Abstract
Asymmetrical intraguild predation (AIGP), which combines both predation and competition between predator species, is pervasive in nature with relative strengths varying by prey availability. But with species redistributions associated with climate change, the response by endemic predators within an AIGP context to changing biotic–abiotic conditions over time (i.e. seasonal and decadal) has yet to be quantified. Furthermore, little is known on AIGP dynamics in ecosystems undergoing rapid directional change such as the Arctic. Here, we investigate the flexibility of AIGP among two predators in the same trophic guild: beluga (Delphinapterus leucas) and Greenland halibut (Reinhardtius hippoglossoides), by season and over 30 years in Cumberland Sound—a system where forage fish capelin (Mallotus villosus) have recently become more available. Using stable isotopes, we illustrate different predator responses to temporal shifts in forage fish availability. On a seasonal cycle, beluga consumed less Greenland halibut and increased consumption of forage fish during summer, contrasting a constant consumption rate of forage fish by Greenland halibut year-round leading to decreased AIGP pressure between predators. Over a decadal scale (1982–2012), annual consumption of forage fish by beluga increased with a concomitant decline in the consumption of Greenland halibut, thereby indicating decreased AIGP pressure between predators in concordance with increased forage fish availability. The long-term changes of AIGP pressure between endemic predators illustrated here highlights climate-driven environmental alterations to interspecific intraguild interactions in the Arctic.
Keywords: climate change, interspecific interactions, predator–prey dynamics, stable isotopes
1. Introduction
Asymmetrical intraguild predation (AIGP), a combination of predation and competition, is widespread in nature and occurs when a relatively larger-bodied predator consumes a sympatric smaller-bodied predator while both compete for a shared prey resource [1]. The availability of this shared resource is a key factor that modulates the extent of predation versus competition between predators and thus the intensity of AIGP; i.e. decreasing prey availability can lead to increased AIGP and vice versa [2]. In turn, the interplay between competitive and predatory AIGP elements can have profound effects on population and community dynamics through altering trophic guild structure [1] and dampening top–down trophic cascades [2]. But how temporal (i.e. seasonal and decadal) changes in the availability and composition of a shared prey resource influence AIGP dynamics between predators has received limited attention.
The Arctic is the fastest-warming ecosystem on the planet, with climate velocities driving marked ecological changes through shifting prey preferences and abundances, and poleward shifts of more-temperate species [3,4]. For example, the forage fish capelin (Mallotus villosus) is considered a sea ‘canary’ for a warming climate because of their ability to use varying depths (1–600 m) and spawning temperatures (2–14°C), and this has led to northward movements coincident with temperature change [5]. Given these conspicuous environmental changes across the Arctic, understanding how endemic predators respond to seasonal and long-term shifts in prey availability and how predator–prey dynamics such as AIGP can influence ecosystem functioning is required.
Two predators in the Arctic, beluga (Delphinapterus leucas) and Greenland halibut (Reinhardtius hippoglossoides), are in the same trophic guild and co-occur in a well-monitored system providing a model to investigate the plasticity of AIGP between predators. Specifically, beluga consume forage fish, Greenland halibut and invertebrates, while Greenland halibut consume forage fish and pelagic invertebrates [6,7]. These predator–competitor dynamics, under a scenario of a northward shift in a temperate forage fish, provide a framework to investigate the plasticity of AIGP between predators with a changing prey species composition over a 30-year time-period. Cumberland Sound, Nunavut, Canada (65°13′0″ N, 65°45′0″ W) is a system where capelin have become increasingly abundant since the mid-2000s (A. Fisk 2007, personal observation; R. Kilabuk from Pangnirtung, Nunavut 2011, personal communication; see electronic supplementary material, figure S1). Traditional ecological knowledge on beluga whales inhabiting waters off Southeast Baffin Island does not report the occurrence of capelin in beluga stomachs in the 1990s [6], supporting a recent shift in capelin availability. Here, we measure time-integrated resource-use of beluga and Greenland halibut over two temporal scales (seasonal and decadal), through stable isotope analysis. We provide the first empirical evidence of seasonal and decadal (1982–2012) shifts in AIGP dynamics between predators coincident with changing prey availability.
