Abstract
Anthropogenic increases in global temperature and agricultural runoff are increasing the prevalence of aquatic hypoxia throughout the world. We investigated the potential for a relatively rapid evolution of hypoxia tolerance using two isolated (for less than 11 000 years) populations of threespine stickleback: one from a lake that experiences long-term hypoxia (Alta Lake, British Columbia) and one from a lake that does not (Trout Lake, British Columbia). Loss-of-equilibrium (LOE) experiments revealed that the Alta Lake stickleback were significantly more tolerant of hypoxia than the Trout Lake stickleback, and calorimetry experiments revealed that the enhanced tolerance of Alta Lake stickleback may be associated with their ability to depress metabolic rate (as indicated by metabolic heat production) by 33% in hypoxia. The two populations showed little variation in their capacities for O2 extraction and anaerobic metabolism. These results reveal that intraspecific variation in hypoxia tolerance can develop over relatively short geological timescales, as can metabolic rate depression, a complex biochemical response that may be favoured in long-term hypoxic environments.
Keywords: hypoxia tolerance, metabolic depression, threespine stickleback, oxygen consumption, calorimetry, fish
1. Introduction
The world's aquatic environments are becoming increasingly hypoxic as a result of elevated water temperatures and increased agricultural runoff [1]. Because oxygen is essential for organisms to generate sufficient ATP to meet their metabolic demands, environmental hypoxia threatens the animal's ability to maintain energy balance, homeostasis and, consequently, life. Despite this, many fishes inhabit environments that naturally become hypoxic and have accordingly evolved strategies that enhance hypoxia tolerance [2], and we can look to these fishes and their strategies to better understand what might facilitate adaptation to an increasingly hypoxic world.
To begin to explore how hypoxia has shaped the evolution of adaptive traits, we collected threespine stickleback (Gasterosteus aculeatus) from populations that have been isolated from one another for approximately 11 000 years in two British Columbia lakes: Alta Lake, which experiences long-term hypoxia due to overwinter freezing [3], and Trout Lake, which does not. We predicted Alta Lake stickleback to be more hypoxia-tolerant than Trout Lake stickleback, and that this difference would result from an increased reliance on metabolic rate depression (MRD) in the Alta Lake fish. We predicted MRD as the causal mechanism because of its effectiveness at maintaining cellular energy balance over long-duration hypoxic bouts [4] and its prevalence of use among ectothermic vertebrate species that inhabit similar winter environments [5–7]. We used time-to-loss of equilibrium (LOE) experiments to assess hypoxia tolerance, calorimetry to measure total metabolic rate as metabolic heat at different PwO2, respirometry to measure aerobic contributions to metabolic rate at different PwO2, and metabolite analyses (glycogen and lactate) to measure anaerobic contributions to metabolic rate following hypoxia exposure.
2. Material and methods
(a). Lakes
Alta Lake (Whistler, BC, Canada; 50°11′42″ N 122°98′11″ W) and Trout Lake (Sechelt, BC, Canada; 49°50′82″ N 123°87′64″ W) are similar oligotrophic water bodies (electronic supplementary material, table S1) that differ in elevation. Alta Lake is surface-frozen for 128 ± 3.64 d yr−1 (mean ± s.e.m.), while Trout Lake does not freeze.
(b). Field collection and husbandry
Stickleback were collected using minnow traps placed on the lake bottom at 1–2 m depth and 3–5 m offshore (N = 73, 1.11 ± 0.05 g). Fish for the calorespirometry experiments were collected in October 2015, and fish for the parallel hypoxic exposures and LOE trials were collected in May 2016.
Fish were held in 100 l recirculating aquaria (dechlorinated Vancouver tap water, 20.6 ± 0.2 kPa PwO2, 17°C) at a density of less than 0.3 g l−1 and under 12 L : 12 D at The University of British Columbia. Twenty-five per cent water changes were carried out every two weeks. Fish were fed bloodworms (Hikari Bio-Pure) daily to satiation and were held for three weeks prior to experimentation to allow adjustment to laboratory conditions, diet and light cycle.
