Abstract
Objectives
To explore potential associations between vitamin D status and risk of chronic low back pain (LBP) in a Norwegian cohort, and to investigate whether relationships depend on the season of blood sample collection.
Design
A nested case–control study in a prospective data set.
Setting
The Norwegian community-based Nord-Trøndelag Health Study (HUNT). Data were collected in the HUNT2 (1995–1997) and HUNT3 (2006–2008) surveys.
Main outcome measure
Chronic LBP, defined as LBP persisting at least 3 months continuously during the past year.
Participants
Among individuals aged 19–55 years without LBP in HUNT2, a data set was generated including 1685 cases with LBP in HUNT3 and 3137 controls without LBP.
Methods
Blood samples from the participants collected in HUNT2 were analysed for serum 25-hydroxyvitamin D (25(OH)D) level. Associations with LBP in HUNT3 were evaluated by unconditional logistic regression analysis with adjustment for age, sex, work status, physical activity at work and in leisure time, education, smoking, and body mass index.
Results
No association between vitamin D status and risk of chronic LBP was found in the total data set (OR per 10 nmol/L 25(OH)D=1.01, 95% CI 0.97 to 1.06) or in individuals with blood samples collected in summer/autumn (OR per 10 nmol/L 25(OH)D=0.99, 95% CI 0.93 to 1.06). For blood samples drawn in winter/spring, associations differed significantly between women and men (p=0.004). Among women a positive association was seen (OR per 10 nmol/L 25(OH)D=1.11, 95% CI 1.02 to 1.20), but among men no significant association was observed (OR per 10 nmol/L 25(OH)D=0.90, 95% CI 0.81 to 1.01).
Conclusions
Overall, no association between vitamin D status and risk of LBP was demonstrated. The association suggested in women for the winter/spring season cannot be regarded as established.
Keywords: epidemiology, vitamin D and low back, back pain
Strengths and limitations of this study.
The study is prospective and the medical condition considered, chronic low back pain at end of follow-up, cannot have influenced vitamin D status at baseline.
Season of blood sample collection for analysis of serum 25-hydroxyvitamin D is taken into account in the analysis.
Vitamin D and back pain status were not registered in the intermediate period between baseline and end of follow-up.
The mean length of time between blood sample collection and assessment of final back pain status was 11 years, and individual vitamin D status may have changed considerably in the meantime.
Introduction
Low back pain (LBP) is one of the most common musculoskeletal disorders and often leads to sick leave and a high degree of disability with substantial costs for society.1 The causes of non-specific LBP are not sufficiently understood.2
Vitamin D is required for absorption of calcium from the intestines, and vitamin D has shown positive health effects on the muscle and skeletal system.3 Skeletal muscles have vitamin D receptors and may require vitamin D for maximum function.4 Associations between vitamin D deficiency and incidence of chronic pain have been suggested in various studies, but the evidence is not conclusive.5 Some studies have found associations between vitamin D deficiency and occurrence of non-specific musculoskeletal pain in patient materials6 and in population-based data sets.7 A meta-analysis8 concluded that vitamin D supplementation can decrease pain scores in chronic widespread pain.
Very few population-based studies have been carried out regarding associations between vitamin D status and occurrence of back pain,9 in particular with a prospective design. Studies of associations between vitamin D status and back pain have mostly been based on relatively small data sets involving patients, with some studies10–14 suggesting an association between low vitamin D levels and back pain, while other studies15–17 were unable to demonstrate any relationships. A cross-sectional study among schoolchildren also indicated such an association.18 A small randomised clinical trial of patients with chronic LBP failed to show any effect of vitamin D supplementation.19
Potential associations between vitamin D deficiency and LBP have partly been ascribed to osteomalacia,12 with an accumulation of osteoid because of defective mineralisation. Poor muscle strength induced by vitamin D deficiency3 may also affect the experience of LBP. It is not certain, however, whether these factors play any role considering the incidence of LBP at the population level.
Only prospective studies can show whether vitamin D levels affect the subsequent risk of experiencing LBP. It is possible that back pain conversely can affect vitamin D status,11 perhaps through modified behaviour influencing exposure to sunlight or through nutritional factors, and for this reason it is essential to base conclusions on results from prospective studies. It is important to carry out adjustment for potential confounders such as obesity, which is related to both vitamin D levels and to risk of LBP.20–22 Vitamin D levels are higher after sun exposure, with the prevalence of vitamin D deficiency varying seasonally,23 so associations should also be assessed separately for the summer/autumn and winter/spring seasons.
This study will investigate whether an association can be established between vitamin D status and risk of LBP in a case–control study nested in a Norwegian cohort. Vitamin D status is based on measurement of the major circulating metabolite 25-hydroxyvitamin D (25(OH)D).24 The importance of seasonality for blood sample collection for vitamin D measurement will be explored.
