Abstract
While a growing body of literature explores the ecological implications of consistent individual variation in the behaviour of wildlife, few studies have looked at the reciprocal influences of personality within interspecific interactions, despite the potentially significant impacts on biodiversity. Here I used two species involved in cleaner-bird behaviour—black-billed magpies (Pica pica) and Rocky mountain elk (Cervus canadensis)—to show that the exhibition of mutualistic behaviour can depend on the personality of the individual involved. I recorded suites of correlated behaviours in both elk and magpies to derive personality gradients from ‘shy’ to ‘bold’, which I compared with observations of interspecific interactions. I measured each half of this mutualistic relationship separately. I found that bold elk were more likely to aggressively reject magpie landings, while shy elk allowed magpies to land and groom them. Contrastingly, I found it was bold magpies that were willing to risk landings, while shy magpies rarely attempted landings. These results show that the exhibition of interspecific behaviour is predicated on the personality of the individuals, and thus likely contributes to the selection and maintenance of personality variation within populations.
Keywords: cleaner-birds, flight response, mutualism, personality
1. Introduction
Mutualism describes an interspecific relationship in which both interacting individuals benefit, and can have important implications for the fitness of each individual [1]. Because there can be consistent individual variation in behaviours related to both intra- and interspecific interactions, personality traits [2,3] may influence the outcome of mutualistic interactions. For example, aggressive Anelosimus studiosus had ammensal, rather than mutualistic relationships with other spiders, while docile A. studiosus had higher fitness [4]. In an example from an aquatic system, cleaner fish (Labroides dimidiatus) with bolder personalities were more likely to cheat when in ostensibly mutualistic relationships with their ‘client’ fish [5]. One well-studied example of mutualism involving mammals is the relationships between large mammalian herbivores and ‘cleaner birds’ that feed on ectoparasites found on the mammal's skin or hair [6,7]. Because this is not obligate behaviour, individual variation persists in both the willingness of large herbivores [8] and cleaner birds [9,10,11] to engage in these interactions, and so these relationships may also be personality-dependent.
Magpies (Pica pica) are among the most intelligent birds in the world [12,13], and have previously been documented landing on and grooming wild ungulates, including moose (Alces alces) [7] and elk (Cervus canadensis) [14]. In these interactions magpies are typically targeting the winter tick (Dermacentor albipictus) [7,14]. Personality traits in elk and magpies have previously explained consistent individual variation in reactivity to stimulus [15], neofilia versus neophobia [12] and the weighing of risk versus reward (i.e. optimality) of differing foraging strategies [16], and so I predicted that the personalities of both magpies and elk would influence whether individuals within each species would engage in cleaner–herbivore behaviour.
2. Methods
I quantified the personality types in wild elk in Jasper (2012–13) and wild magpies in Edmonton (2016) from February to April, when winter tick activity and magpie–ungulate interactions peak [9]. I previously demonstrated that elk personality could be quantified by measuring individual variation in: flight response distance; interior–exterior positioning within groups; social dominance; vigilance; neophobia/neofilia [17]. I used previously collected behavioural data to quantify the behavioural types of 16 marked adult female elk that regularly interacted with magpies [18]. I used non-metric multidimensional scaling to reduce five correlated personality traits (table 1) to a single personality gradient (loss criterion:=0.0459). I opportunistically recorded all instances where magpie attempts to land were either accepted or rejected by the elk (electronic supplementary material, figure S1) and correlated elk acceptance rates with elk personality scores.
Table 1.
Correlation matrices for personality traits and magpie–elk mutualistic behaviour (italicized) for wild elk and magpies from different human-disturbed areas of Alberta, Canada.
| flight response | dominance | neofilia | position | vigilance | accept magpiea | |
|---|---|---|---|---|---|---|
| elk | ||||||
| flight response | 1 | |||||
| dominance | −0.796 | 1 | ||||
| neofilia | −0.717 | 0.723 | 1 | |||
| position | −0.790 | 0.826 | 0.787 | 1 | ||
| vigilance | 0.484 | −0.496 | −0.114 | −0.408 | 1 | |
| acceptance rate | 0.827 | −0.722 | −0.621 | −0.532 | 0.136 | 1 |
| flight response | dominance | neofilia | land-on-elkb | |||
|---|---|---|---|---|---|---|
| magpies | ||||||
| flight response | 1 | |||||
| dominance | −0.717 | 1 | ||||
| neofilia | −0.522 | 0.456 | 1 | |||
| land-on-elk | −0.699 | 0.601 | 0.547 | 1 |
aAllowing a magpie to land and remain on the elk's body.
bMagpie landings on an elk dummy.
