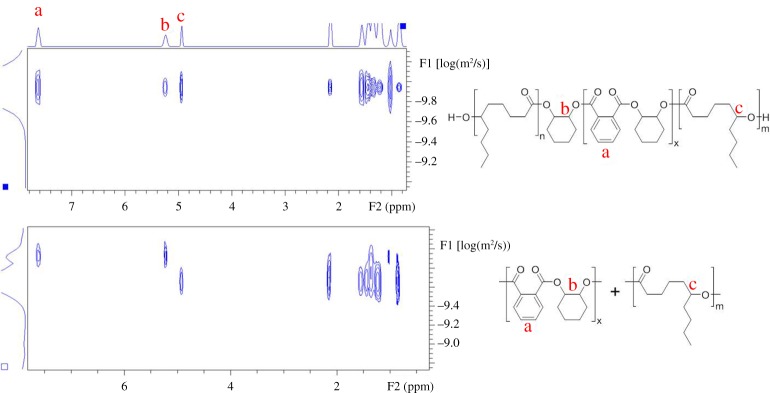
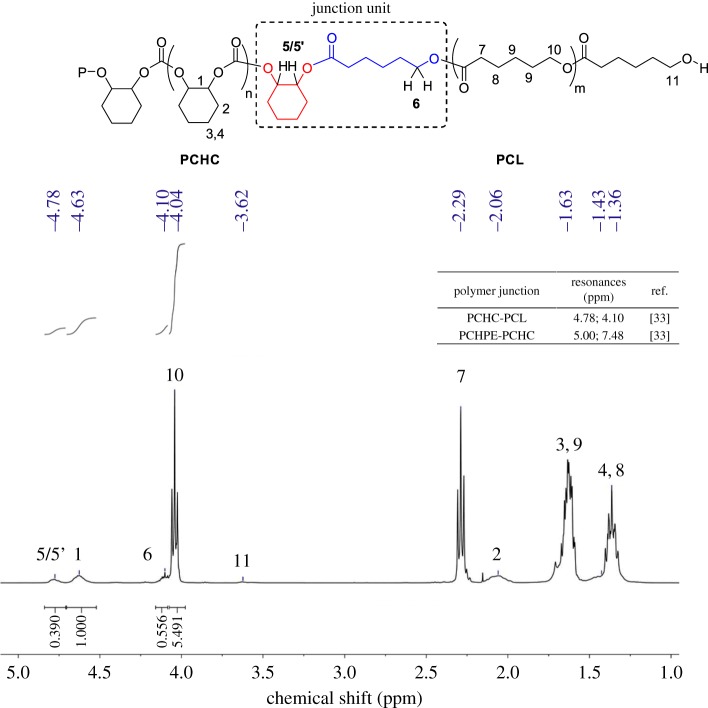
(4) Analysis of block junction signals. For block copolymers, the monomer unit that forms the covalent linkage between the two blocks often has a different chemical environment than the repeating units of the individual blocks and, as such, sometimes shows a distinct chemical shift in the 1H NMR spectrum. The main chain and junction unit signals are often observed in 1H NMR and can be assigned by two-dimensional (2D) NMR techniques. It should be noted that these signals are inherently of weak intensity, with intensity decreasing as the block length increases, as they are only present in a small fraction of the polymer. Nonetheless, analysis of integrals (under a range of relaxation times) can give an indication of the ratio of blocks to junctions and therefore can be used to characterize the polymer product. In the present example, a block ratio of PCL : PCHC of 4 : 1 was obtained, which was within error with the ratio based on the integrals of the repeating unit (5 : 1, figure 7).