Methods
Participants
The present work is based on information from the Nord-Trøndelag Health Study (HUNT). From 1995 to 1997, the large health survey HUNT2 was conducted in Nord-Trøndelag county in Norway. The entire adult population received a health questionnaire and participants underwent a clinical examination, including measurements of body weight and height.25 In particular, each participant provided information on the questionnaire showing whether he or she had experienced chronic LBP during the preceding 12-month period, defined as LBP lasting at least 3 months continuously in that period. Blood samples were drawn at the clinical examination. In the HUNT3 survey, conducted in 2006–2008 in the same county with a corresponding target population, similar questionnaires were distributed.25 Information about residence status was supplied by national registries and linked by use of the unique Norwegian personal identification numbers.
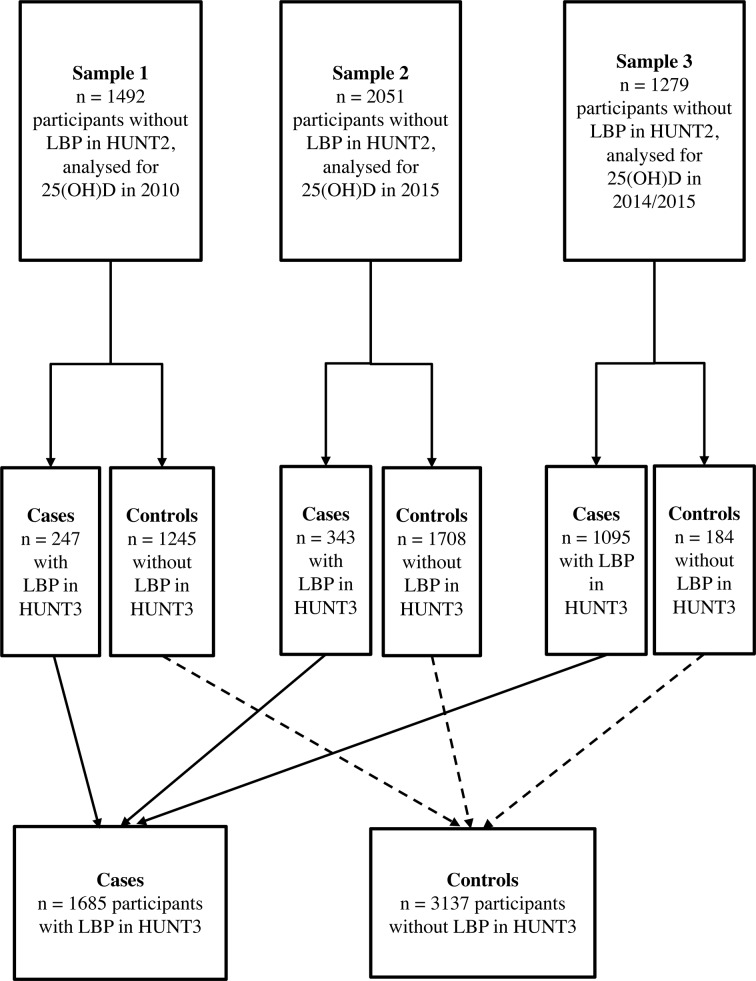
A nested case–control study was conducted using prospective data from HUNT2 regarded as baseline and HUNT3 regarded as follow-up, including 4822 individuals in the age range 19–55 years when attending HUNT2. Vitamin D status was assessed on the basis of blood samples from the participants collected in HUNT2 in 1995–1997, but the actual measurements were only carried out later. Participants belonged to three different subsamples. The first and second subsamples consisted of random samples from HUNT2 and were established in connection with previous studies of the association between vitamin D and asthma and lung function,26 27 but in the current study all individuals with LBP in HUNT2 were excluded. The first subsample was analysed for 25(OH)D in 2010, and included altogether 1492 persons (figure 1), comprising 247 cases (individuals with LBP in HUNT3) and 1245 controls (individuals without LBP in HUNT3). The second subsample with analysis date for 25(OH)D in 2015 included a total of 2051 persons (figure 1), comprising 343 cases and 1708 controls. The third subsample had measurements of 25(OH)D carried out in 2014/2015 and consisted of 1279 persons without LBP in HUNT2. Of these, a total of 1095 cases were selected at random among persons suffering from LBP in HUNT3 and 184 controls were randomly selected among persons without LBP in HUNT3 (figure 1). The third subsample was solely established for the current nested case–control study. None of the individuals included in this study had missing values for any covariate considered.
Figure 1.
Flow chart showing participants for statistical analysis in the nested case–control study. 25(OH)D, 25-hydroxyvitamin D; HUNT, Nord-Trøndelag Health Study; LBP, low back pain.
The original plan was that the nested case–control study would consist of the combined first and third subsamples only, and the size of the third subsample was adapted to power requirements in this data set. The second subsample only became available at a later stage.