I did not quantify personality in the same magpies that were interacting with wild elk because capturing magpies is invariably biased towards bold magpies, and magpies are so mobile that obtaining enough repeated measures of marked magpies that also interacted with marked elk was both unfeasible and unethical. Instead, I conducted 20 separate experimental trials at 20 different locations, and used the 20 different magpie groups as replicates. I attracted magpies to experimental sites by using peanuts placed on a tree stump. I only proceeded with the experiment if 3–5 magpies were present, as I could not reliably monitor ≥6 individual magpies simultaneously.
Previous, repeatable studies have determined that neophobia versus neofilia [12] and flight response distance [19] are personality traits in both magpies and elk [17]. Upon each magpie's initial arrival to the site I walked slowly towards it, from 40 m away, and recorded the distance at which the magpie took flight. I measured neofilia versus neophobia by recording the latency of magpies to land on a bicycle draped with blowing flagging tape and shiny ornaments. I recorded dyadic encounters where there was a clear displacement of one magpie by another and used this to delineate dominance hierarchies within each group. Lastly, at each site I placed an elk dummy treated with elk scent and covered in fur embedded with dogfood. Previous feeding experiments found that magpies' response to dogfood was similar to their reaction to live winter ticks [20]. I recorded individual magpie willingness to land on the artificial elk.
For each behavioural metric I ranked magpie responses relative to that of others within that same trial, and converted these ranks to percentiles to standardize values for different group sizes. This provided 20 separate response gradients for each behaviour [18]. This approach also allowed me to minimize habituating magpies to the artificial elk, novel object, or human observer [13,21]. I used classical multi-dimensional scaling to reduce these three personality traits to two orthogonal axes, dimensions, and used the first (explaining 76.9% of the data) as a gradient of personality (Mardia fit = 0.91). I used Spearman's rank correlation to compare each personality trait with the latencies of magpies to land on the artificial elk, and used logistic regression to determine whether the personality gradient predicted whether magpies land or not.
3. Results
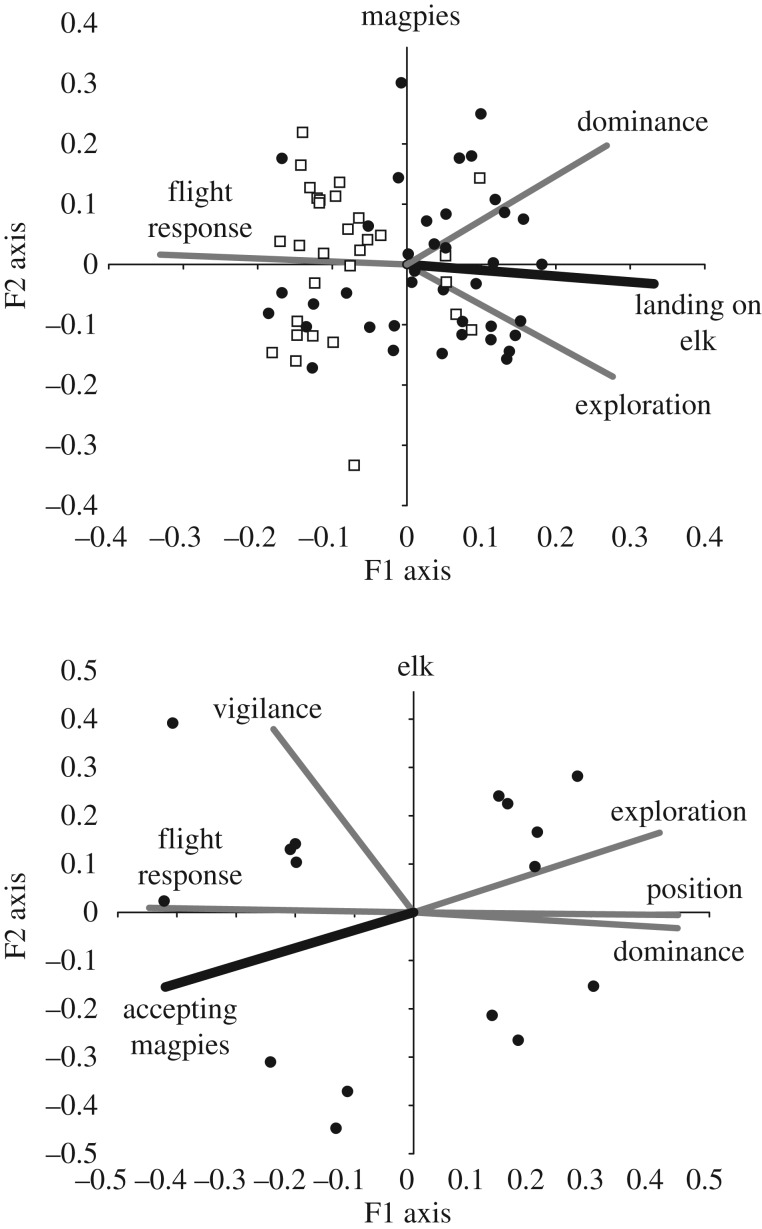
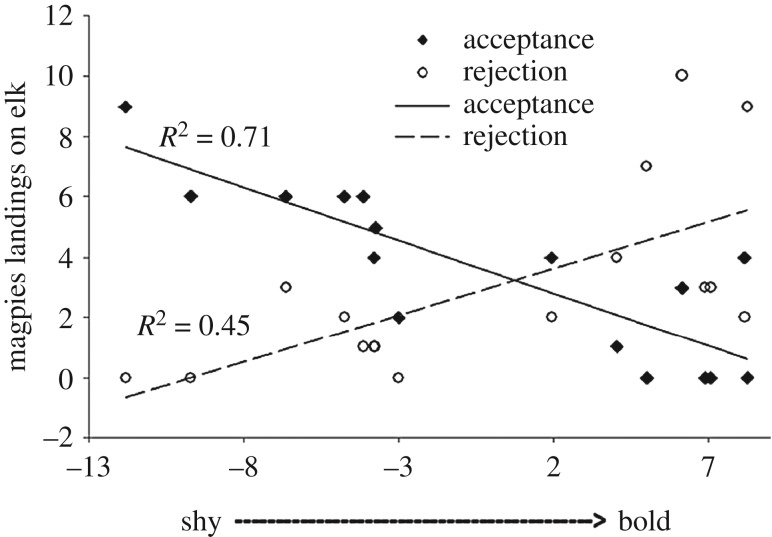
