Abstract
Introduction
Monitoring fungal aerocontamination is an essential measure to prevent severe invasive aspergillosis (IA) infections in hospitals. One central block among 32 blocks of Edouard Herriot Hospital (EHH) was entirely demolished in 2015, while care activities continued in surrounding blocks. The main objective was to undertake broad environmental monitoring and clinical surveillance of IA cases to document fungal dispersion during major deconstruction work and to assess clinical risk.
Methods and analysis
A daily environmental survey of fungal loads was conducted in eight wards located near the demolition site. Air was collected inside and outside selected wards by agar impact samplers. Daily spore concentrations were monitored continuously by volumetric samplers at a flow rate of 10 L.min-1. Daily temperature, wind direction and speed as well as relative humidity were recorded by the French meteorological station Meteociel. Aspergillus fumigatus strains stored will be genotyped by multiple-locus, variable-number, tandem-repeat analysis. Antifungal susceptibility will be assessed by E-test strips on Roswell Park Memorial Institute medium supplemented with agar. Ascertaining the adequacy of current environmental monitoring techniques in hospital is of growing importance, considering the rising impact of fungal infections and of curative antifungal costs. The present study could improve the daily management of IA risk during major deconstruction work and generate new data to ameliorate and redefine current guidelines.
Ethics and dissemination
This study was approved by the clinical research and ethics committees of EHH.
Keywords: invasive aspergillosis, clinical monitoring, environmental monitoring, aspergillus fumigatus, azole resistance, genetic patterns
Strengths and limitations of this study.
This study is one of the largest ongoing prospective studies to evaluate combined approach of environmental and clinical monitoring in hospital during major deconstruction works.
The high frequency and number of samples collected in this study during deconstruction works will allow powerful statistical analysis to evaluate the efficiency of protective measures and reduce fungal contamination.
Non-cultivable methods monitoring outdoor Aspergillus aerocontamination for hospital alerts are evaluated.
Genetic diversity and their antifungal susceptibility profiles of clinical and environmental collected isolates are determined to give complete information on Aspergillus spp dispersion and hopefully give new insights into improvement of environmental monitoring and of hospital guidelines during major demolition work.
Microbiological identification will focus only on Aspergillus spp. because it is the leading pathogen responsible for invasive aspergillosis. Although two air sampling collectors were used in the study, no particle counter was tested.
Introduction
Invasive fungal infections are major threats to immunocompromised patients because of their high incidence and related mortality.1 They occur through inhalation of airborne conidia with various consequences, such as immune-allergic reactions to invasive aspergillosis (IA), a severe opportunistic disease caused mostly by Aspergillus fumigatus (>80%) and, to a lesser extent, by A. flavus, A. niger, A. terreus and A. nidulans.2 3 IA is a very serious condition, with crude lethality ranging from 50% to 90% and greater mortality in haematological patients.4 5 Its epidemiology has changed in recent years, surfacing in non-haematological units, such as intensive care units (ICUs) and increasing in non-neutropenic hosts treated with corticosteroids or lifelong immunosuppressants.6 The burden of patients at risk of IA is growing every year because of longer survival and improved care.7 Azole antifungals are commonly given to combat IA in many countries, with recent studies reporting an emerging, worldwide problem: azole drug resistance of A. fumigatus isolates.8–13
Aspergillus species are opportunistic pathogens widely distributed in the environment and easily transported by air because of their conidia size.13 Construction work in healthcare settings in past decades has been associated with major IA outbreaks.4 Construction involving periods of renovation or deconstruction increases spore release, creating high-risk situations in hospitals.1 14 15 In the 1980s, 22 IA cases arose over a 30-month period during building renovations at Edouard Herriot Hospital (EHH) (Lyon, France).16 A quasi-experimental study, conducted in an adult haematology unit of this hospital, underlined the need for monitoring environmental factors to prevent nosocomial IA.17 A sampling strategy for fungal monitoring in hospital was based on study results.14 18
Recently, an entire block in EHH underwent deconstruction without suspension of care activities in direct proximity to it. The situation prompted the infection control team to monitor fungal dispersion and look for cases of Aspergillus disease. Only a few studies have been performed in this context and under similar conditions so that sampling strategies and analyses differ, indicating varying fungal loads.15–21 All these investigations have highlighted the common need for innovative approaches and tools to improve research in the field.18 Real-time methods may provide warning systems by monitoring outdoor fungal loads.21
The purpose of the present work was to carry out broad environmental monitoring and clinical surveillance of IA cases to better understand fungal dispersion during major deconstruction work.
Methods
Study objectives
The objective of this study is to provide new data for future guidelines on adequate management of invasive fungal infection risk in hospital during major deconstruction work. Its intermediate objectives are to: (1) evaluate non-cultivable methods monitoring outdoor Aspergillus aerocontamination for hospital alerts, (2) assess the impact of meteorological parameters (MP) on Aspergillus aerocontamination and (3) compare the genetic diversity of clinical and environmental A. fumigatus isolates and ascertain their antifungal susceptibility profiles.