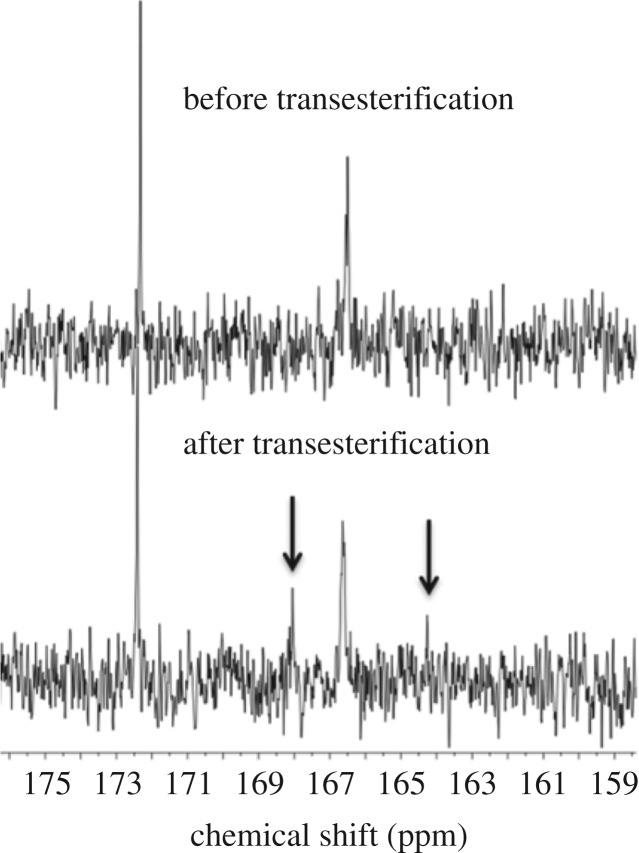
(5) Transesterification (13C NMR and GPC). Transesterification is a side-reaction that could result in block scrambling. The extent of transesterification can be assessed using 13C NMR spectroscopy most commonly in the case of block copoly(ester-carbonate)s or block copolyesters by analysis of the carbonyl region of the spectrum. In particular, control experiments where block copolymers are treated with a catalyst favouring transesterification, such as 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD), can be used to reveal the signals expected for transesterified/randomized copolymer structures. The 13C NMR of the block copolymer and the randomized copolymer can be compared and transesterification should be identified most clearly by changes to the carbonyl region (figure 8). Moreover, extensive transesterification may also be observable by GPC, as broad molecular-weight distributions are expected, sometimes with clear formation of higher/lower molecular-weight species.
(6) Material properties. The macroscopic properties of the resulting block copolymer should also be compared to those of the homopolymers. This includes, but is not restricted to, thermal and mechanical properties, such as glass-transition temperature, decomposition temperature and the modulus of elasticity, which can be characterized using differential scanning calorimetry (DSC), thermal gravimetric analysis (TGA) and tensile tester, respectively. There are often distinct differences in thermal properties for block copolymers compared to mixtures of homopolymers or random structures, but generalizations are not possible, as properties also correlate with block lengths and miscibility.
(7) Physical separation. Another test used to characterize block copolymers is to establish whether the block composition can be changed upon fractionation experiments. These typically involve washing the sample with solvents selective for a particular polymer and/or re-precipitation experiments (from solvent/non-solvent mixtures) and subsequent analysis of the samples using spectroscopy. It should not be possible to change the composition (i.e. ratio of block constituents) for a block copolymer, whereas mixtures of polymers can be separated using such techniques.
Figure 4.
Representative GPC monitoring of ‘Switch’ catalysis. An increase in molecular weight can be observed after the incorporation of DL [34]. (Online version in colour.)
Figure 5.
End-group analysis by 31P{1H} NMR (polymer structures given in scheme 1).
Figure 6.
DOSY of block copolymer (top) versus a mixture of homopolymers (bottom) in toluene-d8 at room temperature [34]. (Online version in colour.)
Figure 7.
An example of a 1H NMR spectrum, illustrating the junction signals [33]. The chemical shifts assigned to common junctions for selected block copolymers (in CDCl3, top right). Polymer structures are given in scheme 1.
Figure 8.
13C NMR showing transesterification of copoly(PDL-b-PCHPE-b-PDL) after the reaction with TBD [34].