2. Material and methods
Predator/prey samples were sourced from tissue archives for two time-periods: 1982–2002 and 2004–2012. These time-periods were selected based on increased availability of capelin (after 2004) and a significant change in beluga δ15N and sympagic carbon source-use in the early 2000s [8]. A two-tissue approach was adopted to discern seasonal (summer, winter) differences in predator diets based on the premise of shorter stable isotope half-lives of cetacean skin (beluga; 14–17 days) [9] and fish liver (Greenland halibut; approx. 39 days) compared to fish and mammal muscle (Greenland halibut: approx. 98; beluga: approx. 202 days based on average adult body mass—1400 kg) [10,11]. Decadal trends were examined using muscle tissue isotope values only. All predator and primary prey (Gonatid squid, shrimp (Pandalus borealis, Lebbeus polaris), Arctic cod (Boreogadus saida) and capelin) tissue samples were collected during Arctic summer/autumn months (May to October) within and near Cumberland Sound (table 1; electronic supplementary material). Anadramous Arctic char (Salvelinus alpinus), a generalist fish predator that co-occurs in the system, were used as a sentinel species to measure shifting prey composition over the study period. Arctic char stomach content data were analysed from fish sampled between 2002–2004 and 2011 (see electronic supplementary material).
Table 1.
Summary of mean δ13C and δ15N (‰) for beluga and Greenland halibut by tissue and time-period.
| common name | species name | n | tissue | δ13C ± s.d. (‰) | δ15N ± s.d. (‰) |
|---|---|---|---|---|---|
| 1982–2002 | |||||
| beluga | Delphinapterus leucas | 63 | muscle | −18.1 ± 0.3 | 17.5 ± 1.0 |
| 57 | skin | −17.9 ± 0.3 | 17.0 ± 0.6 | ||
| Greenland halibut | Reinhardtius hippoglossoides | 14 | muscle | −19.6 ± 0.7 | 16.6 ± 0.4 |
| 2004–2012 | |||||
| beluga | Delphinapterus leucas | 25 | muscle | −18.3 ± 0.4 | 15.9 ± 0.8 |
| 21 | skin | −17.9 ± 0.4 | 16.2 ± 0.5 | ||
| Greenland halibut | Reinhardtius hippoglossoides | 21 | muscle | −19.2 ± 0.4 | 16.4 ± 0.7 |
| 21 | liver | −19.1 ± 0.8 | 15.1 ± 0.6 | ||
Seasonal and decadal contributions of primary prey to beluga and Greenland halibut diet were estimated using Bayesian mixing model analysis in SIAR v. 4.2.2 [12] in R [13] v. 3.3.2 set at 500 000 iterations, a burn-in of 300 000 and thinned by 100. The posterior distribution from the mixing models (2000) was used to estimate the probability (ep) that contributions of a prey item increased/decreased in the predator diet by season (summer versus winter) and time-periods (between 1982–2002 and 2004–2012). See electronic supplementary material for model specifics on diet–tissue discrimination factors and prey groups.
3. Results and discussion
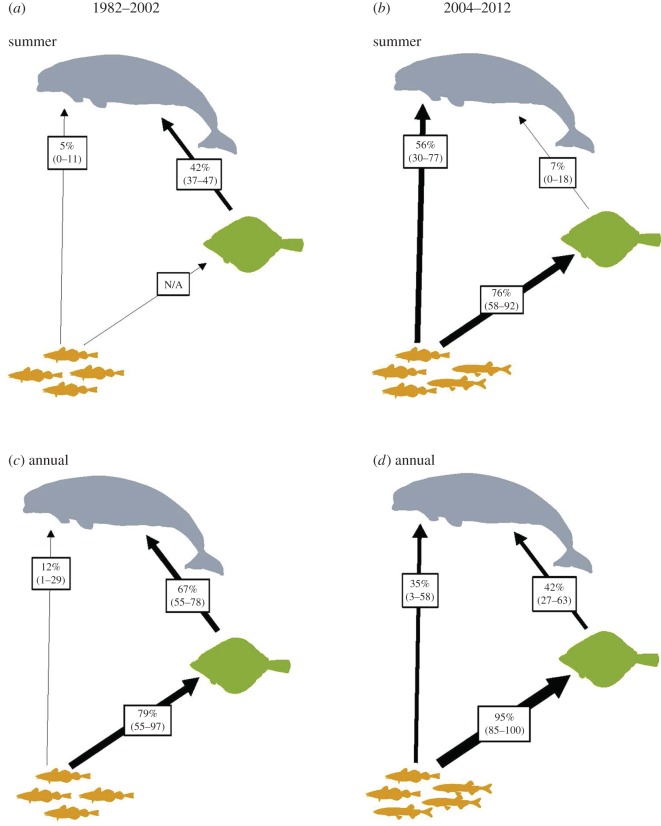
Between 2002 and 2011 there was a marked increase in the abundance of capelin in the stomach contents of Arctic char (0% to 77%; electronic supplementary material, figures S2 and S3), supporting previous observations of their increased presence in Cumberland Sound. Concomitantly, with increasing availability of capelin, the estimated contributions of forage fish to the diet of beluga also showed a marked temporal shift (table 2 and figure 1). On a seasonal basis, beluga increased consumption of forage fish during summer in 2004–2012 based on skin–muscle half-lives (i.e. muscle, year-integrated value; skin, summer-integrated value; ep—89%). Equally, between both time-periods, beluga also increased their consumption of forage fish (ep 86% for muscle and more than 99% for skin), with an associated decline in the consumption of Greenland halibut (ep 98% and more than 99% based on muscle and skin, respectively). For sympatric Greenland halibut, forage fish was identified as the dominant prey over both temporal scales, similar to [14] (table 2 and figure 1; see electronic supplementary material, figures S4 and S5 for stable isotope bi-plots by time-period).