Study site
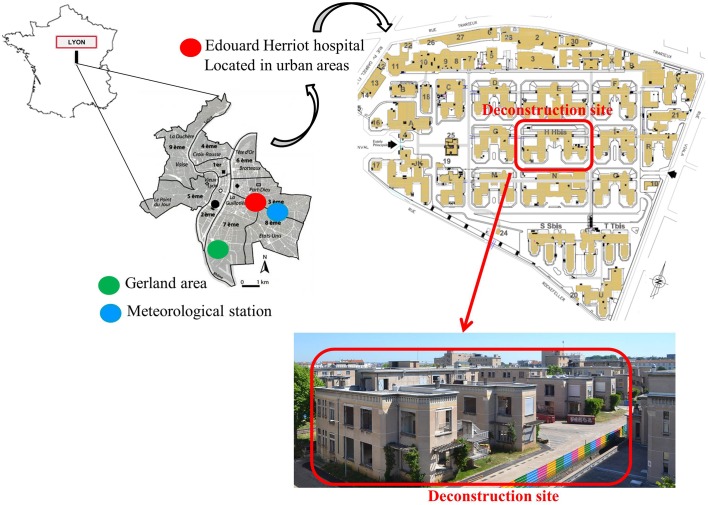
Built in the 1930s in Lyon, Rhône-Alpes, France, on a 0.15-km2 site, EHH is a university-affiliated hospital composed of 32 independent blocks divided by tree-planted walkways and grassy areas. This 850-adult bed tertiary institution provides care to a large panel of immunocompromised patients (solid organ transplantation, haematopoietic stem cell transplantation, immunosuppressive treatment, ICU). The central block that was demolished measured 0.006-km2, representing approximately 12.2% of the area covered by hospital blocks (figure 1, online video of demolition works: https://www.youtube.com/watch?v=Oa7xRufAnhQ). Three deconstruction periods at EHH were scheduled between February and December 2015. The first period, between February and June 2015, consisted of gutting the building and removing asbestos from it. The floors were removed in July and August 2015. Excavation and earthwork took place between September and December 2015. Finally, concrete was poured at the end of December 2015 to allow construction of the new building comprising fully equipped areas (operating rooms, ICUs and heliport) on 2.5 ha.
Figure 1.
Location of Edouard Herriot Hospital, Lyon (France).
Study design
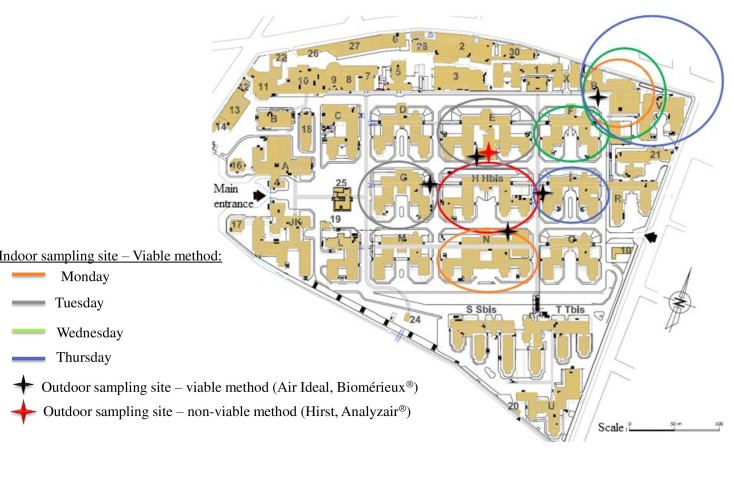
The study was conducted in eight medical wards located around the demolition site: four ICUs, one kidney and pancreas transplantation unit and three medical wards (figure 2). All of them were possibly occupied by at-risk patients. It should be noted that EHH does not have a haematological ward any longer. In a 10-month period, about eight inside and four outside air samples were collected from each unit per week (table 1). Approximately 64 indoor and 48 outdoor air samples were obtained per week, for a total of 3885 air samples. Weekly clinical monitoring of high-risk, hospitalised patients, by infectious control practitioners, was scheduled.
Figure 2.
Detailed map of Edouard Herriot Hospital and sampling sites of environmental monitoring.
Table 1.
Description of manual air sampling sites monitored at Edouard Herriot Hospital
| Medical unit | ICU | Medical unit | Medical unit | Kidney and pancreas transplantation unit | ICU | ICU | |||||||||
| Sampling | Morning | Afternoon | Morning | Afternoon | Morning | Afternoon | Morning | Afternoon | Morning | Afternoon | Morning | Afternoon | Morning | Afternoon | |
| Monday | Outdoor | Building porch | Building porch | Building porch | Building porch | – | – | – | – | – | – | – | – | – | – |
| Indoor | Room+ corridor |
Room+ corridor |
Corridor+ treatment room |
Corridor+ treatment room |
– | – | – | – | – | – | – | – | – | – | |
| Tuesday | Outdoor | – | – | – | – | Building porch | Building porch | – | – | – | – | Building porch | Building porch | – | – |
| Indoor | – | – | – | – | 2 Corridors | 2 Corridors | – | – | – | – | Room+ corridor |
Room+ corridor |
– | – | |
| Wednesday | Outdoor | – | – | – | – | – | – | Building porch | Building porch | Building porch | Building porch | – | – | – | – |
| Indoor | – | – | – | – | – | – | 2 Corridors | 2 Corridors | Room+ corridor |
Room+ corridor |
– | – | – | – | |
| Thursday | Outdoor | Building porch | Building porch | – | – | – | – | – | – | – | – | – | – | Building porch | Building porch |
| Indoor | Room+ corridor |
Room+ corridor |
– | – | – | – | – | – | – | – | – | – | Room+ corridor |
Room+ corridor |
|
| Friday | Outdoor Indoor |
Additional sampling, if needed | |||||||||||||
ICU, intensive care unit.
Protective measures
Several preventive measures were performed: (1) doors and windows in front of the deconstruction site were maintained closed during the day and allowed for opening at night, (2) patient and medical staff movements were limited and special traffic patterns were designed, (3) masks were requested for hospitalised or non-hospitalised immunocompromised patients outside wards to limit fungal exposure, (4) adhesive decontamination carpets were installed at the entry of wards and (5) visitors, patients and medical staff were alerted about fungal exposure due to the deconstruction site. In case of high fungal exposure, after results of environmental survey, intensive biocleaning was performed.