4. Future directions
‘Switch’ catalysis is a method by which monomer mixtures can be selectively transformed into multiblock copolymers using a single catalyst which operates in two distinct catalytic cycles. So far, the method is still in its infancy and has been demonstrated for the production of block copoly(ester-carbonate)s and block copolyesters. This short review has highlighted research from our group in developing the method and also laid out the methods of characterization that should be used to determine whether other catalysts operate by ‘Switch’ catalysis. So far, our investigations have focused exclusively on di-zinc catalysts, coordinated by a macrocyclic ancillary ligand, and mixtures of carbon dioxide, lactone, anhydride and epoxides. Very recently, the first demonstration of ‘Switch’ catalysis using another di-zinc catalyst, coordinated by a β-diiminate ligand, has been reported and used to prepare block copoly(ester-carbonates) [59]. The next phases of the development of the method should seek to explore other known catalysts for ROCOP and ROP reactions; for example, metal salen or porphyrin complexes would be interesting targets. In this context, the use of catalysts able to operate with lower temperatures so as to enable the incorporation of other epoxides, such as propylene oxide, is an important target. The development of ‘Switch’ catalysis should also address expanding the range of monomers able to operate by this mechanism, including functionalized epoxides/anhydrides, many of which are commercially available. As part of long-term studies it is of interest to expand yet wider the range of monomers to include other heterocumulenes, cyclic monomers (cyclic carbonates) and heterocycles, such as aziridines or thiiranes. In parallel with the catalytic development, it is important to fully characterize the new multiblock copolymers formed. One attraction of the ‘Switch’ catalysis method is the ability to form materials featuring ‘hard’ and ‘soft’ domains, which are expected to undergo phase separations and show potential as rigid plastics and/or thermoplastic elastomers. The detailed understanding and study of the polymer properties is important to realize the potential of the catalysis. Finally, the ‘Switch’ catalysis method could be expanded to include other polymerization pathways and mechanisms and, as such, exploring the means to ‘Switch’ catalysts between different pathways remains a less-studied but fascinating future challenge in polymerization catalysis.
Data accessibility
This article has no additional data.
Authors' contributions
All authors have contributed to the review article.
Competing interests
C.K.W. is a director of Econic Technologies.
Funding
Funding to support the research was awarded by EIT, Climate KIC (Project EnCO2re) and the EPSRC (EP/L017393/1; EP/K014668/1).
References
- 1.McNaught AD, Wilkinson A (compilers). 1997. Compendium of chemical terminology--IUPAC recommendations, 2nd edn (the ‘Gold Book’). Oxford, UK: Blackwell Scientific. IUPAC Gold Book. (2006--) Advanced XML online corrected version created by M Nic, J Jirat and B Kosata, with updates compiled by A Jenkins ( 10.1351/goldbook) [DOI]
- 2.Schacher FH, Rupar PA, Manners I. 2012. Functional block copolymers: nanostructured materials with emerging applications. Angew. Chem. Int. Ed. 51, 7898–7921. ( 10.1002/anie.201200310) [DOI] [PubMed] [Google Scholar]
- 3.Ruzette A-V, Leibler L. 2005. Block copolymers in tomorrow's plastics. Nat. Mater. 4, 19–31. ( 10.1038/nmat1295) [DOI] [PubMed] [Google Scholar]