Exposure
Blood samples collected in HUNT2 were stored at −70°C until analysis. The level of 25(OH)D in serum was measured in the years 2010 and in 2014/2015 by chemiluminescent immunoassay methodology, using Liaison 25-OH Vitamin D Total Assay (DiaSorin, Saluggia, Italy). The method has an intra-assay coefficient of variation of 4% and an interassay coefficient of variation of 8%. Another kit from DiaSorin was used from 2014. A total of 118 samples with measurements available from 2010 were reanalysed in 2015 with the new kit. After exclusion of two outliers, a conversion factor for measurements from 2010 to new values from 2014/2015 was established by Passing and Bablok regression.28
Serum 25(OH)D levels were classified into three groups, <50.0 nmol/L, 50.0–74.9 nmol/L and ≥75.0 nmol/L, which are widely used categories in studies of vitamin D levels. Values <50.0 nmol/L are usually regarded as representing vitamin D deficiency.29
Covariate assessment
Baseline age was categorised into 10-year intervals. Four categories of work status were defined, the first comprising people being employed or carrying out professional work. The second category included those temporarily out of work, students and individuals in military service. The third category included pensioners and people receiving social security support, and the fourth category represented women occupied full time with housework. Those currently working supplied information about physical activity at work,30 in four categories representing substantially sedentary work, work involving extensive walking, work leading to both walking and lifting, and work involving particularly strenuous activities. For physical activity in leisure time, including going to work, one category included those engaged in light activity only or hard physical activity (leading to sweating or being out of breath) <1 hour per week.31 Other categories represented hard physical activity 1–2 and ≥3 hours per week.
Education was grouped according to duration as ≤9, 10–12 or ≥13 years. Categories of cigarette smoking represented current daily smoking, previous daily smoking and never daily smoking. Body mass index (BMI), defined as weight/height2 and computed in kg/m2, was subdivided into three groups: <25, 25–29.9 and ≥30.
Season of blood sample collection was categorised as either the summer/autumn season (June through November) or the winter/spring season (December through May). This subdivision gives a marked difference in vitamin D deficiency between 6 months’ periods.23
Statistical methods
Associations between 25(OH)D levels in serum and risk of chronic LBP were assessed by unconditional logistic regression analysis with adjustment for potential confounders. Analyses were performed separately with 25(OH)D levels as categorical and continuous variables. All other variables were considered categorical. First, adjustments were carried out for age and sex and a factor indicating which subsample each individual belonged to. Additional adjustments for work status, physical activity at work and in leisure time, education, smoking, BMI, and season of blood sample collection were then introduced. Separate tests were performed for interaction between continuous 25(OH)D level and all variables adjusted for. Linearity in the association with continuous 25(OH)D level was tested for by adding a quadratic term to the statistical model. All statistical analyses were carried out using IBM SPSS V.23.
Separate analyses were limited to participants who had the blood samples drawn in the summer/autumn season and the winter/spring season.
Results
The age distribution was quite similar among cases and controls (table 1). The percentage of women was higher among cases than among controls. Within the three separate groups of serum 25(OH)D levels categorised as <50.0 nmol/L, 50.0–74.9 nmol/L and ≥75.0 nmol/L, the percentage of persons with and without LBP at end of follow-up was almost the same among cases and controls (table 1).
Table 1.
Number of individuals by age, sex, vitamin D status and case–control status
| Cases, with LBP at end of follow-up | Controls, without LBP at end of follow-up | |||
| n | Per cent | n | Per cent | |
| Age groups (years) | ||||
| 19–29 | 246 | 15 | 504 | 16 |
| 30–39 | 492 | 29 | 951 | 30 |
| 40–49 | 687 | 41 | 1191 | 38 |
| 50–55 | 260 | 15 | 491 | 16 |
| Sex | ||||
| Female | 1043 | 62 | 1677 | 54 |
| Male | 642 | 38 | 1460 | 47 |
| 25(OH)D (nmol/L) | ||||
| <50.0 | 925 | 55 | 1689 | 54 |
| 50.0–74.9 | 619 | 37 | 1126 | 36 |
| ≥75.0 | 141 | 8 | 322 | 10 |
25(OH)D, 25-hydroxyvitamin D; LBP, low back pain.
In the total data set, the mean serum 25(OH)D levels were quite similar among cases and controls (table 2). Both in women and men the serum 25(OH)D levels were higher in the summer/autumn than in the winter/spring season among cases and controls.
Table 2.
Descriptive statistics of 25(OH)D level by case–control status
| Cases, with LBP at end of follow-up | Controls, without LBP at end of follow-up | |||||||
| n | Mean (nmol/L) | SD (nmol/L) | Median (nmol/L) | n | Mean (nmol/L) | SD (nmol/L) | Median (nmol/L) | |
| Total data set | ||||||||
| All seasons | 1685 | 49.4 | 18.2 | 47.9 | 3137 | 50.0 | 19.1 | 48.0 |
| Summer/autumn | 773 | 54.1 | 18.4 | 52.0 | 1424 | 56.7 | 19.0 | 55.2 |
| Winter/spring | 912 | 45.4 | 17.0 | 43.8 | 1713 | 44.4 | 17.4 | 41.9 |
| Women | ||||||||
| All seasons | 1043 | 50.2 | 18.6 | 48.9 | 1677 | 50.5 | 19.0 | 49.0 |
| Summer/autumn | 480 | 53.8 | 18.7 | 52.9 | 784 | 56.2 | 18.7 | 54.4 |
| Winter/spring | 563 | 47.1 | 17.9 | 45.9 | 893 | 45.4 | 17.9 | 43.0 |
| Men | ||||||||
| All seasons | 642 | 48.0 | 17.4 | 45.8 | 1460 | 49.4 | 19.3 | 46.6 |
| Summer/autumn | 293 | 54.5 | 18.0 | 51.3 | 640 | 57.4 | 19.4 | 56.1 |
| Winter/spring | 349 | 42.6 | 14.9 | 40.8 | 820 | 43.2 | 16.7 | 40.4 |
25(OH)D, 25-hydroxyvitamin D; LBP, low back pain.