Preventive measures were also implemented outdoor, at the deconstruction site to limit fungal dispersion. Construction site teams received information and education about IA risks. Humid environment was mandatory for all works completed by regular humidification. Furthermore, rubbles of ruined buildings are humidified and covered for evacuation. Circulation pattern for rubble evacuation is also designed. Damp cleaning of deconstruction site roads are frequently realised with, in addition, cleaning of truck wheels.
Environmental survey
The environmental survey consisted of monitoring air samples inside and outside selected wards (two blocks were monitored per day). Only moulds, in particular Aspergillus spp have been investigated in this study.
Sampling by the non-cultivable method
Outdoor airborne fungal spore concentrations were monitored continuously with 7-day Hirst-type spore traps (VPPS 2000; Lanzoni, Bologna, Italy) at a flow rate of 10 L/min. Air drawn in by suction port was directly impacted on adhesive tape cut daily into segments.
Mean daily fungal spore concentrations were assessed for each segment by optical microscopy (Axiostar; Carl Zeiss, Göttingen, Germany). Spore counts were expressed as spores/m3/day. The Hirst-type spore trap was placed on the rooftop of a block extension consisting of a prefabricated floor located on the north side, just in front of the deconstruction site. It allowed daily monitoring of spore concentrations as total fungal load and Aspergillaceae fungal load (ie, Aspergillus spp + Penicillium spp). A similar Hirst-type spore trap was placed throughout the study in the Gerland area (Lyon, France), located a few kilometre south-west of EHH and served as negative control.
Sampling by the cultivable method
Air samples were collected two times a day outside and inside wards during 11 months, according to a standardised protocol (table 1). Each sample was gathered by agar impact sampler (Air Ideal; bioMérieux, Marcy l’Etoile, France) in 90 mm diameter Petri dishes containing Sabouraud chloramphenicol agar. Air intake velocity of this agar impact sampler was 100 L/min. Two plates were seeded at each sample site. Air sample volume was chosen according to French guidelines environmental fungal risk control.22 23 They recommend a sample volume adapted to the presumed levels of contamination in the environment. Due to the major demolition works ongoing, outdoor air was considered more contaminated by fungi than indoor. So in order to avoid overcrowding on the plates, outdoor plates were seeded for only 1 min corresponding to an air volume of 100 L. Indoor samples were supposed to have an intermediate fungal contamination level due to the preventive measures applied to reduce environmental contamination inside units. Therefore, indoor plates were seeded for 2½ min, resulting in a higher air volume (250 L). One of these plates was incubated for 48 hours at 37°C to grow thermotolerant A. fumigatus species.24 The other plate was incubated for 5 days at 30°C to allow growth of all fungi. Colonies were then counted and identified at the genus level on the basis of macroscopic and microscopic characteristics (lactophenol blue-stained preparations). The data are expressed as colony-forming units per cubic metre.
Prospective clinical survey
Patient inclusion
All hospitalised patients were surveyed prospectively at the hospital level and were eligible for inclusion. IA was classified as proven, probable and possible or excluded according to European Organisation for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group (EORTC) and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (MSG) criteria.25 Only cases diagnosed after hospital admission were included. Cases were categorised into three groups, according to the time between hospital admission and diagnosis: community-acquired, undetermined and nosocomial. Community-acquired cases were defined as incident cases imported from outside the hospital with apportion of clinical symptoms or positive sample in less than 2 days after admission. Undetermined cases were defined as incident cases with lag time ranging from 1 to 9 days between admission and the first IA signs without any previous negative sample. Probable nosocomial cases were defined as incident IA with lag time between admission and symptoms onset of at least 10 days, or if there is some history of negative sample. Clinical manifestations of IA vary widely and may develop in different clinical scenarios.26–28
Case detection
Case detection was based on prospective surveillance of:
Antifungal therapies (eg, voriconazole, posaconazole, itraconazole, caspofungin and amphotericin B distributed by the hospital pharmacy) administered to patients by pharmacy informatics software.
Mycological results positive for Aspergillus spp corresponding to cultures showing colony of A. fumigatus were investigated.
Reporting of suspected cases by hospital clinicians and infection control practitioners.
All suspected cases were investigated by two infection control practitioners (one resident and one physician). External validation was requested in case of uncertain diagnosis by standardised chart, allowing the collection of demographic characteristics, disease history, clinical features, mycological, biological and radiological data, antifungal therapy and disease outcome. If an increased incidence of IA was detected, infections control specialists and mycologists would be solicited to review IA cases.
A. fumigatus collection
During the study, a maximum of four A. fumigatus colonies per day among all A. fumigatus environmental cultures incubated at 37°C were arbitrarily isolated, subcultured on Sabouraud dextrose agar and incubated at 45°C in order to select A. fumigatus before being frozen. All A. fumigatus clinical isolates of interest also were frozen at −20°C. In total, 400 A. fumigatus isolates, corresponding to maximal laboratory capacity and budget, were constituted arbitrarily.
Molecular identification of Aspergillus isolates
All A. fumigatus isolates stored will be identified retrospectively at the species level by sequencing β-tubulin gene (benA, using Bt2a/BT2b primers). Isolates will be subcultured on Sabouraud dextrose chloramphenicol agar for 48 hours at 37°C. A piece of approximately 1 cm2 of culture will be cut and transferred to microtubes for DNA extraction with QIAamp DNA blood mini kits (Qiagen) according to the manufacturer’s instructions. All isolates will be identified by partial sequencing of β-tubulin gene (benA, using Bt2a/BT2b primers). Sequence alignments will be analysed by Chromas Lite, V.2.01 (http://technelysium.com.au) and compared with genome sequences in GenBank and MycoBank. The results will be considered acceptable if homologies with other entries in the databases used for comparison are >99%.