- 4.Hillmyer MA, Tolman WB. 2014. Aliphatic polyester block polymers: renewable, degradable, and sustainable. Acc. Chem. Res. 47, 2390–2396. ( 10.1021/ar500121d) [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Yuan L, Tang C. 2017. Sustainable elastomers from renewable biomass. Acc. Chem. Res. 50, 1762–1773. ( 10.1021/acs.accounts.7b00209) [DOI] [PubMed] [Google Scholar]
- 6.Kataoka K, Harada A, Nagasaki Y. 2001. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv. Drug Deliv. Rev. 47, 113–131. ( 10.1016/S0169-409X(00)00124-1) [DOI] [PubMed] [Google Scholar]
- 7.Park C, Yoon J, Thomas EL. 2003. Enabling nanotechnology with self assembled block copolymer patterns. Polymer 44, 6725–6760. ( 10.1016/j.polymer.2003.08.011) [DOI] [Google Scholar]
- 8.Hamley IW. 2003. Nanostructure fabrication using block copolymers. Nanotechnology 14, R39–R54. ( 10.1088/0957-4484/14/10/201) [DOI] [Google Scholar]
- 9.Zhu Y, Romain C, Williams CK. 2016. Sustainable polymers from renewable resources. Nature 540, 354–362. ( 10.1038/nature21001) [DOI] [PubMed] [Google Scholar]
- 10.Puskas JE, Kaszas G, Kennedy JP, Hager WG. 1992. Polyisobutylene-containing block polymers by sequential monomer addition. IV. New triblock thermoplastic elastomers comprising high Tg styrenic glassy segments: synthesis, characterization and physical properties. J. Polym. Sci. A 30, 41–48. ( 10.1002/pola.1992.080300105) [DOI] [Google Scholar]
- 11.Hirao A, Goseki R, Ishizone T. 2014. Advances in living anionic polymerization: from functional monomers, polymerization systems, to macromolecular architectures. Macromolecules 47, 1883–1905. ( 10.1021/ma401175m) [DOI] [Google Scholar]
- 12.Szwarc M, Levy M, Milkovich R. 1956. Polymerization initiated by electron transfer to monomer. A new method of formation of block polymers. J. Am. Chem. Soc. 78, 2656–2657. ( 10.1021/ja01592a101) [DOI] [Google Scholar]
- 13.Anastasaki A, Waldron C, Wilson P, Boyer C, Zetterlund PB, Whittaker MR, Haddleton D. 2013. High molecular weight block copolymers by sequential monomer addition via Cu(0)-mediated living radical polymerization (SET-LRP): an optimized approach. ACS Macro Lett. 2, 896–900. ( 10.1021/mz4004198) [DOI] [PubMed] [Google Scholar]
- 14.Yasuda T, Aida T, Inoue S. 1984. Synthesis of polyester--polyether block copolymer with controlled chain length from β-lactone and epoxide by aluminum porphyrin catalyst. Macromolecules 17, 2217–2222. ( 10.1021/ma00141a003) [DOI] [Google Scholar]
- 15.Dove AP. 2012. Organic catalysis for ring-opening polymerization. ACS Macro Lett. 1, 1409–1412. ( 10.1021/mz3005956) [DOI] [PubMed] [Google Scholar]
- 16.Ajellal N, Carpentier J-F, Guillaume C, Guillaume SM, Helou M, Poirier V, Sarazin Y, Trifonov A. 2010. Metal-catalyzed immortal ring-opening polymerization of lactones, lactides and cyclic carbonates. Dalton Trans. 39, 8363–8376. ( 10.1039/c001226b) [DOI] [PubMed] [Google Scholar]
- 17.Kamber NE, Jeong W, Waymouth RM, Pratt RC, Lohmeijer BGG, Hedrick JL. 2007. Organocatalytic ring-opening polymerization. Chem. Rev. 107, 5813–5840. ( 10.1021/cr068415b) [DOI] [PubMed] [Google Scholar]
- 18.O'Keefe BJ, Hillmyer MA, Tolman WB. 2001. Polymerization of lactide and related cyclic esters by discrete metal complexes. J. Chem. Soc. Dalton Trans. 2001, 2215–2224. ( 10.1039/b104197p) [DOI] [Google Scholar]
- 19.Longo JM, Sanford MJ, Coates GW. 2016. Ring-opening copolymerization of epoxides and cyclic anhydrides with discrete metal complexes: structure–property relationships. Chem. Rev. 116, 15 167–15 197. ( 10.1021/acs.chemrev.6b00553) [DOI] [PubMed] [Google Scholar]
