Summary
Background
An unmet medical need exists for patients with metastatic renal cell carcinoma (RCC) who have progressed on a vascular endothelial growth factor (VEGF)–targeted therapy plus a mammalian target of rapamycin (mTOR) inhibitor. Fibroblast growth factor (FGF) pathway activation has been proposed as a mechanism of escape from VEGF-targeted therapies. Dovitinib is an oral tyrosine kinase inhibitor that inhibits VEGF and FGF receptors. This open-label, multicenter phase 3 study compared dovitinib with sorafenib as a third-line targeted therapy in metastatic RCC.
Methods
Patients (N = 570) with clear cell metastatic RCC who received one prior VEGF-targeted therapy and one prior mTOR inhibitor were randomized 1:1 to receive dovitinib (500 mg orally on a 5-days-on/2-days-off schedule) or sorafenib (400 mg orally twice daily). Randomization was stratified by risk group and region. The primary endpoint was progression-free survival (PFS) by central review. Secondary endpoints included overall survival (OS) and safety. Biomarker studies were an exploratory endpoint.
Findings
The median PFS was 3·7 months for dovitinib (n = 284) and 3·6 months for sorafenib (n = 286) (hazard ratio [HR], 0·86; 95% CI, 0·72-1·04; one-sided P = 0·063). Median OS was 11·1 months for dovitinib and 11·0 months for sorafenib (HR, 0·96; 95% CI, 0·75-1·22). Diarrhea, nausea, and vomiting were more common with dovitinib, whereas palmar-plantar erythrodysesthesia, hypertension, and alopecia were more common with sorafenib. In both arms, prolonged OS was observed in patients with low baseline plasma levels of FGF2, hepatocyte growth factor, and VEGFA.
Interpretation
Dovitinib demonstrated activity but not superior efficacy compared with sorafenib in patients who progressed on prior VEGF-targeted therapies and mTOR inhibitors. This trial provides landmark outcome data for future studies in this third-line setting.
Funding
Novartis Pharmaceuticals Corporation
Introduction
Renal cell carcinoma (RCC) is a tumor characterized by high vascularity that depends on angiogenesis for growth and survival.1,2 Therapies targeting vascular endothelial growth factor (VEGF) and mammalian target of rapamycin (mTOR) signaling pathways represent standard first- and second-line treatment options in metastatic RCC.3,4 Nearly all patients who initially respond to these therapies acquire resistance, and there is an unmet medical need for new agents targeting angiogenesis and tumor growth in patients with RCC previously treated with VEGF-targeted therapies and mTOR inhibitors.
Fibroblast growth factor (FGF) signaling drives angiogenesis at both the early invasive phase (eg, migration and proliferation) and the late vascular maturation phase (eg, morphogenesis and vessel maturation).5–7 FGF pathway activation has been proposed as a mechanism of escape from VEGF-targeted therapies,8 and increased plasma FGF2 levels were reported in patients with RCC experiencing disease progression while receiving VEGF-targeted therapies.9 Therefore, targeting antiangiogenic escape with FGF pathway inhibition represents one potential strategy in patients with RCC progressing on anti-VEGF therapy.10
Dovitinib (TKI258) is an oral tyrosine kinase inhibitor (TKI) that inhibits FGF receptor (FGFR), as well as VEGF receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR).11 Studies in RCC xenograft models have demonstrated dovitinib activity with trends toward greater tumor reduction compared with sunitinib and sorafenib.12,13 Phase 1 results indicated antitumor activity of dovitinib at the maximum tolerated dose of 500 mg on a 5-days-on/2-days-off schedule in pretreated patients with RCC.12 In phase 2 results, patients previously treated with VEGF and mTOR inhibitors demonstrated median progression-free survival (PFS) and overall survival (OS) of 5·5 and 11·8 months, respectively.14 These data as well as data from phase 2 studies of second- or third-line sorafenib demonstrating median PFS of 3·4 to 4 months15–19 supported studying dovitinib vs sorafenib as a third-line targeted treatment in patients who progressed on therapies targeting VEGF and mTOR.