In the overall data set no association was found between vitamin D status and risk of LBP, neither with adjustment for age, sex and subsample nor with complete adjustment (OR per 10 nmol/L 25(OH)D=1.01, 95% CI 0.97 to 1.06) (table 3). No significant interaction was found, but the interaction with sex was marginally significant (p=0.06). A weak positive association was suggested among women, although the estimated relation among men was in the opposite direction (table 3). Results did not differ significantly between subsamples.
Table 3.
Associations between vitamin D status and risk of chronic LBP
| With adjustment for age, sex and subsample | With complete adjustment* | |||
| OR (95% CI) | p | OR (95% CI) | p | |
| Total data set | ||||
| 25(OH)D (nmol/L) | 0.64† | 0.97† | ||
| <50.0 | 1.00 (Reference) | 1.00 (Reference) | ||
| 50.0–74.9 | 0.94 (0.80 to 1.11) | 1.01 (0.85 to 1.20) | ||
| ≥75.0 | 0.90 (0.68 to 1.18) | 0.98 (0.73 to 1.31) | ||
| Per 10 nmol/L | 0.99 (0.95 to 1.03) | 0.59 | 1.01 (0.97 to 1.06) | 0.59 |
| Women | ||||
| 25(OH)D (nmol/L) | 0.54† | 0.27† | ||
| <50.0 | 1.00 (Reference) | 1.00 (Reference) | ||
| 50.0–74.9 | 1.09 (0.88 to 1.36) | 1.20 (0.96 to 1.51) | ||
| ≥75.0 | 0.91 (0.64 to 1.30) | 1.05 (0.72 to 1.52) | ||
| Per 10 nmol/L | 1.02 (0.97 to 1.08) | 0.48 | 1.06 (1.00 to 1.12) | 0.054 |
| Men | ||||
| 25(OH)D (nmol/L) | 0.10† | 0.16† | ||
| <50.0 | 1.00 (Reference) | 1.00 (Reference) | ||
| 50.0–74.9 | 0.75 (0.57 to 0.98) | 0.76 (0.57 to 1.01) | ||
| ≥75.0 | 0.90 (0.58 to 1.39) | 0.88 (0.55 to 1.41) | ||
| Per 10 nmol/L | 0.94 (0.88 to 1.01) | 0.10 | 0.94 (0.88 to 1.02) | 0.13 |
*Adjustment for age, sex, work status, physical activity at work and in leisure time, education, smoking, body mass index, subsample, and season of blood sample collection.
†For categorical effect.
25(OH)D, 25-hydroxyvitamin D; LBP, low back pain.
Separate analyses of the association between vitamin D status and risk of LBP by season of blood sample collection revealed no effect in either sex in the summer/autumn season (table 4). However, in the winter/spring season, associations differed significantly between women and men (p=0.004). In women a significant positive association was observed (OR per 10 nmol/L 25(OH)D=1.11, 95% CI 1.02 to 1.20) (table 4). In men the estimated association was negative (OR per 10 nmol/L 25(OH)D=0.90, 95% CI 0.81 to 1.01) but did not reach statistical significance. Considering the different OR values from the categorical analyses, a consistent trend was suggested in each sex.
Table 4.
Associations between vitamin D status and risk of chronic LBP by season of blood sample collection*
| Summer/autumn | Winter/spring | |||
| OR (95% CI) | p | OR (95% CI) | p | |
| Total data set | ||||
| 25(OH)D (nmol/L) | 0.87† | 0.79† | ||
| <50.0 | 1.00 (Reference) | 1.00 (Reference) | ||
| 50.0–74.9 | 0.93 (0.72 to 1.20) | 1.08 (0.85 to 1.38) | ||
| ≥75.0 | 0.96 (0.66 to 1.39) | 0.96 (0.58 to 1.58) | ||
| Per 10 nmol/L | 0.99 (0.93 to 1.06) | 0.78 | 1.03 (0.97 to 1.10) | 0.37 |
| Women | ||||
| 25(OH)D (nmol/L) | 0.56† | 0.27† | ||
| <50.0 | 1.00 (Reference) | 1.00 (Reference) | ||
| 50.0–74.9 | 1.13 (0.81 to 1.57) | 1.26 (0.92 to 1.73) | ||
| ≥75.0 | 0.89 (0.53 to 1.47) | 1.34 (0.76 to 2.35) | ||
| Per 10 nmol/L | 1.00 (0.92 to 1.09) | 0.94 | 1.11 (1.02 to 1.20) | 0.012 |
| Men | ||||
| 25(OH)D (nmol/L) | 0.15† | 0.20† | ||
| <50.0 | 1.00 (Reference) | 1.00 (Reference) | ||
| 50.0–74.9 | 0.70 (0.46 to 1.05) | 0.84 (0.56 to 1.27) | ||
| ≥75.0 | 1.01 (0.58 to 1.76) | 0.37 (0.12 to 1.17) | ||
| Per 10 nmol/L | 0.97 (0.88 to 1.08) | 0.58 | 0.90 (0.81 to 1.01) | 0.08 |
*Adjustment for age, sex, work status, physical activity at work and in leisure time, education, smoking, body mass index, and subsample.