(c). Hypoxic exposures, calorespirometry and time-to-loss of equilibrium
Fish were withheld food for 24 h and then transferred to a custom-designed calorespirometer [5]. Following an 18 h habituation period, normoxic routine metabolic heat was measured as in [5], and the PwO2 in the calorespirometer chamber was then reduced to 2.8 kPa (chosen based on preliminary O2 LC50 experiments that determined the PwO2 at which 50% of fish lost dorsal–ventral equilibrium following 8 h exposure; electronic supplementary material, figure S1) over 1 h using compressed N2, and measurements of metabolic heat were made (N = 3–5). Following the 4 h hypoxia exposure, normoxia was re-established for a period of 2 h after which closed-chamber respirometry was performed to measure O2 uptake rate (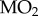 ) and determine the fish's critical O2 tension (Pcrit, the PwO2 below which the fish is unable to extract sufficient environmental O2 to support a stable routine
) and determine the fish's critical O2 tension (Pcrit, the PwO2 below which the fish is unable to extract sufficient environmental O2 to support a stable routine 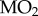 ) according to [5] (N = 4–5).
) according to [5] (N = 4–5).
A parallel set of experiments in which fish were held in opaque 10 l tanks was performed to measure whole body concentrations of glycogen (glycolytic fuel) and lactate (glycolytic end-product). Each tank held four fish and following an 18 h normoxic habituation period, PwO2 was adjusted in two tanks to 2.8 kPa (N = 8) over 1 h and the other two remained normoxic to serve as controls (N = 8). This was done for both populations. At 4 h, we introduced a lethal dose of anaesthetic (buffered MS-222, 0.3 g l−1) and once the fish were unresponsive (approx. 4 min), we removed, weighed and froze them in liquid N2 for later metabolite analyses.
We measured hypoxia tolerance by determining the time-to-LOE at a PwO2 of 1.3 kPa. Eight fish from each population were placed in each of two tanks (N = 16), and following an 18 h normoxic habituation period, we reduced PwO2 to 1.3 kPa over 1 h. We defined time-to-LOE as the time it took after the tank had reached 1.3 kPa for the fish to lose dorsal–ventral equilibrium and become unresponsive to tail prods using a blunt dissection probe. When this point was reached, we removed, weighed and transferred the fish to a well-aerated recovery tank.
(d). Metabolite assays
We measured changes in glycogen and lactate concentrations at the whole body level to be consistent with our whole body measurements of metabolic heat and 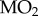 . We prepared whole bodies for metabolite extraction according to [5] and measured glycogen and lactate according to [8].
. We prepared whole bodies for metabolite extraction according to [5] and measured glycogen and lactate according to [8].
(e). Statistical analyses
Statistical analyses were performed using SigmaStat 11.0. For the analysis of metabolic rate, we first assessed the relationships between body mass and both whole-animal 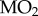 and metabolic heat, neither case showing significant correlation.
and metabolic heat, neither case showing significant correlation. 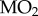 data were analysed using two-way analysis of variance (ANOVA). Metabolic heat data did not exhibit equal variance (even when log-transformed), so we conducted separate analyses on the two populations using one-way repeated-measures ANOVAs with Tukey post hoc tests. For replicate tank exposures (metabolite and LOE experiments), we first tested for differences between tanks (t-test), and in all cases, there were no differences so data were combined. Two-way ANOVA (metabolites) or two-tailed t-tests (time to LOE and Pcrit) were performed as appropriate. Differences were considered significant at p < 0.05. All values are presented as means ± s.e.m.
data were analysed using two-way analysis of variance (ANOVA). Metabolic heat data did not exhibit equal variance (even when log-transformed), so we conducted separate analyses on the two populations using one-way repeated-measures ANOVAs with Tukey post hoc tests. For replicate tank exposures (metabolite and LOE experiments), we first tested for differences between tanks (t-test), and in all cases, there were no differences so data were combined. Two-way ANOVA (metabolites) or two-tailed t-tests (time to LOE and Pcrit) were performed as appropriate. Differences were considered significant at p < 0.05. All values are presented as means ± s.e.m.