Genotyping of A. fumigatus isolates
Isolates will be genotyped by multiple-locus variable-number tandem-repeat analysis (MLVA) based on selected variable-number tandem-repeat (VNTR) polymorphism. The MLVA protocol for A. fumigatus genotyping targeting 10 markers will be adapted from Thierry et al for multiplexing and capillary electrophoresis (CE).29 One primer couple will be modified to provide shorter amplicons while ensuring the absence of overlaps across VNTR loci. Primers targeting new VNTR flanking regions have been designed by Primer3Plus software. MLVA primers for 10 loci and the fluorescent dyes in CE are enumerated in table 2. MLVA PCRs were performed in two multiplexes in a final volume of 50 µL containing: 1–5 ng of DNA, 1X Multiplex PCR Master Mix (Qiagen) and 0.2 µM of each flanking primer. The initial denaturation step at 95°C for 10 min was followed by 35 cycles, consisting of denaturation at 95°C for 30 s, primer annealing at 58°C for 40 s and elongation at 72°C for 10 min. The final extension step was set at 60°C for 10 min. PCR products were diluted 1:50 in deionised water, and 1 µL of the diluted sample was added to 18.5 µL formamide and 0.1 µL of GeneScan 500 LIZ dye size standard (ThermoFisher; Life Technologies, Courtaboeuf, France). All samples were denatured for 5 min at 95°C, then cooled to 4°C before being subjected to CE in a 3130 XL DNA analyser (Applied Biosystems, Courtaboeuf, France) with 3130 POP7 polymer (Applied Biosystems). Each VNTR locus was identified by colour and size in electropherograms by GeneScan analysis (Applied Biosystems). Fragment sizes were converted to repeat numbers based on the formula: number of repeats (bp)=fragment size (bp)—flanking regions (bp)/repeat size (bp). Absent PCR products were designated an allele number, for example, ‘−1’. Phylogenetic relationships between isolates will be studied, generating a minimum spanning tree with PHYLOViZ V.2.0.30
Table 2.
MLVA primers and fluorescence dyes used in each multiplex reaction
| VNTR | Fluorochromes | Primer sequences (5’–3’) | Allele size range (bp) | ||
| Multiplex 1 CE 1 |
Asp 167 | F: R: |
ATTO565- | TGAGATGGTTAACTTACGTAGCGC CGCTCCCACCGTTACCAAC |

|
| Asp 330 | F: R: |
ATTO550- | ATCTGGTCGCGAAATTCCTCT TCTTCGGCCTTTTCATCCC |

|
|
| Asp 345 | F: R: |
Yakima Yellow- | TCTCCAACCCTTCGGACG GCCGGAAGAGCATGAAGACA |
|
|
| Asp 443 | F: R: |
ATTO565- | AAGCTTCGTCTGGCGAAGAG GCACGTGTACGGTGTTCCTG |
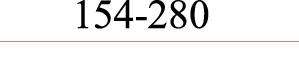
|
|
| Asp 446 | F: R: |
6-FAM- | CGATCATGTTTGCCTGAGGA CCGACAGCATCGAGCAACTA |

|
|
| Multiplex 2 CE 2 |
Asp 20 | F: R: |
6-FAM- | GGGAAGAGAGGAACCGATCC CGCAGTGGGCAGTTTGAAT |

|
| Asp 165 | F: R: |
Yakima Yellow- | TGATGGGCCGCAGTCG GCACCTGCTTGTCGATTCGT |

|
|
| Asp 202 | F: R: |
ATTO565- | AGGATCACTGCCCTCAACCC CCGAAATCCGCGGGA |
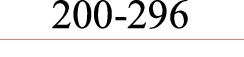
|
|
| Asp 204bis | F: R: |
ATTO565- | ATTGGGAAGAGACGGGGTAT GTCCTCACTTTTGCCTTGGT |

|
|
| Asp 252 | F: R: |
ATTO550- | CAGATTGGAGACACGAAGCG ACCACGGATTGCCAAGGA |

|
|
6-FAM, 6-carboxyfluorescein; CE, capillary electrophoresis; F, forward; MLVA, multiple-locus variable-number tandem-repeat analysis; R, reverse; VNTR, variable-number tandem repeat.
Antifungal susceptibility testing
Briefly, the antifungal susceptibility of stored A. fumigatus isolates to itraconazole, voriconazole and amphotericin B will be analysed by Etest on RPMI medium supplemented with 2% glucose, according to the manufacturer’s instructions. A conidial suspension adjusted at 0.5 McFarland will be inoculated on RPMI 1640 agar plates (bioMérieux). Etest strips (bioMérieux) will then be applied, and the plates incubated for 48 hours at 37°C. Minimal inhibitory concentrations (MICs) of amphotericin B, itraconazole, voriconazole, posaconazole and will be tested after 24 hours and 48 hours incubation, respectively, depending on growth rate.31 They will be estimated visually as no-growth endpoints, where the edge of the inhibition ellipse intersects the side of the Etest strips.
Meteorological surveillance
Meteorological conditions monitored were temperature (degree celsius), relative humidity (percentage), wind direction and speed (kilometre per hour). They were recorded every 2 hours by Meteociel, the French regional meteorological station at Bron, 5 km from EHH (figure 1).
Data analysis
Data quality control
Location of and information on sampling sites were recorded on paper and electronic database. To avoid typing errors, a computer model was created to clean up the electronic database. Every detected error was discussed by the infection control team and screened to assess its reliability. A. fumigatus isolates stored for data collection were correctly identified in a strain bank. Antifungal treatments, delivered by the hospital pharmacy, and microbiological data were extracted every week from infection control practitioners. Deconstruction work meetings involving clinicians, infection control practitioners and engineers, held every month, to ensure conformity with and respect of French deconstruction work guidelines.
