Abstract
The major histocompatibility complex (MHC) plays a key role in vertebrate immunity, and pathogen-mediated selection often favours certain allelic combinations. Assessing potential mates' MHC profiles may provide receivers with genetic benefits (identifying MHC-compatible mates and producing optimally diverse offspring) and/or material benefits (identifying optimally diverse mates capable of high parental investment). Oscine songbirds learn songs during early life, such that song repertoire content can reflect population of origin while song complexity can reflect early life condition. Thus birdsong may advertise the singer's genetic dissimilarity to others in the population (and, presumably, compatibility with potential mates), or individual genetic diversity (and thus condition-dependent material benefits). We tested whether song repertoire content and/or complexity signal MHC class IIβ dissimilarity and/or diversity in male song sparrows (Melospiza melodia). Pairwise dissimilarity in repertoire content did not predict MHC dissimilarity between males, suggesting that locally rare songs do not signal rare MHC profiles. Thus, geographical variation in song may not facilitate MHC-mediated inbreeding or outbreeding. Larger repertoires were associated with intermediate MHC diversity, suggesting intermediate rather than maximal MHC diversity is optimal. This could reflect trade-offs between resisting infection and autoimmune disorders. Song complexity may advertise optimal MHC diversity, a trait affecting disease resistance and capacity for parental care.
Keywords: birdsong, compatible genes, genetic diversity, major histocompatibility complex, song sparrow, Melospiza melodia
1. Introduction
The major histocompatibility complex (MHC) plays a fundamental role in vertebrate immunity. MHC molecules recognize exogenous peptides (antigens) and present them to T cells, initiating an immune response [1]. Because MHC genotype determines the suite of antigens that can be recognized, pathogen-mediated selection often favours particular alleles or allelic combinations. Evolutionary arms races with pathogens can impose selection favouring rare alleles, while balancing selection (e.g. heterozygote advantage) often favours individuals with multiple alleles at MHC [2]. Thus, receivers should benefit by assessing potential mates' MHC profiles [1–3]. Choosing mates with MHC alleles that are dissimilar to one's own or locally rare should yield offspring that are MHC-diverse or possess rare alleles, conferring genetic benefits via disease resistance [1–4]. Choosing mates that are themselves MHC-diverse may also enhance offspring MHC diversity [5] and/or inheritance of rare alleles [6]. Moreover, MHC-diverse mates may provide enhanced parental investment [7] owing to superior condition. Signals of locally rare MHC profiles, and of individual diversity at MHC, are thus likely to be salient to mating decisions [3].
In mammals, fish and seabirds, groups with well-developed chemical communication, receivers identify specific (e.g. locally rare) MHC alleles through olfactory cues from sweat, urine or preen oil [8–10]. Conversely, because individual diversity at MHC affects disease resistance, condition-dependent ornaments may signal MHC diversity [11,12]. In oscine songbirds, however, learned birdsong could theoretically reflect similarity to the surrounding population as well as individual diversity at MHC. Song learning is generally restricted to early life, meaning that song repertoire content can advertise population of origin [13]. Conversely, song complexity can advertise early life condition [14] and/or adult immunocompetence [15]. Thus, song repertoire content and complexity may signal the singer's MHC similarity versus dissimilarity to others in the population (and, presumably, compatibility with potential mates), and/or individual diversity at MHC (and, presumably, condition and capacity for parental care).
In song sparrows (Melospiza melodia), males learn song during early life. Song varies geographically, and locally typical repertoires are associated with locally typical microsatellite genotypes [16], suggesting that repertoire content reflects population of origin. Females prefer local over non-local song [17], and larger over smaller song repertoires [18]. We characterized the peptide-binding region of MHC class IIβ to test whether pairwise dissimilarity in song repertoire content reflects MHC dissimilarity to other males in the population (a proxy for locally rare alleles), and whether song repertoire size reflects individual diversity at MHC.
2. Material and methods
(a). Field sampling
The subjects were 32 male song sparrows at a single breeding site (less than 1 km diameter, and not physically isolated from other suitable habitat) near Newboro, Ontario, Canada (44.633° N, 76.330° W). Between 13 April and 3 May 2015, we captured sparrows in seed-baited traps, collected blood for genetic analysis, applied individually unique colour band combinations for field identification, then released birds.