3. Results and discussion
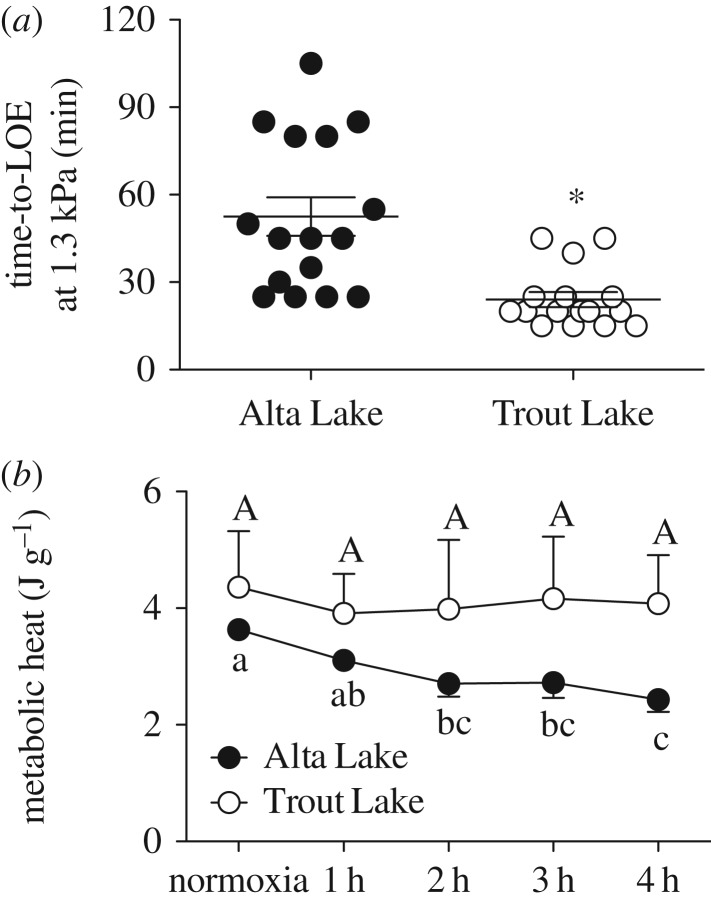
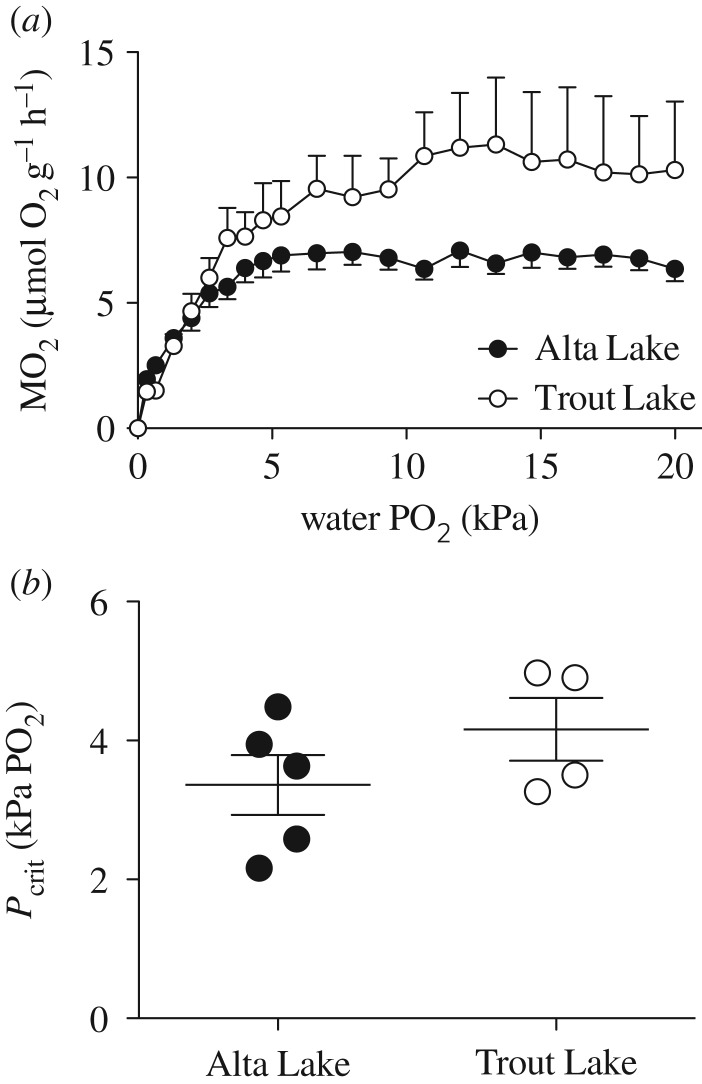
We predicted that stickleback native to Alta Lake (long-term hypoxia) would be more hypoxia-tolerant than stickleback native to Trout Lake (no long-term hypoxia), a difference resulting from a reliance on MRD in the Alta Lake fish. The results generally agree with these predictions. Alta Lake stickleback lasted approximately twice as long at 1.3 kPa PwO2 than Trout Lake stickleback (t-test, p < 0.001, figure 1a), and calorimetry experiments suggest that this difference in hypoxia tolerance is associated with the Alta Lake stickleback's use of MRD in hypoxia (one-way ANOVA, p < 0.001, figure 1b), something the Trout Lake stickleback did not do (one-way ANOVA, p = 0.9634, figure 1b). Two-way ANOVA of the respirometry results revealed that 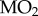 declined in both populations with declining PwO2, but that there were differences between populations resulting from the Trout Lake population's 55% higher average routine
declined in both populations with declining PwO2, but that there were differences between populations resulting from the Trout Lake population's 55% higher average routine 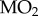 (assessed as the
(assessed as the 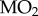 during the oxy-regulation stage above 15 kPa PwO2; population p < 0.001, PwO2
p < 0.001, interaction p = 0.331; figure 2a). Despite this, the two populations did not differ in Pcrit (t-test, p = 0.245, figure 2b), though these results should be viewed cautiously due to the low number of fish available from Trout Lake. Finally, the metabolite analyses revealed that Alta Lake fish had higher overall glycogen stores, but 4 h exposure to 2.8 kPa PwO2 had no effect on either population's use of anaerobic glycolysis as indicated by glycogen depletion and lactate accumulation (table 1).
during the oxy-regulation stage above 15 kPa PwO2; population p < 0.001, PwO2