Statistical analysis
Descriptive statistics, such as means, SD (or medians and quartiles), numbers and percentages, have been obtained for indoor and outdoor fungal loads. Incidence rates will be calculated as the ratio of detected IA cases over the population at risk during the study period. Generalised linear models (appropriate for outcomes) will examine correlations between MPs and fungal contamination. To compare results obtained with cultivable and non-cultivable methods, Pearson correlation coefficients or Spearman’s rank correlation coefficients (r) will be applied according to data normality. Time series, by the non-cultivable method, autocorrelation and cross-correlation across sites (EHH and Gerland), including meteorological factors at different time scales and time lag, will be studied. P<0.05 values will be considered to be statistically significant. Statistical analyses will be performed with R language (V.3.0.2).
Discussion
Clinical and environmental monitoring was reinforced during deconstruction work, according to standard French recommendations.32 No specific prophylaxis strategy was implemented at our hospital for immunocompromised patients. Only patients with acute leukaemia were susceptible to received prophylaxis therapy according to their condition. Outdoor and indoor environmental monitoring of contamination was implemented at EHH to prevent high-risk airborne fungal situations.
The present study addresses several questions. Its main objective is to detect clinical cases as soon as possible to adequately manage invasive fungal infection risk in hospital during major deconstruction work and report new findings for future guidelines. Are national recommendations on protective measures in high-risk units efficient in shielding patients from A. fumigatus aerocontamination? Totally, 3885 air samples were collected between 23 February 2015 and 17 December 2015. Air specimens, assessed by the cultivable method, were grouped into 2141 indoor and 1744 outdoor samples. A total of 296 days of recording were undertaken with the non-cultivable method. The high frequency and number of samples collected in this study during the three deconstruction periods will allow powerful statistical analysis to evaluate the efficiency of protective measures and reduce fungal contamination.
One of its objectives is to scrutinise the performance of non-cultivable methods of outdoor Aspergillus aerocontamination monitoring, such as Hirst-type spore traps, and to ascertain the impact of MP on Aspergillus aerocontamination. One preliminary study demonstrated potential interest in monitoring outdoor contamination.21 Complete data on airborne fungal concentration will be analysed by cultivable and non-cultivable sampling methods.33 34 Non-cultivable methods allow the sampling of numerous spores, useful to carry out surveys but have limitation in fungal spore’s identification only possible at the genus level.35 Cultivable method can identify spores at the species level but is time-consuming and depends on the substrate plated and culture condition applied.36 Cultivable methods generally allow the identification at the species level. Cultivable method is required to perform genotyping and evaluation of antifungal susceptibility of the isolates. Cultivable and non-cultivable methods will be compared, which results could improve current prevention guidelines by providing early warning systems to hospitals.
Part of the study analysis evaluated the impact of MP on airborne fungal contamination. The relationship between MPs and fungal spore concentrations has already been scrutinised.37 38 However, no study has investigated the role of meteorological factors in outdoor and indoor air contamination during construction works. A better understanding of MP impact on airborne fungi is needed to see if it could help to assess high-risk situations during construction work in hospital.
In addition, the present study compares the genetic diversity of clinical and environmental A. fumigatus isolates and profiles their antifungal susceptibility patterns. The aim is to describe A. fumigatus diversity over deconstruction periods along with genetic correlations between environmental and clinical strains collected. Correlation between environmental contamination and the occurrence of IA is hardly ever established due to limitations of both environmental sampling and genotyping.39 40 Only a few authors have demonstrated links between clinical and environmental genotypes, and only a small number of environmental strains were included in the analysis, limiting the possibility of finding matches.41 42
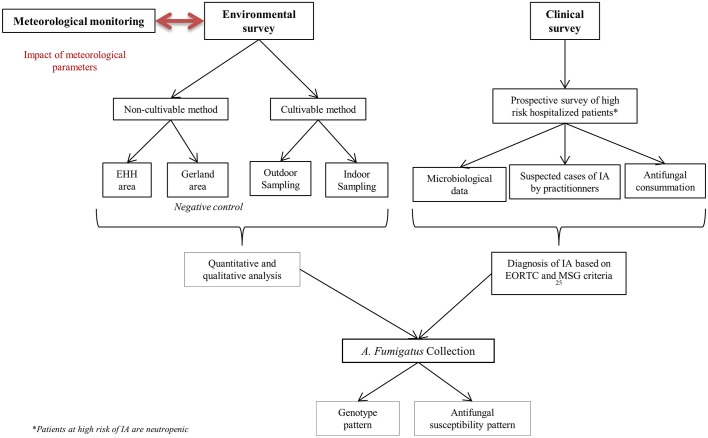
The present work provides considerable data on potential IA outbreaks and determines genetic patterns among A. fumigatus isolates (figure 3). Principally two methods of genotyping have emerged for study of medically important fungi: multilocus sequence typing and method bases on short tandem repeats.43 Comparison of several A. fumigatus typing techniques has suggested that methods based on short tandem repeats combine high discriminatory power, unambiguous interpretation and significant interlaboratory reproducibility.44 45 MLVA based on 10 VNTR markers will be tested in this study: it has already permitted to evaluate A. fumigatus dispersion and diversity in the environment.29 The MLVA-10 protocol normally employs singleplex PCRs and agarose gel electrophoresis (AE).29 While traditional AE is relatively cheap, it is also time-consuming. In this study, a multiplex PCR system with multicolour CE for the MLVA-10 panel was designed and provided 97% typeability of isolates, good reproducibility and high discriminatory power. The main advantage of this new MLVA is the high throughput that is facilitated by multiplex PCR and CE.
Figure 3.
Flow chart of protocol study.