(b). Song analysis
We recorded song onto Marantz Professional PMD 671 recorders using Telinga Twin Science Pro parabolic microphones. Recording 200 songs per individual, not necessarily consecutive, is sufficient in most cases to characterize complete repertoires in this population [19]. To be conservative, we recorded 300 songs per individual and confirmed by accumulation curves that a plateau had occurred. We digitized recordings in Raven Pro 1.5 (Cornell Lab of Ornithology), inspected spectrograms to identify song types, and noted song repertoire size as the number of different song types each individual produced. We identified a total of 235 syllables (i.e. one or more traces on a spectrogram that always occurred together [16]) across all song types. As detailed elsewhere [16], we screened each individual's repertoire for each syllable, constructed a presence–absence syllable matrix and calculated pairwise Jaccard dissimilarity coefficients adjusted for differences in syllable repertoire size.
(c). Genetic analysis
We used primers SospMHCint1f [20] and Int2r.1 [21] to amplify MHC class II, exon 2 (β subunit). Details of polymerase chain reaction (PCR) and sequencing conditions are available in Dryad, and hereafter referred to as ‘electronic supplementary material’ [22]. We sorted sequences into stacks of identical reads using a pipeline [23] and removed chimeras using UCHIME [24]. As detailed elsewhere [20,25], we used a 1% threshold frequency to remove rare reads that could represent PCR or sequencing errors, and compared a subset of reads to complementary DNA (cDNA)-derived sequences to confirm transcription of at least some alleles.
We trimmed sequences to remove introns, translated them into amino acid sequences of 70–74 codons, and removed apparent pseudogenes based on premature termination codons. Based on a maximum-likelihood allele phylogeny with WAG substitution and five discrete gamma categories, we used the unweighted UniFrac algorithm in the R package GUniFrac [26] to calculate pairwise genetic distances between individuals. Alleles in the same clade are presumably similar functionally, so to be conservative in estimating genetic diversity, we clustered them into ‘superalleles’ based on well-defined clade membership [25]. We scored each individual's MHC diversity as the number of different superalleles.
(d). Data analysis
To test whether song dissimilarity signals MHC dissimilarity to other males in the population, we assessed the correlation between Jaccard dissimilarity and UniFrac genetic distance, using a Mantel test with 9999 permutations (mantel in vegan [27]).
To test whether song complexity varies with MHC diversity, we compared support for three models predicting song repertoire size: a linear model (number of superalleles), a quadratic model (number of superalleles + squared number of superalleles), and a null model. We ranked models using Akaike's corrected information criterion (AICc), setting a threshold of 2 AICc units for model averaging. We checked models for highly influential points, and confirmed Cook's distance was less than 0.5 in all cases.
Analyses were performed in R v. 3.4.0 [28]; values reported are means ± s.e.m.
3. Results
We detected 186 unique alleles at MHC IIβ, which clustered to 91 superalleles (electronic supplementary material, fig. S1 [22]; 13.6 ± 0.5 superalleles per individual). Repertoire size ranged from 5 to 12 song types (7.8 ± 0.3 per individual).
Song dissimilarity was not associated with genetic distance at MHC (Mantel's r = −0.01, p = 0.55).
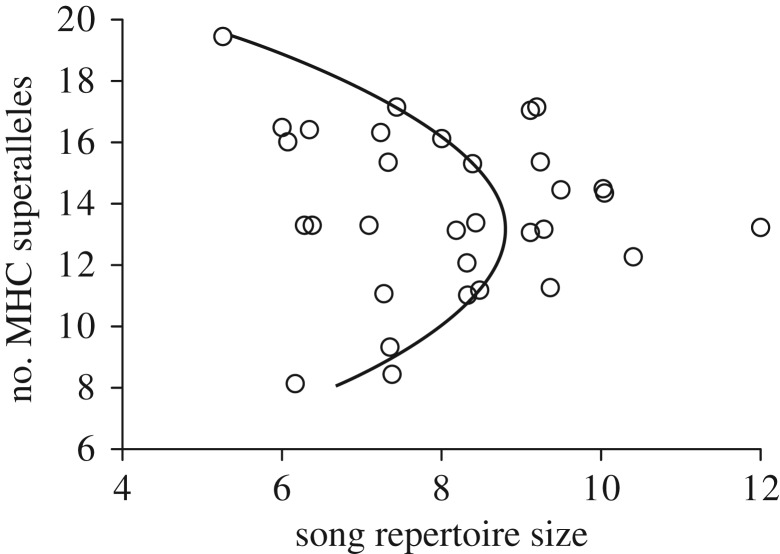
In predicting song repertoire size, the quadratic model received 5–16 times more support than the null or linear models (table 1; parameter estimates in table 2). The largest song repertoires occurred in males with intermediate MHC diversity (figure 1). Excluding one individual with low repertoire size and high MHC diversity (figure 1, upper leftmost point) did not qualitatively alter significance of results (electronic supplementary material, tables S1 and S2 [22]).