Table 2.
Mean of δ13C and δ15N (‰) for potential prey items and their median contribution (95% Bayesian credible interval) to beluga and Greenland halibut diet by time-period. Bold font represents dietary estimates derived from beluga skin and Greenland halibut liver.
| common name | species name | n | δ13C ± s.d. | δ15N ± s.d. | contribution to beluga diet (%) | contribution to Greenland halibut diet (%) |
|---|---|---|---|---|---|---|
| 1982–2002 | ||||||
| squid | Gonatid sp. | 7 | −20.3 ± 0.9 | 11.4 ± 0.9 | 1 (0–2) | 6 (0–18) |
| 51 (47–55) | — | |||||
| shrimp | Pandalus borealis | 10 | −18.7 ± 0.5 | 13.3 ± 1.0 | 21 (7–33) | 13 (0–38) |
| 2 (0–6) | — | |||||
| Arctic cod | Boreogadus saida | 8 | −19.2 ± 0.5 | 14.1 ± 1.2 | 12 (1–29) | 79 (55–97) |
| 5 (0–11) | — | |||||
| Greenland halibut | Reinhardtius hippoglossoides | 14 | −19.6 ± 0.7 | 16.6 ± 0.4 | 67 (55–78) | — |
| 42 (37–47) | — | |||||
| 2004–2012 | ||||||
| squid | Gonatid sp. | 5 | −19.8 ± 0.6 | 11.2 ± 1.3 | 14 (0–31) | 2 (0–8) |
| 34 (19–48) | 16 (5–28) | |||||
| shrimp | Lebbeus polaris | 7 | −18.2 ± 0.2 | 13.9 ± 0.4 | 8 (0–18) | 2 (0–10) |
| 2 (0–10) | 7 (0–21) | |||||
| Arctic cod/capelin | Boreogadus saida/Mallotus villosus | 22 | −20.0 ± 0.4 | 13.7 ± 0.8 | 35 (3–58) | 95 (87–100) |
| 56 (30–77) | 76 (58–92) | |||||
| Greenland halibut | Reinhardtius hippoglossoides | 21 | −19.4 ± 0.4 | 16.4 ± 0.7 | 42 (27–63) | — |
| 7 (0–18) | — | |||||
Figure 1.
Median contributions (95% Bayesian credible interval) to beluga and Greenland halibut diet estimated from stable isotope mixing models from 1982–2002 and 2004–2012 in an AIGP context derived from skin–liver (summer) (a,b) and muscle (annual) (c,d), respectively. Symbols represent beluga (grey), Greenland halibut (green) and forage fish (Arctic cod 1982–2002, and capelin/Arctic cod 2004–2012; yellow).
Our study, for the first time to our knowledge, provides data to illustrate the temporal (seasonal and decadal) plasticity of AIGP between predators with a change in the availability of a shared prey resource as a result of climate change. Previous studies have typically examined AIGP dynamics during a single time-period [1,2], but given species redistributions across the globe [3,5], we demonstrate associated impacts on interspecific intraguild interactions between endemic predators over time. Northward-expanding capelin, a prevalent forage fish in Cumberland Sound since 2004, promotes opportunistic, flexible foraging behaviour among predators to take advantage of increased capelin abundance in the system. As a result, a decrease in AIGP pressure, which acts as a stabilizing mechanism in ecological communities by hindering trophic cascades [15,16], has occurred between beluga and Greenland halibut. This decrease in AIGP pressure and increased reliance on pelagic forage fish by both predators could, in theory, increase the susceptibility for ecosystem perturbations in the pelagic energy channel.