The present study evaluates the antifungal susceptibility pattern of environmental and clinical isolates collected. The past decade has seen worldwide reports of azole drug resistance, with prevalence depending on country.11 46–48 Recently, Choukri et al 12 observed that the overall prevalence of azole resistance in France was about 1.8% in unselected clinical A. fumigatus isolates. Resistance has also been encountered in the environment, but possible linkage between clinical resistance and environmental usage of azole fungicides is still unclear.49 50 Environmental acquisition and dispersion of resistance have been reinforced by IA attributed to azole-resistant A. fumigatus isolates in patients who have never ever been subjected to azole therapy.51 Determination of the prevalence of azole resistance may contribute to better management of IA cases.
Azole resistance and genetic patterns can provide experimental data to evaluate correlations between clinical and environmental isolates and clearly understand A. fumigatus dispersion during large demolition programme.
This work had some limitations. Adjustment of MP could change the data analysis and conclusions. As IA incubation time is unknown or variable, ranging from a few days to 3 months, its diagnosis may involve some bias.52–56 Precise definition by EORTC/MSG was respected to identify IA cases and reduce misclassification.25 This protocol could not be implemented during a baseline period before construction work began. Microbiological identification will focus only on Aspergillus spp because it is the leading pathogen responsible for IA.3 To improve study outcomes, data on airborne fungal colonisation in hospital need to be analysed in association with indoor air-handling systems. Although two air sampling collectors were used in the study, no particle counter was tested. Klassen et al have underlined the existence of possible genetic differentiation and variable recombination rates of A. fumigatus which could prevent correct analysis of genotyping result.57
A combined approach could deliver meaningful conclusions on Aspergillus spp dispersion and hopefully give new insights into improvement of environmental monitoring and of hospital guidelines during major demolition work. This study fits in with larger, ongoing exposome research based on increased knowledge of connections between environmental exposure and health.58
Supplementary Material
Acknowledgments
The authors thank all hospital department chiefs for study participation. They also thank the EA 7380 Dynamyc Team from Université Paris Est, France, the Mycology and Parasitology Institute of Lyon and Analyzair Society, Lyon, France, for providing invaluable advice. PhD salary support of the first author by Laboratoires ANIOS is acknowledged.
Footnotes
Contributors: STL, EM, CD, LH and TB acquired the original data for this study. STL, EM, CD, TB, JG, FB, M-PG and PV formulated study methodology. STL, EM, M-PG and PV designed the protocol. CD, TB, PC, DD and MW helped with manuscript writing and language review. All authors contributed to revisions and approved the final manuscript version.
Funding: This study was supported by Laboratoires ANIOS and the Department of Environment and Public Health, Faculty of Medicine, Université de Lyon, France, and Edouard Herriot Hospital, Lyon, France. The funder provided support in the form of salaries for the author STL but did not have any additional role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interests: All authors approved the final version of the manuscript and vouch that it is not being considered for publication elsewhere.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Denning DW. Invasive aspergillosis. Clin Infect Dis 1998;26:781–803. 10.1086/513943 [DOI] [PubMed] [Google Scholar]
- 2. Marr KA, Patterson T, Denning D. Aspergillosis. Pathogenesis, clinical manifestations, and therapy. Infect Dis Clin North Am 2002;16:875–94. [DOI] [PubMed] [Google Scholar]
- 3. Garcia-Vidal C, Upton A, Kirby KA, et al. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis 2008;47:1041–50. 10.1086/591969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nicolle MC, Bénet T, Thiebaut A, et al. Invasive aspergillosis in patients with hematologic malignancies: incidence and description of 127 cases enrolled in a single institution prospective survey from 2004 to 2009. Haematologica 2011;96:1685–91. 10.3324/haematol.2011.044636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hadrich I, Makni F, Sellami H, et al. Invasive aspergillosis: epidemiology and environmental study in haematology patients (Sfax, Tunisia). Mycoses 2010;53:443–7. 10.1111/j.1439-0507.2009.01710.x [DOI] [PubMed] [Google Scholar]
- 6. López-Medrano F, Silva JT, Fernández-Ruiz M, et al. Risk Factors associated with early invasive pulmonary aspergillosis in kidney transplant recipients: results from a multinational matched case-control study. Am J Transplant 2016;16:2148–57. 10.1111/ajt.13735 [DOI] [PubMed] [Google Scholar]
- 7. Lortholary O, Gangneux JP, Sitbon K, et al. Epidemiological trends in invasive aspergillosis in France: the SAIF network (2005-2007). Clin Microbiol Infect 2011;17:1882–9. 10.1111/j.1469-0691.2011.03548.x [DOI] [PubMed] [Google Scholar]
- 8. Fuhren J, Voskuil WS, Boel CH, et al. High prevalence of azole resistance in Aspergillus fumigatus isolates from high-risk patients. J Antimicrob Chemother 2015;70:2894–8. 10.1093/jac/dkv177 [DOI] [PubMed] [Google Scholar]