Methods
Study design
The study (Global Oncologic Learnings for Dovitinib in RCC [GOLD RCC]) was a multicenter, open-label, randomized phase 3 trial comparing dovitinib vs sorafenib in patients with metastatic RCC. The primary endpoint was PFS, as assessed by central radiological review according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1·1.20 The key secondary endpoint was OS; additional secondary endpoints included overall response rate, time to definitive worsening of Karnofsky performance status (decrease by ≥ 10 points from baseline), and safety. Biomarker analyses were an exploratory endpoint.
Patients received dovitinib (500 mg, orally on a 5-days-on/2-days-off schedule) or sorafenib (400 mg, orally, twice daily) until disease progression, unacceptable toxicity, death, or withdrawal of consent. Treatment crossover was not permitted on study; following radiological confirmation of disease progression, the investigator could prescribe any treatment(s) deemed appropriate. Drug-related toxicities could be managed with dose interruptions (up to 21 days) or reductions (dovitinib: 400 mg, then 300 mg on the 5-days-on/2-days-off schedule; sorafenib: 400 mg once daily, then 400 mg every other day).
The study protocol was reviewed and approved by each site's institutional review board/independent ethics committee/research ethics board. All patients provided written informed consent. The trial was conducted in accordance with the ICH Harmonised Tripartite Guidelines for Good Clinical Practice, with applicable local regulations, and with the ethical principles laid down in the Declaration of Helsinki.
Randomization and masking
Patients were randomized 1:1 to receive either dovitinib or sorafenib using an interactive web and voice response system stratified by risk group (favorable, intermediate, and poor) based on the Memorial Sloan-Kettering Cancer Center risk criteria21 and geographic region (Japan, Asia Pacific, Europe/Middle East, and Americas).
Patients
Eligible patients had metastatic RCC with clear cell histology and had received one prior VEGF-targeted therapy (eg, sunitinib, bevacizumab) plus one prior mTOR inhibitor (eg, everolimus, temsirolimus) in either sequence. Patients must have had disease progression on or within 6 months of last therapy (VEGF-targeted agent and/or mTOR inhibitor therapy). Prior treatment with cytokines or certain specified anticancer therapies was permitted. Additional inclusion criteria included measurable disease (RECIST 1·1), Karnofsky performance status ≥ 70%, and adequate hematologic, renal, and hepatic function. Exclusion criteria included previous sorafenib or dovitinib, brain metastases, clinically significant cardiac diseases, or uncontrolled hypertension.
Assessments
Tumors were evaluated using RECIST 1·1 via computed tomography scan or magnetic resonance imaging every 8 weeks during the first year of treatment and every 12 weeks thereafter. Patients who had disease progression, as determined by the local investigator, continued to receive study drug until disease progression was confirmed by a blinded central review. Following confirmation, the patient discontinued study treatment. If there was discordance between the local and central radiologist, the patient could continue study treatment and was reassessed every 8 weeks or as frequently as considered appropriate. This review to facilitate investigator response determination was a separate process from the independent blinded review to assess the primary endpoint. Patients who discontinued study treatment (for reasons other than centrally confirmed disease progression, death, or lost to follow-up) continued to have tumor assessments until the start of new anticancer therapy or up to 4 months after discontinuation of study treatment.
Safety assessments included regular monitoring of hematology, coagulation, blood chemistry, physical examination, electrocardiogram, and multiple gated acquisition scan or echocardiogram. Adverse events (AEs) were assessed according to the Common Terminology Criteria for Adverse Events version 4·03 throughout the study and 30 days after the last dose of study treatment. Blood samples for dovitinib trough concentrations were collected predose on day 5 of weeks 2, 4, and 6.
Biomarkers
Blood samples for pharmacodynamic analyses were collected predose on days 1, 26, and 57 and subsequently every 12 weeks (on day 1 of that week) and at the end of study treatment. Circulating growth factors VEGFA, FGF2, placental growth factor (PLGF), and hepatocyte growth factor (HGF) were evaluated by immunoassay (Meso Scale Discovery, Rockville, MD, USA). Details on biomarker analysis and statistics are provided in the appendix.