The degree of AIGP between beluga and Greenland halibut increased with season as beluga shifted from primarily consuming forage fish in the summer to consuming more Greenland halibut in late-autumn/winter. Our estimates of seasonal prey contributions are further supported by telemetry data. Beluga dive to shallower depths (0–100 m) during the summer than late-autumn and winter, when dives deeper than 400 m are more common [17]. These dive data suggest a seasonal switch from foraging on capelin in the summer to Greenland halibut in the winter [17]. As such, beluga likely expend more energy on foraging during the less-productive winter compared to summer, but may acquire a higher energy pay-off due to Greenland halibut being larger, on average, than capelin despite similar energy densities (4.7 and 5.5 kJ g−1) [18].
From 1982 to 2012, beluga consumed less Greenland halibut, while their diet of forage fish increased. Given increased accessibility to capelin in shallower waters during summer, beluga likely select not to expend high amounts of energy deep-diving for Greenland halibut. The cost of deep-diving to reach Greenland halibut, which typically reside at depths of approximately 1000 m in the summer [19], likely drives reduced predation pressure on Greenland halibut at this time. Greenland halibut are primarily piscivorous [7] and feed on a high proportion of forage fish when compared with beluga. Greenland halibut consequently represent a superior competitor than beluga to exploit shared forage fish resources—a common attribute in AIGP systems that promotes co-existence between predators [2]. Furthermore, with a declining Cumberland Sound beluga population [20], their increased reliance on forage fish through increased capelin availability and an increase in Greenland halibut abundance over time [21], Greenland halibut have likely undergone a mesopredator release.
Our data suggest a warming Arctic will continue to lead to altered interspecific intraguild interactions, thereby modifying predator–competitor–prey dynamics between endemic Arctic species. These ecosystem modifications have the potential to have reverberating effects across all trophic guilds in the Arctic, with ramifications on its ecosystem-level processes in our rapidly warming world.
Supplementary Material
Supplementary Material
Acknowledgements
We thank the Pangnirtung HTA for collecting marine mammal samples, and the R/V Paamiut, Margaret Treble, Kevin Hedges, Gregg Tomy, Bailey McMeans and Cortney Watt for fish and zooplankton samples/data.
Ethics
All tissue samples were acquired through Fisheries and Oceans Canada Licences to Fish for Scientific Purposes (FWI-ACC-06-07-010).
Data accessibility
Data supporting our results are provided in electronic supplementary material.
Authors' contributions
D.J.Y., N.E.H., A.T.F. and S.H.F. conceived and designed the study. K.L.I. and R.F.T. acquired Arctic char data and samples. D.J.Y. analysed, interpreted the data and wrote the manuscript, with all authors providing input and approving the final version. All authors agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by NSERC-Discovery and ArcticNet to S.H.F. and A.T.F., The Government of Nunavut to N.E.H. and A.T.F., and The W. Garfield Weston Foundation to D.J.Y.