- 20.Paul S, Zhu Y, Romain C, Brooks R, Saini PK, Williams CK. 2015. Ring-opening copolymerization (ROCOP): synthesis and properties of polyesters and polycarbonates. Chem. Commun. 51, 6459–6479. ( 10.1039/C4CC10113H) [DOI] [PubMed] [Google Scholar]
- 21.Kember MR, Buchard A, Williams CK. 2011. Catalysts for CO2/epoxide copolymerisation. Chem. Commun. 47, 141–163. ( 10.1039/C0CC02207A) [DOI] [PubMed] [Google Scholar]
- 22.Trott G, Saini PK, Williams CK. 2016. Catalysts for CO2/epoxide ring-opening copolymerization. Phil. Trans. R. Soc. A 374, 20150085 ( 10.1098/rsta.2015.0085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kember MR, White AJP, Williams CK. 2009. Di- and tri-zinc catalysts for the low-pressure copolymerization of CO2 and cyclohexene oxide. Inorg. Chem. 48, 9535–9542. ( 10.1021/ic901109e) [DOI] [PubMed] [Google Scholar]
- 24.Kember MR, Knight PD, Reung PTR, Williams CK. 2009. Highly active dizinc catalyst for the copolymerization of carbon dioxide and cyclohexene oxide at one atmosphere pressure. Angew. Chem. Int. Ed. 48, 931–933. ( 10.1002/anie.200803896) [DOI] [PubMed] [Google Scholar]
- 25.Kember MR, Copley J, Buchard A, Williams CK. 2012. Triblock copolymers from lactide and telechelic poly(cyclohexene carbonate). Polym. Chem. 3, 1196–1201. ( 10.1039/c2py00543c) [DOI] [Google Scholar]
- 26.Wu G-P, Darensbourg DJ, Lu X-B. 2012. Tandem metal-coordination copolymerization and organocatalytic ring-opening polymerization via water to synthesize diblock copolymers of styrene oxide/CO2 and lactide. J. Am. Chem. Soc. 134, 17 739–17 745. ( 10.1021/ja307976c) [DOI] [PubMed] [Google Scholar]
- 27.Wu G-P, Darensbourg DJ. 2016. Mechanistic insights into water-mediated tandem catalysis of metal-coordination CO2/epoxide copolymerization and organocatalytic ring-opening polymerization: one-pot, two steps, and three catalysis cycles for triblock copolymers synthesis. Macromolecules 49, 807–814. ( 10.1021/acs.macromol.5b02752) [DOI] [Google Scholar]
- 28.Darensbourg DJ. 2017. Switchable catalytic processes involving the copolymerization of epoxides and carbon dioxide for the preparation of block polymers. Inorg. Chem. Front. 4, 412–419. ( 10.1039/C7QI00013H) [DOI] [Google Scholar]
- 29.Li Y, Hong J, Wei R, Zhang Y, Tong Z, Zhang X, Du B, Xu J, Fan Z. 2015. Highly efficient one-pot/one-step synthesis of multiblock copolymers from three-component polymerization of carbon dioxide, epoxide and lactone. Chem. Sci. 6, 1530–1536. ( 10.1039/C4SC03593C) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kröger M, Folli C, Walter O, Döring M. 2006. Alternating copolymerization of carbon dioxide and cyclohexene oxide and their terpolymerization with lactide catalyzed by zinc complexes of N,N ligands. Adv. Synth. Catal. 348, 1908–1918. ( 10.1002/adsc.200606075) [DOI] [Google Scholar]
- 31.Song P, Xu H, Mao X, Liu X, Wang L. 2017. A one-step strategy for aliphatic poly(carbonate-ester)s with high performance derived from CO2, propylene oxide and l-lactide. Polym. Adv. Technol 28, 736–741. ( 10.1002/pat.3974) [DOI] [Google Scholar]
- 32.Romain C, Zhu Y, Dingwall P, Paul S, Rzepa HS, Buchard A, Williams CK. 2016. Chemoselective polymerizations from mixtures of epoxide, lactone, anhydride, and carbon dioxide. J. Am. Chem. Soc. 138, 4120–4131. ( 10.1021/jacs.5b13070) [DOI] [PubMed] [Google Scholar]
- 33.Paul S, Romain C, Shaw J, Williams CK. 2015. Sequence selective polymerization catalysis: a new route to ABA block copoly(ester-b-carbonate-b-ester). Macromolecules 48, 6047–6056. ( 10.1021/acs.macromol.5b01293) [DOI] [Google Scholar]