Statistics
The distribution of PFS was estimated using the Kaplan-Meier method. A total of 411 PFS events were needed for a one-sided log-rank test at 2·5% significance level to have ≥ 96% power to show a statistically significant difference when the true hazard ratio (HR) is 0·67 (eg, when the median PFS in dovitinib was 6 months and the median PFS in sorafenib was 4 months). Assumptions for the median PFS of sorafenib were based on results of clinical trials and consideration of the disease condition after progression from two lines of prior anticancer treatments.18,22
The study incorporated an interim analysis for PFS, which took place after 175 PFS events (per central review) were documented. Both futility and efficacy analyses were performed by an independent statistician at the interim analysis. An α-spending function according to Lan-DeMets group sequential design with an O'Brien-Fleming type stopping boundary was used to construct the efficacy stopping boundaries. A user-defined gamma function (γ = 3) was used as β-spending function to determine the futility boundary. Interim analyses for OS were conducted concurrently with the interim and final PFS analyses. The final OS analysis will take place after 386 deaths are recorded, to provide a one-sided log-rank test at 2·5% significance level to have ≥ 80% cumulative power to show a statistically significant difference when the true HR is 0·75.
This study is registered with ClinicalTrials.gov, number NCT01223027.
Role of the funding source
Novartis Pharmaceuticals Corporation was the funding source and with the study steering committee participated in study design and data collection, analysis, and interpretation. All authors had full access to the primary data. RJM had the final responsibility for the decision to submit for publication.
Results
Patient characteristics
A total of 764 patients were assessed for eligibility. Most of the 194 patients not proceeding to randomization were considered screening failures (n = 169). The most common reason for screening failure was the presence of brain metastases (n = 59). Between March 2011 and September 2012, 570 patients were randomized at 199 sites to receive dovitinib (n = 284) or sorafenib (n = 286; Figure 1). Of these, 280 and 284 patients received at least one dose of dovitinib and sorafenib, respectively. As of the January 2013 data cutoff, 440 PFS (central) and 265 OS events were reported.
Figure 1.
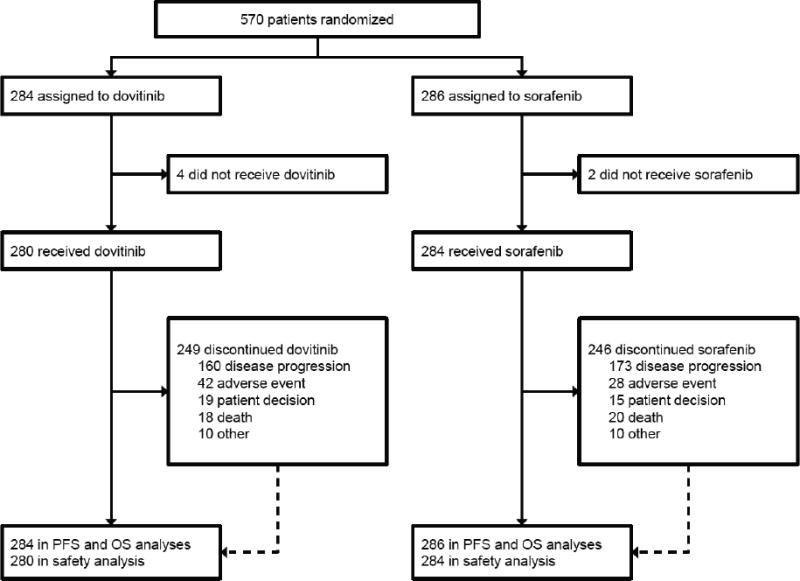
Trial profile.
OS, overall survival; PFS, progression-free survival.
Patient characteristics were balanced between the arms (Table 1). The most common prior VEGF inhibitor was sunitinib (90% overall), and the most common mTOR inhibitor was everolimus (87% overall). Most patients (92%) received a VEGF inhibitor followed by an mTOR inhibitor. As of the data cutoff, the majority of patients (87%) discontinued treatment, most commonly due to disease progression (58%; Figure 1).