Table 1.
Ranked set of candidate models predicting song sparrow song repertoire size. Predictors were number of MHC superalleles and squared number of MHC superalleles.
| model | d.f. | logLik | AICc | ΔAICc | model weight |
|---|---|---|---|---|---|
| quadratic: no. MHC + (no. MHC)2 | 4 | −54.8 | 119.0 | 0 | 0.78 |
| null: intercept only | 2 | −58.9 | 122.2 | 3.20 | 0.16 |
| linear: no. MHC | 3 | −58.8 | 124.4 | 5.40 | 0.05 |
Table 2.
Parameter estimates from the best-supported model predicting song sparrow song repertoire size. Repertoire size increased with number of MHC class IIβ superalleles, but decreased with squared number of superalleles. R2 = 0.23, F2,29 = 4.24.
| parameter | β ± s.e. | 95% CI |
|---|---|---|
| intercept | −5.27 ± 4.97 | −15.4, 4.89 |
| no. MHC | 2.12 ± 0.76 | 0.56, 3.67 |
| (no. MHC)2 | −0.082 ± 0.029 | −0.14, −0.02 |
Figure 1.
Relationship between song repertoire size and MHC class IIβ superallele diversity. Curve depicts best-supported model described in tables 1 and 2.
4. Discussion
MHC-related mating preferences have been observed in all vertebrate classes [3], raising the question of how animals assess MHC profiles. Cues of compatibility and diversity are generally studied in the contexts of chemosignalling [8–10] and visual ornaments [6,11,12], respectively. We investigated birdsong, an acoustic ornament, as a signal of MHC class IIβ dissimilarity to other individuals in the population (a proxy for locally rare genotypes), and individual genetic diversity. Geographical variation in song has long been proposed to advertise population of origin, suggesting receivers might use song to achieve an optimal balance between inbreeding and outbreeding [13,29]. Finding no relationship between song and MHC dissimilarity could reflect low genetic differentiation at MHC in this system [25]: if MHC does not vary with population of origin, song is unlikely to signal MHC dissimilarity. However, we examined only one class of MHC and cannot rule out associations between song repertoire content and class I loci, whose products interact with intracellular pathogens such as viruses [1].
Whereas MHC class IIβ dissimilarity to other males at the site was not associated with song dissimilarity, individual diversity at MHC explained 23% of the variation in song repertoire size. This supports previous findings that MHC diversity influences ornamentation [6,11,12], but an association with an acoustic ornament is novel (although see [15] for evidence that song repertoire size advertises cell-mediated immunity in this species). Also notable is the nonlinear nature of this relationship: larger song repertoires were associated with intermediate, not maximal, MHC diversity. This might reflect trade-offs between susceptibility to pathogens versus autoimmune disorders [1,2], or a dilution effect whereby overly diverse MHC profiles have too few copies of protective alleles. In choosing social mates with complex song, females may obtain material benefits through increased paternal investment. To the extent that males with optimal MHC diversity produce optimally diverse offspring, preferences for complex song may also confer genetic benefits.
Acknowledgements
We thank the Queen's University Biological Station for logistic support; André Lachance, Bryan Neff and Ben Rubin for advice; Tosha Kelly and Alannah Lymburner for field assistance.
Ethics
Animal work was approved by the University of Western Ontario Animal Use Subcommittee (protocol 2008-054) and conducted under the required federal permits.
Data accessibility
MHC sequences are on GenBank (accession nos KX263957-KX264148; KX375230-KX375341; MF197785-MF197843). Supporting data and other electronic supplementary material are posted to the Dryad Digital Repository [22].