Compared to shyer elk, bolder elk had shorter flight response distance, spent less time on vigilance behaviour, adopted more peripheral positions within the herd, expressed more neofilia and were more dominant (figure 1). I presumed that these behaviours reflected the contrasting personalities of shy versus bold elk. Compared to shyer magpies, bolder magpies had shorter flight response distance, expressed more neofilia and were more dominant (figure 1). Adult elk accepted 53.8% of 104 attempted landings by magpies. Shyness of elk personality was strongly correlated with the rate of acceptance of landings by magpies (GLM: F1,14 = 32.29, adj. R2 = 0.68, p < 0.001; figure 2).
Figure 1.
Gabriel biplots showing correlations between different personality traits and elk–magpie mutualism behaviour (bold lines) for magpies (a) and elk (b). Solid dots indicate magpies that landed and fed off an artificial elk; hollow boxes indicate those that did not.
Figure 2.
Correlation between elk personality (x-axis) and frequency (y-axis) that individual elk accepted or rejected attempts by magpies to land on it.
Forty-eight of 77 individual magpies landed on the artificial elk (62.3%). The index ranking magpies on their latency to land was correlated with flight response distance (r = 0.84), neofilia (r = 0.73) and dominance (0.60; figure 1). The gradient of shyness to boldness of personality strongly predicted whether a magpie would land on the artificial elk or not (log likelihood = −32.81, χ2 = 39.6, pseudo R2 = 0.38, p < 0.001). In both elk and magpies, shy individuals had higher flight response distances, were neophobic and were submissive to bolder individuals.
4. Discussion
I found that cleaner–host interactions between magpies and elk were dependent on the personality of each individual. Contrastingly, it was shy elk but bold magpies that were most likely to interact with the other species. I can only speculate as to why some elk consistently rejected magpie landings, while others consistently did not. Ticks presumably affect all elk in the same way that arthropod parasites affect other large mammalian hosts [6] by disrupting feeding and resting, and altering blood chemistry [7]. However, habitual groomers like elk maintain lower tick loads than ‘stimulus groomers’ such as moose [9], so magpie landings may sometimes be only to the advantage of the magpie and may be better described as parasitic [22]. It is not known whether shy and bold elk have different probabilities of being parasitized by ticks, but if so, the occurrence of mutualism likely varies because of individual reactions to the stimuli magpies provide. Bold elk have increased rates of aggression towards conspecifics [17] and other species (e.g. humans [21]) and magpies may be eliciting similar aggression.