- 9. Chowdhary A, Kathuria S, Xu J, et al. Emergence of azole-resistant aspergillus fumigatus strains due to agricultural azole use creates an increasing threat to human health. PLoS Pathog 2013;9:e1003633 10.1371/journal.ppat.1003633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vermeulen E, Lagrou K, Verweij PE. Azole resistance in Aspergillus fumigatus: a growing public health concern. Curr Opin Infect Dis 2013;26:493–500. 10.1097/QCO.0000000000000005 [DOI] [PubMed] [Google Scholar]
- 11. Lavergne RA, Morio F, Favennec L, et al. First description of azole-resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation in France. Antimicrob Agents Chemother 2015;59:4331–5. 10.1128/AAC.00127-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choukri F, Botterel F, Sitterlé E, et al. Prospective evaluation of azole resistance in Aspergillus fumigatus clinical isolates in France. Med Mycol 2015;53:593–6. 10.1093/mmy/myv029 [DOI] [PubMed] [Google Scholar]
- 13. O’Gorman CM. Airborne Aspergillus fumigatus conidia: a risk factor for aspergillosis. Fungal Biol Rev 2011;25:151–7. 10.1016/j.fbr.2011.07.002 [DOI] [Google Scholar]
- 14. Gangneux JP, Adjidé CC, Bernard L, et al. [Quantitative assessment of fungal risk in the case of construction works in healthcare establishments: Proposed indicators for the determination of the impact of management precautions on the risk of fungal infection]. J Mycol Med 2012;22:64–71. 10.1016/j.mycmed.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 15. Kanamori H, Rutala WA, Sickbert-Bennett EE, et al. Review of fungal outbreaks and infection prevention in healthcare settings during construction and renovation. Clin Infect Dis 2015;61:433–44. 10.1093/cid/civ297 [DOI] [PubMed] [Google Scholar]
- 16. Perraud M, Piens MA, Nicoloyannis N, et al. Invasive nosocomial pulmonary aspergillosis: risk factors and hospital building works. Epidemiol Infect 1987;99:407–12. 10.1017/S0950268800067893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bénet T, Nicolle MC, Thiebaut A, et al. Reduction of invasive aspergillosis incidence among immunocompromised patients after control of environmental exposure. Clin Infect Dis 2007;45:682–6. 10.1086/521378 [DOI] [PubMed] [Google Scholar]
- 18. Méheust D, Le Cann P, Reboux G, et al. Indoor fungal contamination: health risks and measurement methods in hospitals, homes and workplaces. Crit Rev Microbiol 2014;40:248–60. 10.3109/1040841X.2013.777687 [DOI] [PubMed] [Google Scholar]
- 19. Reboux G, Gbaguidi-Haore H, Bellanger AP, et al. A 10-year survey of fungal aerocontamination in hospital corridors: a reliable sentinel to predict fungal exposure risk? J Hosp Infect 2014;87:34–40. 10.1016/j.jhin.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 20. Fournel I, Sautour M, Lafon I, et al. Airborne Aspergillus contamination during hospital construction works: efficacy of protective measures. Am J Infect Control 2010;38:189–94. 10.1016/j.ajic.2009.07.011 [DOI] [PubMed] [Google Scholar]
- 21. Dananché C, Gustin MP, Cassier P, et al. Evaluation of hirst-type spore trap to monitor environmental fungal load in hospital. PLoS One 2017;12:e0177263 10.1371/journal.pone.0177263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gangneux JP, Bousseau A, Cornillet A, et al. Control of fungal environmental risk in French Hospitals. Journal de Mycologie Médicale 2006;16:204–11. [Google Scholar]
- 23. Méheust D, Gangneux JP, Cann PL. Comparative evaluation of three impactor samplers for measuring airborne bacteria and fungi concentrations. J Occup Environ Hyg 2013;10:455–9. 10.1080/15459624.2013.800955 [DOI] [PubMed] [Google Scholar]
- 24. Boff C, Brun CP, Miron D, et al. Technical note: the effect of different incubation temperatures on the recovery of Aspergillus species from hospital air. Am J Infect Control 2012;40:1016–7. 10.1016/j.ajic.2012.01.029 [DOI] [PubMed] [Google Scholar]
- 25. De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin Infect Dis 2008;46:1813–21. 10.1086/588660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis 2005;5:609–22. 10.1016/S1473-3099(05)70238-3 [DOI] [PubMed] [Google Scholar]
- 27. Nicolle MC, Bénet T, Vanhems P. Nosocomial or community-acquired? Med Mycol 2011;49:24–9. [DOI] [PubMed] [Google Scholar]
- 28. Bénet T, Voirin N, Nicolle MC, et al. Estimation of the incubation period of invasive aspergillosis by survival models in acute myeloid leukemia patients. Med Mycol 2013;51:214–8. 10.3109/13693786.2012.687462 [DOI] [PubMed] [Google Scholar]
- 29. Thierry S, Wang D, Arné P, et al. Multiple-locus variable-number tandem repeat analysis for molecular typing of Aspergillus fumigatus . BMC Microbiol 2010;10:315 10.1186/1471-2180-10-315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Francisco AP, Vaz C, Monteiro PT, et al. PHYLOViZ: phylogenetic inference and data visualization for sequence based typing methods. BMC Bioinformatics 2012;13:87 10.1186/1471-2105-13-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Espinel-Ingroff A, Arendrup M, Cantón E, et al. Multicenter study of method-dependent epidemiological cutoff values for detection of resistance in Candida spp. and Aspergillus spp. to Amphotericin B and echinocandins for the Etest Agar diffusion method. Antimicrob Agents Chemother 2017;61:e01792-16–16. 10.1128/AAC.01792-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Risque infectieux fongique et travaux en établissements de santé. Identification du risque et mise en place de mesures de gestion. Hygiène 2009;19:1–53. [Google Scholar]
- 33. Fernández-Rodríguez S, Tormo-Molina R, Maya-Manzano JM, et al. Outdoor airborne fungi captured by viable and non-viable methods. Fungal Ecol 2014;7:16–26. 10.1016/j.funeco.2013.11.004 [DOI] [Google Scholar]