Authors' contributions
Study design: J.W.G.S. and E.A.M.-S. Fieldwork: J.W.G.S., M.J.W. and E.A.M.-S. Genetic analysis: J.W.G.S. and M.J.W. Song analysis: J.W.G.S. Data analysis: J.W.G.S. Manuscript preparation and revision: J.W.G.S., E.A.M.-S. and M.J.W. All authors approved the final version of the manuscript, and agree to be accountable for this work's accuracy and integrity.
Competing interests
We have no competing interests.
Funding
This study was supported by award 293123-2012RGPIN from the Natural Sciences and Engineering Research Council of Canada (NSERC) to EAM-S, and a Hesse Award from the American Ornithologists' Union and a Taverner Award from the Society for Canadian Ornithologists to J.W.G.S.
References
- 1.Klein J. 1986. Natural history of the major histocompatibility complex. New York, NY: John Wiley and Sons. [Google Scholar]
- 2.Piertney SB, Oliver MK. 2006. The evolutionary ecology of the major histocompatibility complex. Heredity 96, 7–21. ( 10.1038/sj.hdy.6800724) [DOI] [PubMed] [Google Scholar]
- 3.Kamiya T, O'Dwyer K, Westerdahl H, Senior A, Nakagawa S. 2014. A quantitative review of MHC-based mating preference: the role of diversity and dissimilarity. Mol. Ecol. 23, 5151–5163. ( 10.1111/mec.12934) [DOI] [PubMed] [Google Scholar]
- 4.Potts WK, Manning CJ, Wakeland EK. 1991. Mating patterns in seminatural populations of mice influenced by MHC genotype. Nature 352, 619–621. ( 10.1038/352619a0) [DOI] [PubMed] [Google Scholar]
- 5.Reusch TBH, Häberli MA, Aeschlimann PB, Milinski M. 2001. Female sticklebacks count alleles in a strategy of sexual selection explaining MHC polymorphism. Nature 414, 300–302. ( 10.1038/35104547) [DOI] [PubMed] [Google Scholar]
- 6.Hale ML, Verduijn MH, Møller AP, Wolff K, Petrie M. 2009. Is the peacock's train an honest signal of genetic quality at the major histocompatibility complex? J. Evol. Biol. 22, 1284–1294. ( 10.1111/j.1420-9101.2009.01746.x) [DOI] [PubMed] [Google Scholar]
- 7.Knafler GJ, Clark JA, Boersma PD, Bouzat JL. 2012. MHC diversity and mate choice in the Magellanic penguin, Spheniscus magellanicus. J. Hered. 103, 759–768. ( 10.1093/jhered/ess054) [DOI] [PubMed] [Google Scholar]
- 8.Penn D, Potts W. 1998. How do major histocompatibility complex genes influence odor and mating preferences? Adv. Immunol. 69, 411–436. ( 10.1016/S0065-2776(08)60612-4) [DOI] [PubMed] [Google Scholar]
- 9.Milinski M, Griffiths S, Wegner KM, Reusch TBH, Haas-Assenbaum A, Boehm T. 2005. Mate choice decisions of stickleback females predictably modified by MHC peptide ligands. Proc. Natl Acad. Sci. USA 102, 4414–4418. ( 10.1073/pnas.0408264102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leclaire S, Strandh M, Mardon J, Westerdahl H, Bonadonna F. 2017. Odour-based discrimination of similarity at the major histocompatibility complex in birds. Proc. R. Soc. B 284, 20163466 ( 10.1098/rspb.2016.2466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schantz T, Wittzell H, Göransson G, Grahn M. 1997. Mate choice, male condition-dependent ornamentation and MHC in the pheasant. Hereditas 127, 133–140. ( 10.1111/j.1601-5223.1997.t01-1-00133.x) [DOI] [Google Scholar]
- 12.Whittingham LA, Freeman-Gallant CR, Taff CC, Dunn PO. 2015. Different ornaments signal male health and MHC variation in two populations of a warbler. Mol. Ecol. 24, 1584–1595. ( 10.1111/mec.13130) [DOI] [PubMed] [Google Scholar]
- 13.Podos J, Warren PS. 2007. The evolution of geographic variation in birdsong. Adv. Stud. Behav. 37, 403–458. ( 10.1016/S0065-3454(07)37009-5) [DOI] [Google Scholar]
- 14.Schmidt KL, Moore SD, MacDougall-Shackleton EA, MacDougall-Shackleton SA. 2013. Early-life stress affects song complexity, song learning, and volume of the brain nucleus RA in adult male song sparrows. Anim. Behav. 86, 25–35. ( 10.1016/j.anbehav.2013.03.036) [DOI] [Google Scholar]