Experiments have shown that magpies land on cervids to access protein-rich ticks [20]. If the benefits are thus equal for all magpies, an individual's willingness to land must depend on the costs (i.e. risk) each individual perceives. Landing on large herbivores presents a risk to cleaner-birds [23]. Oxpeckers (Buphagus spp.) prefer landing where the ungulate is less able to fend off the attempt, such as on the necks of giraffes (Giraffa spp.) and backs of (elk-sized) greater kudu (Tragelaphus strepsiceros) [8]. Studies of fallow deer (Dama dama) found magpies preferred grooming bedded individuals [24]. My results support the idea that risk-taking is a personality trait conserved across taxa [15], as it was only magpies with bolder personalities that engaged in potentially risky interactions with elk. However, this risk-taking may be state-dependent, as hunger may drive an animal to take greater risks.
I found marked elk accepted only 53.8% of magpie landings, which is lower than the 83.9% by fallow deer [21] or 82.8% recorded for kudu accepting oxpeckers [8]. However, those studies observed unmarked ungulates, which preclude measurement of individual tendencies. Accepted landings result in longer-duration events than rejected attempts, so observations of unmarked ungulates will be biased towards accepted landings, and inflate acceptance rates. My observations of unmarked elk found an acceptance rate of 82.6%, similar to those other studies of unmarked ungulates [8].
Hosts and parasites are known to exert reciprocal influences on each other [25], but while I did not measure these influences simultaneously, I separately showed that each half of the mutualistic relationship was personality-dependent. Personality variation can develop and persist in populations of captive elk, in which ticks are entirely absent, but individual variation in parasite load may still influence the expression of personality traits [17]. At the same time, however, by having greater tolerance for magpies, shy elk may reduce their tick loads compared to bold elk, and this can help them compensate for being out-competed for forage by the more dominant, bold elk. These results may be important for further understanding the impacts of mutualism on biodiversity, and this is a promising avenue for further research.
Supplementary Material
Acknowledgements
I thank Parks Canada for support, all field assistants, Pete Hurd and Evie Merrill for insights and feedback, and Colleen St Clair for guidance and collaboration throughout the larger project from which this work stemmed.
Ethics
Ethical approval for the collection of animal behaviour data was obtained from the University of Alberta Ethics for Animal Use Committee, Protocol no. 7121112.
Data accessibility
Competing interests
I declare I have no competing interests.
Funding
This work was supported by Alberta Conservation Association (no. 0000334393), Alberta Sports, Parks, Recreation and Wildlife Foundation (no. 0008780), Canadian Circumpolar Institute (no. 0010338), and the Natural Science and Engineering Research Council.
References
- 1.Boucher DH, James S, Keeler KH. 1982. The ecology of mutualism. Annu. Rev. Ecol. Syst. 13, 315–347. ( 10.1146/annurev.es.13.110182.001531) [DOI] [Google Scholar]
- 2.Sih A, Cote J, Evans M, Fogarty S, Pruitt J. 2012. Ecological implications of behavioural syndromes. Ecol. Lett. 15, 278–289. ( 10.1111/j.1461-0248.2011.01731.x) [DOI] [PubMed] [Google Scholar]
- 3.Dochtermann NA, Dingemanse NJ. 2013. Behavioural syndromes as evolutionary constraints. Behav. Ecol. 24, 806–811. ( 10.1093/beheco/art002) [DOI] [Google Scholar]
- 4.Pruitt JN, Ferrari MCO. 2011. Intraspecific trait variants determine the nature of interspecific interactions in a habitat-forming species. Ecology 92, 1902–1908. ( 10.1890/11-0701.1) [DOI] [PubMed] [Google Scholar]
- 5.Wilson ADM, Krause J, Herbert-Read JE, Ward AJW. 2014. The personality behind cheating: behavioural types and the feeding ecology of cleaner fish. Ethology 120, 904–912. ( 10.1111/eth.12262) [DOI] [Google Scholar]