†For categorical effect.
25(OH)D, 25-hydroxyvitamin D; LBP, low back pain.
No significant deviations from linearity were observed in the relationships with 25(OH)D considered as a continuous variable.
Discussion
The association between vitamin D status and chronic LBP was examined in a case–control study nested in a population-based follow-up of a Norwegian cohort. No association between vitamin D status and risk of LBP was found overall. For measurements in the winter/spring season, associations differed significantly between women and men. Among women a positive association was seen, but among men no significant association was observed.
A strength of the study is that the overwhelming majority of participants belonged to a homogeneous ethnic group.32 Information was available on potential confounders, which made it possible to carry out accurate adjustments. The risk factor considered is represented by the vitamin D status measured in blood samples drawn at the clinical examination in HUNT2. However, the blood samples were stored for many years at low temperature, and the measurements of serum 25(OH)D levels were carried out in 2010 or in 2014/2015. Despite attempts to standardise the measurements, use of different instruments in the two periods may have led to minor systematic deviations in the 25(OH)D levels. To some extent, this was accounted for by adjusting all statistical analyses for subsample. This procedure was also essential in view of the different sampling procedures applied to establish the subsamples. The Liaison immunoassay method may still underestimate true 25(OH)D levels,33 and a direct comparison with values found by other methods may not be justified.34
A limitation is the lack of information about back pain occurring at other times in the 11-year follow-up interval between HUNT2 and HUNT3. The case definition only refers to the last year before collection of information in HUNT3. Thus all cases must have experienced incident chronic LBP during follow-up but so may some of the controls in the intervening period if they later recovered. Any real association between vitamin D status and LBP should be present anyhow but may be more difficult to detect. Furthermore, the extent of back pain was not characterised by the participants.
Another potential problem is the relatively long period between collection of information about risk factors and the recording of LBP status. In view of the weaker associations seen between 25(OH)D levels and mortality and cancer risk in prospective studies with longer follow-periods, it has been suggested that 25(OH)D levels should be measured at regular intervals in prospective studies, perhaps every 2–4 years.35 Such information was not available in the present study. However, vitamin D levels have been shown to be relatively stable over periods as long as 14 years in the Norwegian population,36 and large data sets in other countries have shown a definite degree of stability over somewhat shorter periods.37 38 In data with measurements from the HUNT2 survey, low 25(OH)D levels showed a clear association with all-cause mortality in a prospective study with a median follow-up time of 18.5 years.39 Thus potential relationships between 25(OH)D levels and disease should still persist in a study such as the current one with 11 years of follow-up, although associations may be attenuated. The state of relevant confounders may also change during a long follow-up. An alternative to the design used here would be a cross-sectional case–control study based on both 25(OH)D levels and reports of chronic LBP from the HUNT2 survey. Problems concerning 25(OH)D levels changing over time would be eliminated, but it would not be possible to rule out an influence of disease on 25(OH)D status.
There are few population-based studies of the relationships between vitamin D status and LBP.9 Some studies have investigated such relationships among patients,11 13 16 but population-based studies of risk of LBP with a prospective design have been lacking. The present study is to our knowledge the only prospective study of the association between vitamin D status and risk of LBP.
In cross-sectional population-based studies, an association between low level of 25(OH)D and prevalence of back pain has been found in older women9 and in schoolchildren.18 Some case–control studies have shown an association between low levels of vitamin D and occurrence of back pain,12 14 but others do not support an association.15 17 In a recent small Swedish case–control study, no difference in vitamin D levels could be established between participants with chronic LBP and matched controls.17 This is consistent with the overall results found in our study.
Among women with blood samples drawn in the winter/spring season, we found that high levels of vitamin D were associated with an increased risk of LBP, which is the opposite of what was initially hypothesised. In a Danish cross-sectional study of patients with LBP, an association in the same direction was found, and normal levels of vitamin D, as opposed to vitamin D deficiency, were associated with more Modic changes in the lumbar vertebral end plates as seen on MRI.16
An association between vitamin D deficiency and adiposity has been found in several studies.21 This was also confirmed in a cross-sectional study in the HUNT population.23 In a prospective study using data from HUNT2 and HUNT3, overweight and obesity were found to be associated with a predisposition to chronic LBP.22 This suggests that BMI could be a confounder in the association with 25(OH)D. However, our complete analyses included adjustment for BMI, and it is not to be expected that any substantial relationship should remain because of the association with adiposity.