- 15.Reid JM, Arcese P, Cassidy ALEV, Marr AB, Smith JNM, Keller LF. 2005. Hamilton and Zuk meet heterozygosity? Song repertoire size indicates inbreeding and immunity in song sparrows (Melospiza melodia). Proc. R. Soc. B 272, 481–487. ( 10.1098/rspb.2004.2983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart KA, MacDougall-Shackleton EA. 2008. Local song elements indicate local genotypes and predict physiological condition in song sparrows Melospiza melodia. Biol. Lett. 4, 240–242. ( 10.1098/rsbl.2008.0010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Searcy WA, Nowicki S, Hughes M, Peters S. 2002. Geographic song discrimination in relation to dispersal distances in song sparrows. Am. Nat. 159, 221–230. ( 10.1086/338509) [DOI] [PubMed] [Google Scholar]
- 18.Searcy WA. 1984. Song repertoire size and female preferences in song sparrows. Behav. Ecol. Sociobiol. 14, 281–286. ( 10.1007/BF00299499) [DOI] [Google Scholar]
- 19.Potvin DA, Crawford PW, MacDougall-Shackleton SA, MacDougall-Shackleton EA. 2015. Song repertoire size, not territory location, predicts reproductive success and territory tenure in a migratory songbird. Can. J. Zool. 93, 627–633. ( 10.1139/cjz-2015-0039) [DOI] [Google Scholar]
- 20.Slade JWG, Watson MJ, Kelly TR, Gloor GB, Bernards MA, MacDougall-Shackleton EA. 2016. Chemical composition of preen wax reflects major histocompatibility complex similarity in songbirds. Proc. R. Soc. B 283, 20161966 ( 10.1098/rspb.2016.1966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards SV, Gasper J, March M. 1998. Genomics and polymorphism of Agph-DAB1, an Mhc class IIB gene in red-winged blackbirds (Agelaius phoeniceus). Mol. Biol. Evol. 15, 236–250. ( 10.1093/oxfordjournals.molbev.a025921) [DOI] [PubMed] [Google Scholar]
- 22.Slade JWG, Watson MJ, MacDougall-Shackleton EA.. 2017. Data from: Birdsong signals individual diversity at MHC Dryad Digital Repository. ( 10.5061/dryad.8g1bb) [DOI] [PMC free article] [PubMed]
- 23.Gloor GB, Hummelen R, Macklaim JM, Dickson RJ, Fernandes AD, MacPhee R, Reid G. 2010. Microbiome profiling by Illumina sequencing of combinatorial sequence-tagged PCR products. PLoS ONE 5, e15406 ( 10.1371/journal.pone.0015406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. 2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200. ( 10.1093/bioinformatics/btr381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slade JWG, Sarquis-Adamson Y, Gloor GB, Lachance MA, MacDougall-Shackleton EA. 2017. Population differences at MHC do not explain enhanced resistance of song sparrows to local parasites. J. Hered. 108, 127–134. ( 10.1093/jhered/esw082) [DOI] [PubMed] [Google Scholar]
- 26.Chen J. 2012. GUniFrac: generalized UniFrac distances. R Package Version 1. Vienna, Austria: R Foundation for Statistical Computing; (https://cran.r-project.org/web/packages/GUniFrac/index.html) [Google Scholar]
- 27.Dixon P. 2003. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 14, 927–930. ( 10.1111/j.1654-1103.2003.tb02228.x) [DOI] [Google Scholar]
- 28.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (https://cran.r-project.org/mirrors.html) (https://www.r-project.org/) [Google Scholar]
- 29.Nottebohm F. 1969. The song of the chingolo, Zonotrichia capensis, in Argentina: description and evaluation of a system of dialects. Condor 71, 299–315. ( 10.2307/1366306) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Slade JWG, Watson MJ, MacDougall-Shackleton EA.. 2017. Data from: Birdsong signals individual diversity at MHC Dryad Digital Repository. ( 10.5061/dryad.8g1bb) [DOI] [PMC free article] [PubMed]
Data Availability Statement
MHC sequences are on GenBank (accession nos KX263957-KX264148; KX375230-KX375341; MF197785-MF197843). Supporting data and other electronic supplementary material are posted to the Dryad Digital Repository [22].