- 34.Zhu Y, Romain C, Williams CK. 2015. Selective polymerization catalysis: controlling the metal chain end group to prepare block copolyesters. J. Am. Chem. Soc. 137, 12 179–12 182. ( 10.1021/jacs.5b04541) [DOI] [PubMed] [Google Scholar]
- 35.Romain C, Williams CK. 2014. Chemoselective polymerization control: from mixed-monomer feedstock to copolymers. Angew. Chem. Int. Ed. 53, 1607–1610. ( 10.1002/anie.201309575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guillaume SM, Kirillov E, Sarazin Y, Carpentier J-F. 2015. Beyond stereoselectivity, switchable catalysis: some of the last frontier challenges in ring-opening polymerization of cyclic esters. Chem. Eur. J. 21, 7988–8003. ( 10.1002/chem.201500613) [DOI] [PubMed] [Google Scholar]
- 37.Teator AJ, Lastovickova DN, Bielawski CW. 2016. Switchable polymerization catalysts. Chem. Rev. 116, 1969–1992. ( 10.1021/acs.chemrev.5b00426) [DOI] [PubMed] [Google Scholar]
- 38.Jeske RC, Rowley JM, Coates GW. 2008. Pre-rate-determining selectivity in the terpolymerization of epoxides, cyclic anhydrides, and CO2: a one-step route to diblock copolymers. Angew. Chem. Int. Ed. 47, 6041–6044. ( 10.1002/anie.200801415) [DOI] [PubMed] [Google Scholar]
- 39.Ren W-M, Zhang X, Liu Y, Li J-F, Wang H, Lu X-B. 2010. Highly active, bifunctional Co(III)-salen catalyst for alternating copolymerization of CO2 with cyclohexene oxide and terpolymerization with aliphatic epoxides. Macromolecules 43, 1396–1402. ( 10.1021/ma902321g) [DOI] [Google Scholar]
- 40.Shi L, Lu X-B, Zhang R, Peng X-J, Zhang C-Q, Li J-F, Peng X-M. 2006. Asymmetric alternating copolymerization and terpolymerization of epoxides with carbon dioxide at mild conditions. Macromolecules 39, 5679–5685. ( 10.1021/ma060290p) [DOI] [Google Scholar]
- 41.Darensbourg DJ, Poland RR, Escobedo C. 2012. Kinetic studies of the alternating copolymerization of cyclic acid anhydrides and epoxides, and the terpolymerization of cyclic acid anhydrides, epoxides, and CO2 catalyzed by (salen)CrIIICl. Macromolecules 45, 2242–2248. ( 10.1021/ma2026385) [DOI] [Google Scholar]
- 42.Huijser S, HosseiniNejad E, Sablong R, de Jong C, Koning CE, Duchateau R. 2011. Ring-opening co- and terpolymerization of an alicyclic oxirane with carboxylic acid anhydrides and CO2 in the presence of chromium porphyrinato and salen catalysts. Macromolecules 44, 1132–1139. ( 10.1021/ma102238u) [DOI] [Google Scholar]
- 43.Darensbourg DJ, Poland RR, Strickland AL. 2012. (Salan)CrCl, an effective catalyst for the copolymerization and terpolymerization of epoxides and carbon dioxide. J. Polym. Sci. A 50, 127–133. ( 10.1002/pola.24996) [DOI] [Google Scholar]
- 44.Wu G-P, Xu P-X, Zu Y-P, Ren W-M, Lu X-B. 2013. Cobalt(III)-complex-mediated terpolymerization of CO2, styrene oxide, and epoxides with an electron-donating group. J. Polym. Sci. A 51, 874–879. ( 10.1002/pola.26444) [DOI] [Google Scholar]
- 45.Duan Z, Wang X, Gao Q, Zhang L, Liu B, Kim I. 2014. Highly active bifunctional cobalt-salen complexes for the synthesis of poly(ester-block-carbonate) copolymer via terpolymerization of carbon dioxide, propylene oxide, and norbornene anhydride isomer: roles of anhydride conformation consideration. J. Polym. Sci. A 52, 789–795. ( 10.1002/pola.27057) [DOI] [Google Scholar]
- 46.Darensbourg DJ, Wang Y. 2015. Terpolymerization of propylene oxide and vinyl oxides with CO2: copolymer cross-linking and surface modification via thiol-ene click chemistry. Polym. Chem. 6, 1768–1776. ( 10.1039/C4PY01612B) [DOI] [Google Scholar]