- 34. Grinn-Gofroń A. Airborne Aspergillus and Penicillium in the atmosphere of Szczecin, (Poland) (2004-2009). Aerobiologia 2011;27:67–76. 10.1007/s10453-010-9177-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fernández-Rodríguez S, Tormo-Molina R, Maya-Manzano JM, et al. Outdoor airborne fungi captured by viable and non-viable methods. Fungal Ecol 2014;7:16–26. 10.1016/j.funeco.2013.11.004 [DOI] [Google Scholar]
- 36. Fernández Rodríguez S, Tormo Molina R, Silva Palacios I, et al. Two sampling methods for the Petri dish detection of airborne fungi. Grana 2011;50:202–7. 10.1080/00173134.2011.596218 [DOI] [Google Scholar]
- 37. Cavallo M, Andreoni S, Martinotti MG, et al. Monitoring environmental Aspergillus spp. contamination and meteorological factors in a haematological unit. Mycopathologia 2013;176:387–94. 10.1007/s11046-013-9712-6 [DOI] [PubMed] [Google Scholar]
- 38. Mahieu LM, De Dooy JJ, Van Laer FA, et al. A prospective study on factors influencing aspergillus spore load in the air during renovation works in a neonatal intensive care unit. J Hosp Infect 2000;45:191–7. 10.1053/jhin.2000.0773 [DOI] [PubMed] [Google Scholar]
- 39. Bart-Delabesse E, Cordonnier C, Bretagne S. Usefulness of genotyping with microsatellite markers to investigate hospital-acquired invasive aspergillosis. J Hosp Infect 1999;42:321–7. 10.1053/jhin.1998.0590 [DOI] [PubMed] [Google Scholar]
- 40. Menotti J, Waller J, Meunier O, et al. Epidemiological study of invasive pulmonary aspergillosis in a haematology unit by molecular typing of environmental and patient isolates of Aspergillus fumigatus . J Hosp Infect 2005;60:61–8. 10.1016/j.jhin.2004.10.009 [DOI] [PubMed] [Google Scholar]
- 41. Guinea J, García de Viedma D, Peláez T, et al. Molecular epidemiology of Aspergillus fumigatus: an in-depth genotypic analysis of isolates involved in an outbreak of invasive aspergillosis. J Clin Microbiol 2011;49:3498–503. 10.1128/JCM.01159-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peláez T, Muñoz P, Guinea J, et al. Outbreak of invasive aspergillosis after major heart surgery caused by spores in the air of the intensive care unit. Clin Infect Dis 2012;54:e24–e31. 10.1093/cid/cir771 [DOI] [PubMed] [Google Scholar]
- 43. Alanio A, Bretagne S. Challenges in microbiological diagnosis of invasive Aspergillus infections. F1000Res 2017;6:157 10.12688/f1000research.10216.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de Valk HA, Meis JF, Klaassen CH. Microsatellite based typing of Aspergillus fumigatus: strengths, pitfalls and solutions. J Microbiol Methods 2007;69:268–72. 10.1016/j.mimet.2007.01.009 [DOI] [PubMed] [Google Scholar]
- 45. de Valk HA, Meis JF, Bretagne S, et al. Interlaboratory reproducibility of a microsatellite-based typing assay for Aspergillus fumigatus through the use of allelic ladders: proof of concept. Clin Microbiol Infect 2009;15:180–7. 10.1111/j.1469-0691.2008.02656.x [DOI] [PubMed] [Google Scholar]
- 46. van der Linden JW, Snelders E, Kampinga GA, et al. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007-2009. Emerg Infect Dis 2011;17:1846–54. 10.3201/eid1710.110226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lelièvre L, Groh M, Angebault C, et al. Azole resistant Aspergillus fumigatus: an emerging problem. Med Mal Infect 2013;43:139–45. 10.1016/j.medmal.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 48. Vermeulen E, Maertens J, Schoemans H, et al. Azole-resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation emerging in Belgium, July 2012. Euro Surveill 2012;17:pii: 20326. [PubMed] [Google Scholar]
- 49. Snelders E, Huis In ’t Veld RA, Rijs AJ, et al. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl Environ Microbiol 2009;75:4053–7. 10.1128/AEM.00231-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kano R, Sobukawa H, Murayama SY, et al. In vitro resistance of Aspergillus fumigatus to azole farm fungicide. J Infect Chemother 2016;22:133–6. 10.1016/j.jiac.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 51. van der Linden JW, Camps SM, Kampinga GA, et al. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin Infect Dis 2013;57:513–20. 10.1093/cid/cit320 [DOI] [PubMed] [Google Scholar]
- 52. Balloy V, Huerre M, Latgé JP, et al. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infect Immun 2005;73:494–503. 10.1128/IAI.73.1.494-503.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gregg KS, Kauffman CA. Invasive Aspergillosis: Epidemiology, Clinical Aspects, and Treatment. Semin Respir Crit Care Med 2015;36:662–72. 10.1055/s-0035-1562893 [DOI] [PubMed] [Google Scholar]
- 54. Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax 2015;70:270–7. 10.1136/thoraxjnl-2014-206291 [DOI] [PubMed] [Google Scholar]
- 55. Meersseman W, Vandecasteele SJ, Wilmer A, et al. Invasive aspergillosis in critically ill patients without malignancy. Am J Respir Crit Care Med 2004;170:621–5. 10.1164/rccm.200401-093OC [DOI] [PubMed] [Google Scholar]
- 56. American Academy of Pediatrics. Committee on Infectious Diseases. Red Book. 28th edition Elk Grove Village: IL, 2009. [Google Scholar]
- 57. Klaassen CH, Gibbons JG, Fedorova ND, et al. Evidence for genetic differentiation and variable recombination rates among Dutch populations of the opportunistic human pathogen Aspergillus fumigatus . Mol Ecol 2012;21:57–70. 10.1111/j.1365-294X.2011.05364.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Escher BI, Hackermüller J, Polte T, et al. From the exposome to mechanistic understanding of chemical-induced adverse effects. Environ Int 2017;99:97–106. 10.1016/j.envint.2016.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.