p < 0.001, interaction p = 0.331; figure 2a). Despite this, the two populations did not differ in Pcrit (t-test, p = 0.245, figure 2b), though these results should be viewed cautiously due to the low number of fish available from Trout Lake. Finally, the metabolite analyses revealed that Alta Lake fish had higher overall glycogen stores, but 4 h exposure to 2.8 kPa PwO2 had no effect on either population's use of anaerobic glycolysis as indicated by glycogen depletion and lactate accumulation (table 1).
Figure 1.
(a) Time taken for two populations of threespine stickleback to lose dorsal–ventral equilibrium and become unresponsive to gentle tail prods when exposed to severe hypoxia (1.3 kPa PwO2). Points represent individual values, horizontal lines represent mean values, error bars represent s.e.m. and asterisk indicates significant difference (t-test, p < 0.001). (b) Metabolic heat as a function of time in severe hypoxia (2.8 kPa PwO2). Points represent average values and error bars represent s.e.m. Values sharing a letter are not significantly different (Alta Lake: p < 0.001, N = 5; Trout Lake: heat p = 0.963, N = 3).
Figure 2.
(a) Closed-chamber 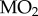 measurements as a function of PwO2 for two populations of threespine stickleback. (b) Average Pcrit values for two populations of threespine stickleback, calculated for each fish from its closed-chamber
measurements as a function of PwO2 for two populations of threespine stickleback. (b) Average Pcrit values for two populations of threespine stickleback, calculated for each fish from its closed-chamber 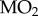 trace (t-test, p = 0.245). Points represent individual values, horizontal lines represent mean value and error bars represent s.e.m. Alta Lake: N = 5; Trout Lake: N = 4.
trace (t-test, p = 0.245). Points represent individual values, horizontal lines represent mean value and error bars represent s.e.m. Alta Lake: N = 5; Trout Lake: N = 4.