- 47.Buchard A, Bakewell CM, Weiner J, Williams CK. 2012. In Organometallics and renewables (eds Meier MAR, Weckhuysen BM, Bruijnincx PCA), pp. 175–224. Berlin, Germany: Springer. [Google Scholar]
- 48.Platel RH, Hodgson LM, Williams CK. 2008. Biocompatible initiators for lactide polymerization. Polym. Rev. 48, 11–63. ( 10.1080/15583720701834166) [DOI] [Google Scholar]
- 49.Jutz F, Buchard A, Kember MR, Fredriksen SB, Williams CK. 2011. Mechanistic investigation and reaction kinetics of the low-pressure copolymerization of cyclohexene oxide and carbon dioxide catalyzed by a dizinc complex. J. Am. Chem. Soc. 133, 17 395–17 405. ( 10.1021/ja206352x) [DOI] [PubMed] [Google Scholar]
- 50.Buchard A, Jutz F, Kember MR, White AJP, Rzepa HS, Williams CK. 2012. Experimental and computational investigation of the mechanism of carbon dioxide/cyclohexene oxide copolymerization using a dizinc catalyst. Macromolecules 45, 6781–6795. ( 10.1021/ma300803b) [DOI] [Google Scholar]
- 51.Chapman AM, Keyworth C, Kember MR, Lennox AJJ, Williams CK. 2015. Adding value to power station captured CO2: tolerant Zn and Mg homogeneous catalysts for polycarbonate polyol production. ACS Catal. 5, 1581–1588. ( 10.1021/cs501798s) [DOI] [Google Scholar]
- 52.Kember MR, Jutz F, Buchard A, White AJP, Williams CK. 2012. Di-cobalt(II) catalysts for the copolymerisation of CO2 and cyclohexene oxide: support for a dinuclear mechanism? Chem. Sci. 3, 1245–1255. ( 10.1039/c2sc00802e) [DOI] [Google Scholar]
- 53.Kember MR, Williams CK. 2012. Efficient magnesium catalysts for the copolymerization of epoxides and CO2; using water to synthesize polycarbonate polyols. J. Am. Chem. Soc. 134, 15 676–15 679. ( 10.1021/ja307096m) [DOI] [PubMed] [Google Scholar]
- 54.Kember MR, White AJP, Williams CK. 2010. Highly active di- and trimetallic cobalt catalysts for the copolymerization of CHO and CO2 at atmospheric pressure. Macromolecules 43, 2291–2298. ( 10.1021/ma902582m) [DOI] [Google Scholar]
- 55.Garden JA, Saini PK, Williams CK. 2015. Greater than the sum of its parts: a heterodinuclear polymerization catalyst. J. Am. Chem. Soc. 137, 15 078–15 081. ( 10.1021/jacs.5b09913) [DOI] [PubMed] [Google Scholar]
- 56.Saini PK, Fiorani G, Mathers RT, Williams CK. 2017. Zinc versus magnesium: orthogonal catalyst reactivity in selective polymerizations of epoxides, bio-derived anhydrides and carbon dioxide. Chem. Eur. J. 23, 4260–4265. ( 10.1002/chem.201605690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu Y, Romain C, Poirier V, Williams CK. 2015. Influences of a dizinc catalyst and bifunctional chain transfer agents on the polymer architecture in the ring-opening polymerization of ε-caprolactone. Macromolecules 48, 2407–2416. ( 10.1021/acs.macromol.5b00225) [DOI] [Google Scholar]
- 58.Romain C, Garden JA, Trott G, Buchard A, White AJP, Williams CK. 2017. Di-zinc–aryl complexes: CO2 insertions and applications in polymerisation catalysis. Chem. Eur. J. 23, 7367–7376. ( 10.1002/chem.201701013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reiter M, Vagin S, Kronast A, Jandl C, Rieger B. 2017. A Lewis acid β-diiminato-zinc-complex as all-rounder for co- and terpolymerisation of various epoxides with carbon dioxide. Chem. Sci. 8, 1876–1882. ( 10.1039/C6SC04477H) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spyros A, Argyropoulos DS, Marchessault RH. 1997. A study of poly(hydroxyalkanoate)s by quantitative 31P NMR spectroscopy: molecular weight and chain cleavage. Macromolecules 30, 327–329. ( 10.1021/ma9601979) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.