- 6.Sutherst RW. 1987. Ectoparasites and herbivore nutrition. In The nutrition of herbivores (eds Hacker JB, Ternouth JH), pp. 191–209. Sydney, Australia: Academic Press. [Google Scholar]
- 7.Glines MV, Samuel WM. 1984. The development of the winter tick, Dermacentor albipictus, and its effect on the hair coat of moose, Alces alces, of central Alberta, Canada. In Acarology, VI (eds Griffiths DA, Bowman CE), pp. 1208–1214. Chichester, UK: Ellis Horwood Ltd. [Google Scholar]
- 8.Ndlovu M, Combrink L. 2015. Feeding preferences of Oxpeckers in Kruger National Park, South Africa. Koedoe 57, 1–6. ( 10.4102/koedoe.v57i1.1316) [DOI] [Google Scholar]
- 9.Mooring MS, Samuel WM. 1998. The biological basis of grooming in moose: programmed versus stimulus-driven grooming. Anim. Behav. 56, 1561–1570. ( 10.1006/anbe.1998.0915) [DOI] [PubMed] [Google Scholar]
- 10.Plantan T, Howitt M, Kotze A, Gaines M. 2012. Feeding preference of the red-billed oxpecker, Buphagus erythrorhynchus: a parasitic mutualist? Afr. J. Ecol. 51, 325–336. ( 10.1111/aje.12042) [DOI] [Google Scholar]
- 11.Bishop AL, Bishop RP. 2013. Resistance of wild African ungulates to foraging by red-billed oxpeckers (Buphagus erythrorhynchus): evidence that this behaviour modulates a potentially parasitic interaction. Afr. J. Ecol. 52, 103–110. ( 10.1111/aje.12093) [DOI] [Google Scholar]
- 12.Rokka K, Pihlaja M, Siitari H, Soulsbury CD. 2014. Sex-specific differences in offspring personalities across the laying order in magpies Pica pica. Behav. Processes 107, 79–87. ( 10.1016/j.beproc.2014.07.019) [DOI] [PubMed] [Google Scholar]
- 13.Lee WY, Lee S-I, Choe JC, Jablonski PG. 2011. Wild birds recognize individual humans: experiments on magpies, Pica pica. Anim. Cogn. 14, 817–825. ( 10.1007/s10071-011-0415-4) [DOI] [PubMed] [Google Scholar]
- 14.Welch DA, Samuel WM, Wilke CJ. 1991. Suitability of moose, elk, mule deer, and white-tailed deer as hosts for winter ticks (Dermacentor albipictus). Can. J. Zool. 69, 2300–2305. ( 10.1139/z91-323) [DOI] [Google Scholar]
- 15.Koolhaas JM, de Boer SF, Buwalda B, van Reenan K. 2007. Individual variation in coping with stress: a multidimensional approach of ultimate and proximate mechanisms. Brain Behav. Evol. 70, 218–226. ( 10.1159/000105485) [DOI] [PubMed] [Google Scholar]
- 16.Chapman BB, Hulthen K, Blomqvist DR, Hansson L-A, Nilson J-A, Brodersen J, Nilsson PA, Skov C, Bronmark C. 2011. To boldly go: individual differences in boldness influence migratory tendency. Ecol. Lett. 14, 871–876. ( 10.1111/j.1461-0248.2011.01648.x) [DOI] [PubMed] [Google Scholar]
- 17.Found R, St. Clair CC. 2016. Behavioural syndromes influence migratory strategies in elk. Anim. Behav. 115, 35–46. ( 10.1016/j.anbehav.2016.02.007) [DOI] [Google Scholar]
- 18.Found R. 2017. Data from: Interactions between cleaner-birds and ungulates are personality dependent. ( 10.6084/m9.figshare.4876847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gravolin I, Key M, Lill A. 2014. Boldness of urban Australian magpies and local traffic volume. Avian Biol. Res. 7, 244–250. ( 10.3184/175815514X14151981691872) [DOI] [Google Scholar]
- 20.Samuel WM, Welch DA. 1991. Winter ticks on moose and other ungulates: factors influencing their population size. Alces 27, 169–182. [Google Scholar]
- 21.Kenney SP, Knight RL. 1992. Flight distances of black-billed magpies in different regimes of human density and persecution. Condor 94, 545–547. ( 10.2307/1369231) [DOI] [Google Scholar]
- 22.Musante AR, Pekins PJ, Scarpitti DL. 2007. Metabolic impacts of winter tick infestations on calf moose. Alces 43, 101–110. [Google Scholar]
- 23.Weeks P. 2000. Red-billed oxpeckers: vampires or tickbirds? Behav. Ecol. 11, 154–160. ( 10.1093/beheco/11.2.154) [DOI] [Google Scholar]
- 24.Genov PV, Gigantesco P, Massei G. 1998. Interactions between black-billed magpie and fallow deer. Condor 100, 177–179. ( 10.2307/1369914) [DOI] [Google Scholar]
- 25.Ezenwa VO, Archie EA, Craft ME, Hawley DM, Martin LB, Moore J, White L. 2016. Host behaviour–parasite feedback: an essential link between animal behaviour and disease ecology. Proc. R. Soc. B 283, 20153078 ( 10.1098/rspb.2015.3078) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.