Table 1.
Whole body concentrations of glycogen and lactate in two populations of threespine stickleback. Measurements were made following a 4 h exposure to normoxia or hypoxia (2.8 kPa PwO2). N = 8 for each treatment.
| Alta Lake |
Trout Lake |
two-way ANOVA p-values |
|||||
|---|---|---|---|---|---|---|---|
| normoxia | hypoxia | normoxia | hypoxia | pop. | PwO2 | interaction | |
| glycogen | 1.37 ± 0.19 | 1.32 ± 0.11 | 0.89 ± 0.17 | 1.19 ± 0.10 | 0.044 | 0.355 | 0.292 |
| lactate | 0.15 ± 0.05 | 0.30 ± 0.06 | 0.21± 0.07 | 0.35 ± 0.11 | 0.461 | 0.068 | 0.878 |
Concentrations in μmol g−1 tissue; values are mean ± s.e.m.
(a). Metabolic rate depression and hypoxia tolerance
By 4 h exposure to 2.8 kPa PwO2, the Alta Lake stickleback had depressed their metabolic rates (as indicated by metabolic heat) by an average of 33% relative to their normoxic routine values. At this same time point and PwO2, the average metabolic rate of the Trout Lake stickleback was approximately twice that of the Alta Lake fish (although direct statistical comparisons between populations cannot be made due to unequal variance). This agrees with the twofold longer tolerance time of the Alta Lake fish in the LOE experiment. An MRD of 33% would improve hypoxic survival by reducing energetic demand, and is a deeper MRD than other hypoxia-native fish species such as zebrafish (Danio rerio) are capable of [9]. Despite this, our results may underestimate the true capacity of the Alta Lake fish in the wild. Because our analyses were carried out at 17°C (to maximize calorimetric signal : noise) instead of the near-freezing water temperature that would occur in Alta Lake in the winter (electronic supplementary material, table S1), the 33% MRD measured in our study likely underestimates the total metabolic savings that are actually accrued by the Alta Lake fish under long-term hypoxic conditions in the wild. In addition, acclimation effects stemming from three weeks' normoxic laboratory acclimation, and field collection when persistent hypoxia was not present in Alta Lake, may further underestimate the Alta Lake population's winter hypoxia tolerance.
To our knowledge, these results represent the first time that MRD use has been shown to vary between geographically isolated populations of the same species. While MRD is a complex biochemical phenotype requiring a reorganization of cellular processes, the fact that Alta Lake and Trout Lake populations have been isolated for a maximum of 11 000 years [10] suggests it is a phenotype that can evolve rapidly. This is consistent with the rapidity that marine stickleback naturally evolve their freshwater-distinctive, genetically based morphological features de novo (less than 50 years; [11]). However, an intriguing alternative hypothesis is that MRD may be a developmentally plastic neonatal characteristic similar to the hypoxic ventilatory response of mammals [12], and supporting this are findings that zebrafish employ hypoxia-induced MRD as embryos, but not as adults [9,13].
4. Conclusion
Our results demonstrate significant variation in the hypoxia tolerances of two isolated populations of threespine stickleback. The tolerant population is native to an environment that experiences long-term hypoxia (Alta Lake), suggesting this may underlie the enhanced tolerance and associated mechanisms of a lower O2 demand and the use of hypoxia-induced MRD. Furthermore, the Alta Lake population's use of MRD is consistent with MRD's use among ectothermic vertebrates that inhabit similar winter environments [5–7]. This suggests MRD is a strategy employed in particularly severe and/or long-lasting hypoxic environments, and one that may even evolve over relatively short geological timescales. Assessing the generality of these relationships, their adaptive value and their connection to other biotic and abiotic environmental factors that may differ between lakes will require investigating additional independent populations.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Bob Brett, Taylor Gibbons, Jordan Rosenfeld, Seth Rudman, Tara Shaufele and the three anonymous reviewers.