In view of the seasonal variation in sun exposure, it is important to look at the time when blood samples are collected to define vitamin D deficiency.40 Thus in a study of bone mineral density, the summer season was found to be the best period to determine the serum 25(OH)D level.41 This contrasts with our results, with a positive association between LBP and vitamin D status observed in the winter/spring season for women only. Elevated levels of 25(OH)D may be harmful in other respects.42 In a study of mortality among hospitalised patients, a U-shaped relationship was found, with both low and high values of 25(OH)D associated with increased mortality.42 43 However, the 25(OH)D levels had to be considerably above the typical values in the present study to be associated with higher mortality and a causal relationship was not inferred, so such effects at the upper end of the range for 25(OH)D are hardly relevant here. False U-shaped relationships between 25(OH)D levels and health problems may easily appear when individuals with poor health have only recently started using vitamin D supplement and are thus essentially misclassified.44
Gender and sex hormone levels are important variables that can influence a possible effect of vitamin D in rheumatic diseases.45 In a Danish population receiving ultraviolet B treatment, the decline in 25(OH)D varied over time between sexes, with women maintaining a greater half-life of 25(OH)D.46 It is not evident, however, how such differences can explain the sex contrast seen in the present study in the association with risk of LBP.
Measurements of 25(OH)D levels made during the winter/spring season may possibly capture variability of vitamin D in a better way, because vitamin D values in winter are not so dependent on sunlight exposure. It is possible, however, that women tending to report LBP in this population are particularly health-conscious and use more vitamin D supplements during the winter/spring season, creating a false-positive association. Cod liver oil supplement has traditionally represented a major source of vitamin D in Norway,47 with about 35% of both the male and female populations using such supplement in the 1990s.48 Whole-year usage among women is associated with poor perceived health but is otherwise associated with a healthy diet,47 suggesting that use of supplements is not in general matched to the vitamin D needs.47 In any case, the significant relationship observed among women may be spurious due to the multiple statistical tests carried out. Thus this association cannot yet be regarded as causal.
A basic biological relationship between vitamin D status and risk of LBP would be expected to be similar in different populations. Unless the lack of overall association seen in the present study represents a chance finding, the result should also apply to other populations. However, the population distribution of vitamin D status may vary substantially between countries because of differences in sunlight exposure and dietary conditions. Thus it is not obvious that our main result can be generalised to other widely different populations, and further studies are still called for.
Conclusions
In this population-based, nested case–control study, no overall association between vitamin D status and risk of LBP was found. The positive association observed in women with blood samples drawn in the winter/spring season needs confirmation from other studies.
Supplementary Material
Acknowledgments
The Nord-Trøndelag Health Study (the HUNT study) is a collaboration between the HUNT Research Centre, Faculty of Medicine and Health Science, the Norwegian University of Science and Technology (NTNU); Norwegian Institute of Public Health; Central Norway Health Authority; and the Nord-Trøndelag County Council. Laboratory measurements were carried out at facilities owned by the Nord-Trøndelag Hospital Trust.
Footnotes
Contributors: InH, IvH, KH, X-MM, AL and J-AZ contributed to the study design. InH and IvH contributed to analysis and interpretation of data. InH wrote the paper. IvH, KH, X-MM, AL and J-AZ all revised the manuscript. All the authors have read and approved the paper.
Funding: X-MM received funding from the Research Council of Norway for the measurements of vitamin D in the first and second subsamples.
Competing interests: None declared.
Ethics approval: The work was approved by the Regional Committee for Medical and Health Research Ethics in Central Norway, and HUNT was also approved by the Norwegian Data Inspectorate.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The data set analysed belongs to a third party, the HUNT study (the Nord-Trøndelag Health Study). The authors of the current manuscript are not affiliated with the project as such, but have been given permission to analyse the data after obtaining the necessary Norwegian permits. Because of the confidentiality requirements according to Norwegian law, a data set of this kind with information from a complete county at the individual level cannot be made public. However, research groups wishing to analyse data from the HUNT study may apply to the HUNT organisation (http://www.ntnu.edu/hunt) to get access to the data, after having obtained the permits needed according to Norwegian law.