References
- 1.Polis GA, Myers CA, Holt RD. 1989. The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu. Rev. Ecol. Syst. 20, 297–330. ( 10.1146/annurev.es.20.110189.001501) [DOI] [Google Scholar]
- 2.Polis GA, Holt RD. 1992. Intraguild predation: the dynamics of complex trophic interactions. Trends Ecol. Evol. 7, 151–154. ( 10.1016/0169-5347(92)90208-S) [DOI] [PubMed] [Google Scholar]
- 3.Pinsky ML, Worm B, Fogarty MJ, Sarmiento JL, Levin SA. 2013. Marine taxa track climate velocities. Science 341, 1239–1242. ( 10.1126/science.1239352) [DOI] [PubMed] [Google Scholar]
- 4.Fossheim M, Primicerio R, Johanessen E, Ingvaldsen RB, Aschan MM, Dolgov AV. 2015. Recent warming leads to rapid borealization of fish communities in the Arctic. Nat. Clim. Change 5, 673–677. ( 10.1038/nclimate2647) [DOI] [Google Scholar]
- 5.Rose GA. 2005. Capelin (Mallotus villosus) distribution and climate: a sea ‘canary’ for marine ecosystem change. ICES J. Mar. Sci. 62, 1524–1530. ( 10.1016/j.icesjms.2005.05.008) [DOI] [Google Scholar]
- 6.Kilabuk P. 1998. A study of Inuit knowledge of southeast Baffin beluga. Iqaluit, NU: Nunavut Wildlife Management Board. [Google Scholar]
- 7.Hovde SC, Albert OT, Nilssen EM. 2002. Spatial, seasonal and ontogenetic variation in diet of Northeast Arctic Greenland halibut (Reinhardtius hippoglossoides). ICES J. Mar. Sci. 59, 421–437. ( 10.1006/jmsc.2002.1171) [DOI] [Google Scholar]
- 8.Brown TA, Chrystal E, Ferguson SH, Yurkowski DJ, Watt CA, Hussey NE, Kelley TC, Belt ST. 2017. Coupled changes between the H-Print biomarker and δ15N indicates a variable sea ice carbon contribution to the diet of Cumberland Sound beluga whales. Limnol. Oceanogr. 62, 1606–1619. ( 10.1002/lno.10520) [DOI] [Google Scholar]
- 9.Browning NE, Dold C, I-Fan J, Worthy GAJ. 2014. Isotope turnover rates and diet–tissue discrimination in skin of ex situ bottlenose dolphins (Tursiops truncatus). J. Exp. Biol. 217, 214–221. ( 10.1242/jeb.093963) [DOI] [PubMed] [Google Scholar]
- 10.MacNeil MA, Drouillard KG, Fisk AT. 2006. Variable uptake and elimination of stable nitrogen isotopes between tissues in fish. Can. J. Fish. Aquat. Sci. 63, 345–353. ( 10.1139/f05-219) [DOI] [Google Scholar]
- 11.Vander Zanden MJ, Clayton MK, Moody EK, Solomon CT, Weidel BC.. 2015. Stable isotope turnover and half-life in animal tissues: a literature synthesis. PLoS ONE 10, e0116182 ( 10.1371/journal.pone.0116182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parnell AC, Jackson A. 2013. SIAR: Stable isotope analysis in R. R package version 4.2. See http://CRAN.R-project.org/package=siar. (accessed 8 October 2016)
- 13.R Development Core Team 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.Rproject.org) [Google Scholar]
- 14.Dennard ST, McMeans BC, Fisk AT. 2009. Preliminary assessment of Greenland halibut diet in Cumberland Sound using stable isotopes. Polar Biol. 32, 941–945. ( 10.1007/s00300-009-0624-3) [DOI] [Google Scholar]
- 15.Finke DL, Deno RF. 2005. Predator diversity and the functioning of ecosystems: the role of intraguild predation in dampening trophic cascades. Ecol. Lett. 8, 1299–1306. ( 10.1111/j.1461-0248.2005.00832.x) [DOI] [Google Scholar]
- 16.Holt RD, Polis GA. 1992. A theoretical framework for intraguild predation. Am. Nat. 149, 745–764. ( 10.1086/286018) [DOI] [Google Scholar]
- 17.Watt CA, Orr J, Ferguson SH. 2016. A shift in foraging behaviour of beluga whales Delphinapterus leucas from the threatened Cumberland Sound population may reflect a changing Arctic food web. Endanger. Species Res. 31, 259–270. ( 10.3354/esr00768) [DOI] [Google Scholar]
- 18.Lawson JW, Magalhães AM, Miller EH. 1998. Important prey species of marine vertebrate predators in the northwest Atlantic: proximate composition and energy density. Mar. Ecol. Prog. Ser. 164, 13–20. ( 10.3354/meps164013) [DOI] [Google Scholar]
- 19.Hussey NE, et al. 2017. Movements of a deep-water fish: establishing marine fisheries management boundaries in coastal Arctic waters. Ecol. Appl. 27, 687–704. ( 10.1002/eap.1485) [DOI] [PubMed] [Google Scholar]
- 20.Marcoux MM, Hammill MO.2016. Model estimates of Cumberland Sound beluga (Delphinapterus leucas) population size and total allowable removals. DFO Can. Sci. Res. Doc. 2016/077, iv+35p. See http://waves-vagues.dfo-mpo.gc.ca/Library/40573850.pdf .
- 21.Treble MA.2017. Report on Greenland halibut caught during the 2016 trawl survey in Divisions 0A and 0B. NAFO SCR Doc. 17-028. Northwest Atlantic Fisheries Organization.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting our results are provided in electronic supplementary material.