Ethics
Fish were collected under BC Fish Collection permit SU15-195700 and housed under UBC Animal Care permit A13-0309.
Data accessibility
Raw data available at: https://doi.org/10.5061/dryad.d27gv [14].
Authors' contributions
M.D.R. and J.G.R. conceived the study. All authors designed the experiments. I.S.G. collected the data with help from M.D.R. M.D.R. wrote the manuscript and all authors commented, approve the final version of the manuscript and agree to be held accountable for its content.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by an NSERC Discovery grant (no. 312029) to J.G.R. I.S.G. was supported by a UBC Science Undergraduate Research Award. M.D.R. was supported by an NSERC postgraduate scholarship.
References
- 1.Intergovernmental Panel on Climate Change, IPCC. 2014. Climate change 2014—impacts, adaptation and vulnerability: regional aspects. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Hochachka PW, Lutz PL. 2001. Mechanism, origin, and evolution of anoxia tolerance in animals. Comp. Biochem. Physiol. B 130, 435–459. ( 10.1016/S1096-4959(01)00408-0) [DOI] [PubMed] [Google Scholar]
- 3.Jacques Whitford/AXYS Ltd. 2007. Alta lake limnology study. Resort Municipality of Whistler, Whistler.
- 4.Hochachka PW, Buck LT, Doll CJ, Land SC. 1996. Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc. Natl Acad. Sci. USA 93, 9493–9498. ( 10.1073/pnas.93.18.9493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regan MD, Gill IS, Richards JG. 2017. Calorespirometry reveals that goldfish prioritize aerobic metabolism over metabolic rate depression in all but near-anoxic environments. J. Exp. Biol. 220, 564–572. ( 10.1242/jeb.145169) [DOI] [PubMed] [Google Scholar]
- 6.Jackson DC. 1968. Metabolic depression and oxygen depletion in the diving turtle. J. Appl. Physiol. 24, 503–509. [DOI] [PubMed] [Google Scholar]
- 7.Johansson D, Nilsson G, Touml Rnblom E. 1995. Effects of anoxia on energy metabolism in crucian carp brain slices studied with microcalorimetry. J. Exp. Biol. 198, 853–859. [DOI] [PubMed] [Google Scholar]
- 8.Bergmeyer HU, Bergmeyer J, Grassl M. 1983. Methods of enzymatic analysis. Weinheim, Germany: Verlag Chemie. [Google Scholar]
- 9.Stangl P, Wegener G. 1996. Calorimetric and biochemical studies on the effects of environmental hypoxia and chemicals on freshwater fish. Thermochim. Acta 271, 101–113. ( 10.1016/0040-6031(95)02586-3) [DOI] [Google Scholar]
- 10.Bell MA, Foster SA. 1994. The evolutionary biology of the threespine stickleback. Oxford, UK: Oxford University Press. [Google Scholar]
- 11.Lescak EA, Bassham SL, Catchen J, Gelmond O, Sherbick ML, von Hippel FA, Cresko WA. 2015. Evolution of stickleback in 50 years on earthquake-uplifted islands. Proc. Natl Acad. Sci. USA 112, E7204–E7212. ( 10.1073/pnas.1512020112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teppema LJ, Dahan A. 2010. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol. Rev. 90, 675–754. ( 10.1152/physrev.00012.2009) [DOI] [PubMed] [Google Scholar]
- 13.Padilla PA, Roth MB. 2001. Oxygen deprivation causes suspended animation in the zebrafish embryo. Proc. Natl Acad. Sci. USA 98, 7331–7335. ( 10.1073/pnas.131213198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Regan MD, Gill IS, Richards JG. 2017. Data from: Metabolic depression and the evolution of hypoxia tolerance in threespine stickleback, Gasterosteus aculeatus Dryad Digital Repository. ( 10.5061/dryad.d27gv) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Regan MD, Gill IS, Richards JG. 2017. Data from: Metabolic depression and the evolution of hypoxia tolerance in threespine stickleback, Gasterosteus aculeatus Dryad Digital Repository. ( 10.5061/dryad.d27gv) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Raw data available at: https://doi.org/10.5061/dryad.d27gv [14].