References
- 1. Lambeek LC, Bosmans JE, Van Royen BJ, et al. Effect of integrated care for sick listed patients with chronic low back pain: economic evaluation alongside a randomised controlled trial. BMJ 2010;341:c6414 10.1136/bmj.c6414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet 2017;389:736–47. 10.1016/S0140-6736(16)30970-9 [DOI] [PubMed] [Google Scholar]
- 3. Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- 4. Wintermeyer E, Ihle C, Ehnert S, et al. Crucial role of vitamin D in the musculoskeletal system. Nutrients 2016;8:319 10.3390/nu8060319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Straube S, Moore RA, Derry S, et al. Vitamin D and chronic pain. Pain 2009;141:10–13. 10.1016/j.pain.2008.11.010 [DOI] [PubMed] [Google Scholar]
- 6. Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc 2003;78:1463–70. 10.4065/78.12.1463 [DOI] [PubMed] [Google Scholar]
- 7. McBeth J, Pye SR, O’Neill TW, et al. Musculoskeletal pain is associated with very low levels of vitamin D in men: results from the European Male Ageing Study. Ann Rheum Dis 2010;69:1448–52. 10.1136/ard.2009.116053 [DOI] [PubMed] [Google Scholar]
- 8. Yong WC, Sanguankeo A, Upala S. Effect of vitamin D supplementation in chronic widespread pain: a systematic review and meta-analysis. Clin Rheumatol 2017. (Published Online First: 15 Aug 2017) 10.1007/s10067-017-3754-y [DOI] [PubMed] [Google Scholar]
- 9. Hicks GE, Shardell M, Miller RR, et al. Associations between vitamin D status and pain in older adults: the Invecchiare in Chianti study. J Am Geriatr Soc 2008;56:785–91. 10.1111/j.1532-5415.2008.01644.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al Faraj S, Al Mutairi K. Vitamin D deficiency and chronic low back pain in Saudi Arabia. Spine 2003;28:177–9. 10.1097/00007632-200301150-00015 [DOI] [PubMed] [Google Scholar]
- 11. Kim TH, Lee BH, Lee HM, et al. Prevalence of vitamin D deficiency in patients with lumbar spinal stenosis and its relationship with pain. Pain Physician 2013;16:165–76. [PubMed] [Google Scholar]
- 12. Lotfi A, Abdel-Nasser AM, Hamdy A, et al. Hypovitaminosis D in female patients with chronic low back pain. Clin Rheumatol 2007;26:1895–901. 10.1007/s10067-007-0603-4 [DOI] [PubMed] [Google Scholar]
- 13. Ghai B, Bansal D, Kapil G, et al. High prevalence of hypovitaminosis D in Indian chronic low back patients. Pain Physician 2015;18:E853–62. [PubMed] [Google Scholar]
- 14. Lodh M, Goswami B, Mahajan RD, et al. Assessment of vitamin D status in patients of chronic low back pain of unknown etiology. Indian J Clin Biochem 2015;30:174–9. 10.1007/s12291-014-0435-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heidari B, Shirvani JS, Firouzjahi A, et al. Association between nonspecific skeletal pain and vitamin D deficiency. Int J Rheum Dis 2010;13:340–6. 10.1111/j.1756-185X.2010.01561.x [DOI] [PubMed] [Google Scholar]
- 16. Johansen JV, Manniche C, Kjaer P. Vitamin D levels appear to be normal in Danish patients attending secondary care for low back pain and a weak positive correlation between serum level vitamin D and Modic changes was demonstrated: a cross-sectional cohort study of consecutive patients with non-specific low back pain. BMC Musculoskelet Disord 2013;14:78 10.1186/1471-2474-14-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thörneby A, Nordeman LM, Johanson EH. No association between level of vitamin D and chronic low back pain in Swedish primary care: a cross-sectional case-control study. Scand J Prim Health Care 2016;34:196–204. 10.1080/02813432.2016.1183557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alghadir AH, Gabr SA, Al-Eisa ES. Mechanical factors and vitamin D deficiency in schoolchildren with low back pain: biochemical and cross-sectional survey analysis. J Pain Res 2017;10:855–65. 10.2147/JPR.S124859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sandoughi M, Zakeri Z, Mirhosainee Z, et al. The effect of vitamin D on nonspecific low back pain. Int J Rheum Dis 2015;18:854–8. 10.1111/1756-185X.12172 [DOI] [PubMed] [Google Scholar]
- 20. Mai XM, Chen Y, Camargo CA, et al. Cross-sectional and prospective cohort study of serum 25-hydroxyvitamin D level and obesity in adults: the HUNT study. Am J Epidemiol 2012;175:1029–36. 10.1093/aje/kwr456 [DOI] [PubMed] [Google Scholar]
- 21. Earthman CP, Beckman LM, Masodkar K, et al. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes 2012;36:387–96. 10.1038/ijo.2011.119 [DOI] [PubMed] [Google Scholar]
- 22. Heuch I, Heuch I, Hagen K, et al. Body mass index as a risk factor for developing chronic low back pain: a follow-up in the Nord-Trøndelag Health Study. Spine 2013;38:133–9. 10.1097/BRS.0b013e3182647af2 [DOI] [PubMed] [Google Scholar]
- 23. Larose TL, Chen Y, Camargo CA, et al. Factors associated with vitamin D deficiency in a Norwegian population: the HUNT Study. J Epidemiol Community Health 2014;68:165–70. 10.1136/jech-2013-202587 [DOI] [PubMed] [Google Scholar]
- 24. Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest 2006;116:2062–72. 10.1172/JCI29449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krokstad S, Langhammer A, Hveem K, et al. Cohort Profile: the HUNT Study, Norway. Int J Epidemiol 2013;42:968–77. 10.1093/ije/dys095 [DOI] [PubMed] [Google Scholar]
- 26. Mai XM, Langhammer A, Camargo CA, et al. Serum 25-hydroxyvitamin D levels and incident asthma in adults: the HUNT Study. Am J Epidemiol 2012;176:1169–76. 10.1093/aje/kws235 [DOI] [PubMed] [Google Scholar]
- 27. Larose TL, Langhammer A, Chen Y, et al. Serum 25-hydroxyvitamin D levels and lung function in adults with asthma: the HUNT Study. Eur Respir J 2015;45:1019–26. 10.1183/09031936.00069714 [DOI] [PubMed] [Google Scholar]
- 28. Passing H, Bablok W. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I. J Clin Chem Clin Biochem 1983;21:709–20. 10.1515/cclm.1983.21.11.709 [DOI] [PubMed] [Google Scholar]
- 29. Ross AC, Manson JE, Abrams SA, et al. The 2011 Dietary Reference Intakes for Calcium and Vitamin D: what dietetics practitioners need to know. J Am Diet Assoc 2011;111:524–7. 10.1016/j.jada.2011.01.004 [DOI] [PubMed] [Google Scholar]
- 30. Heuch I, Heuch I, Hagen K, et al. Physical activity level at work and risk of chronic low back pain: A follow-up in the Nord-Trøndelag Health Study. PLoS One 2017;12:e0175086 10.1371/journal.pone.0175086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heuch I, Heuch I, Hagen K, et al. Is there a U-shaped relationship between physical activity in leisure time and risk of chronic low back pain? A follow-up in the HUNT Study. BMC Public Health 2016;16:306 10.1186/s12889-016-2970-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holmen J, Midthjell K, Krüger Ø, et al. The Nord-Trøndelag Health Study 1995–97 (HUNT 2): objectives, contents, methods and participation. Nor Epidemiol 2003;13:19–32. [Google Scholar]
- 33. Schöttker B, Jansen EH, Haug U, et al. Standardization of misleading immunoassay based 25-hydroxyvitamin D levels with liquid chromatography tandem-mass spectrometry in a large cohort study. PLoS One 2012;7:e48774 10.1371/journal.pone.0048774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carter GD, Berry J, Durazo-Arvizu R, et al. Hydroxyvitamin D assays: An historical perspective from DEQAS. J Steroid Biochem Mol Biol 2017. (Published Online First: 20 Jul 2017) 10.1016/j.jsbmb.2017.07.018 [DOI] [PubMed] [Google Scholar]
- 35. Grant WB. Effect of follow-up time on the relation between prediagnostic serum 25-hydroxyvitamin D and all-cause mortality rate. Dermatoendocrinol 2012;4:198–202. 10.4161/derm.20514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jorde R, Sneve M, Hutchinson M, et al. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol 2010;171:903–8. 10.1093/aje/kwq005 [DOI] [PubMed] [Google Scholar]
- 37. Saliba W, Barnett O, Stein N, et al. The longitudinal variability of serum 25(OH)D levels. Eur J Intern Med 2012;23:e106–e111. 10.1016/j.ejim.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 38. Schöttker B, Haug U, Schomburg L, et al. Strong associations of 25-hydroxyvitamin D concentrations with all-cause, cardiovascular, cancer, and respiratory disease mortality in a large cohort study. Am J Clin Nutr 2013;97:782–93. 10.3945/ajcn.112.047712 [DOI] [PubMed] [Google Scholar]
- 39. Sun YQ, Langhammer A, Skorpen F, et al. Serum 25-hydroxyvitamin D level, chronic diseases and all-cause mortality in a population-based prospective cohort: the HUNT Study, Norway. BMJ Open 2017;7:e017256 10.1136/bmjopen-2017-017256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schramm S, Lahner H, Jöckel KH, et al. Impact of season and different vitamin D thresholds on prevalence of vitamin D deficiency in epidemiological cohorts—a note of caution. Endocrine 2017;56:658–66. 10.1007/s12020-017-1292-7 [DOI] [PubMed] [Google Scholar]
- 41. Michaëlsson K, Wolk A, Byberg L, et al. The seasonal importance of serum 25-hydroxyvitamin D for bone mineral density in older women. J Intern Med 2017;281:167–78. 10.1111/joim.12563 [DOI] [PubMed] [Google Scholar]
- 42. Drake MT. Vitamin D and the goldilocks principle: too little, too much, or just right? J Clin Endocrinol Metab 2014;99:1164–6. 10.1210/jc.2014-1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Amrein K, Quraishi SA, Litonjua AA, et al. Evidence for a U-shaped relationship between prehospital vitamin D status and mortality: a cohort study. J Clin Endocrinol Metab 2014;99:1461–9. 10.1210/jc.2013-3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grant WB, Karras SN, Bischoff-Ferrari HA, et al. Do studies reporting ‘U’-shaped serum 25-hydroxyvitamin D-health outcome relationships reflect adverse effects? Dermatoendocrinol 2016;8:e1187349 10.1080/19381980.2016.1187349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vasile M, Corinaldesi C, Antinozzi C, et al. Vitamin D in autoimmune rheumatic diseases: A view inside gender differences. Pharmacol Res 2017;117:228–41. 10.1016/j.phrs.2016.12.038 [DOI] [PubMed] [Google Scholar]
- 46. Datta P, Philipsen PA, Olsen P, et al. The half-life of 25(OH)D after UVB exposure depends on gender and vitamin D receptor polymorphism but mainly on the start level. Photochem Photobiol Sci 2017;16:985–95. 10.1039/C6PP00258G [DOI] [PubMed] [Google Scholar]
- 47. Brustad M, Braaten T, Lund E. Predictors for cod-liver oil supplement use—the Norwegian Women and Cancer Study. Eur J Clin Nutr 2004;58:128–36. 10.1038/sj.ejcn.1601759 [DOI] [PubMed] [Google Scholar]
- 48. Alexander J, Frøyland L, Hemre GI, et al. A comprehensive assessment of fish and other seafood in the Norwegian diet. Oslo: Norwegian Scientific Committee for Food Safety, 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.