Table 1. Baseline demographics and disease characteristics.
| Dovitinib (n = 284) | Sorafenib (n = 286) | |
|---|---|---|
| Age, years, median (range) | 61 (29–89) | 62 (18–81) |
| Age ≥ 65 years, n (%) | 97 (34) | 121 (42) |
| Sex, n (%) | ||
| Male | 213 (75) | 219 (77) |
| Female | 71 (25) | 67 (23) |
| Race, n (%) | ||
| White | 233 (82) | 232 (81) |
| Asian | 42 (15) | 40 (14) |
| Black | 3 (1) | 5 (2) |
| Other/unknown | 6 (2) | 9 (3) |
| Region, n (%) | ||
| Europe and Middle East | 171 (60) | 170 (59) |
| America | 66 (23) | 67 (23) |
| Asia (excluding Japan) | 28 (10) | 28 (10) |
| Japan | 19 (7) | 21 (7) |
| Karnofsky performance score, n (%) | ||
| 100 | 83 (29) | 73 (26) |
| 90 | 93 (33) | 101 (35) |
| 80 | 73 (26) | 83 (29) |
| 70 | 35 (12) | 29 (10) |
| MSKCC risk group, n (%)21 | ||
| Favorable | 58 (20) | 59 (21) |
| Intermediate | 164 (58) | 162 (57) |
| Poor | 62 (22) | 65 (23) |
| Metastatic site of cancer, n (%) | ||
| Lung | 224 (79) | 216 (76) |
| Lymph nodes | 144 (51) | 147 (51) |
| Bone | 99 (35) | 119 (42) |
| Liver | 94 (33) | 94 (33) |
| Prior therapy, n (%) | ||
| Prior nephrectomy | 272 (96) | 260 (91) |
| Radiotherapy | 66 (23) | 91 (32) |
| Cytokines | 20 (7) | 23 (8) |
| Targeted therapy | ||
| VEGF targeted | 284 (100) | 286 (100) |
| Sunitinib | 260 (92) | 253 (88) |
| Bevacizumab | 10 (4) | 11 (4) |
| Axitinib | 3 (1) | 6 (2) |
| Pazopanib | 10 (4) | 11 (4) |
| Other investigational agent | 1 (< 1) | 5 (2) |
| mTOR inhibitor | 284 (100) | 286 (100) |
| Everolimus | 247 (87) | 247 (86) |
| Temsirolimus | 35 (12) | 39 (14) |
| Other investigational agent | 2 (1) | 0 |
| Number of prior treatment regimens, n (%) | ||
| 2 | 264 (93) | 259 (91) |
| 3 | 16 (6) | 25 (9) |
| ≥ 4 | 4 (1) | 2 (1) |
| Best response to last VEGF-targeted agent, n (%) | ||
| Complete response | 7 (2) | 5 (2) |
| Partial response | 76 (27) | 72 (25) |
| Stable disease | 121 (43) | 120 (42) |
| Disease progression | 50 (18) | 42 (15) |
| Unknown/not applicable | 29 (10) | 42 (15) |
| Best response to last mTOR inhibitor, n (%) | ||
| Complete response | 0 | 1 (< 1) |
| Partial response | 19 (7) | 18 (6) |
| Stable disease | 123 (43) | 105 (37) |
| Disease progression | 108 (38) | 116 (41) |
| Unknown/not applicable | 32 (11) | 46 (16) |
MSKCC, Memorial Sloan-Kettering Cancer Center; mTOR, mammalian target of rapamycin; VEGF, vascular endothelial growth factor.
Efficacy
The median PFS by central radiologist review was 3·7 and 3·6 months in the dovitinib and sorafenib arms, respectively (HR, 0·86; 95% CI, 0·72-1·04; one-sided P = 0·063; Figure 2). By investigator assessment, the median PFS was 3·9 months in both arms (HR, 1·00; 95% CI, 0·82-1·21); appendix Figure 1). Analysis of PFS by patient demographics and disease characteristics showed 95% CIs that crossed unity (appendix Figure 2). The best overall response by central review was partial response (4% in both arms; stable disease was 52% in both arms; appendix Table 1). The best percentage change from baseline in sum of diameters as per central review by waterfall plot was evaluable in 230 and 241 patients and showed tumor reductions in 117 (51%) and 111 (46%) patients in the dovitinib and sorafenib arms, respectively (Figure 3).
Figure 2.
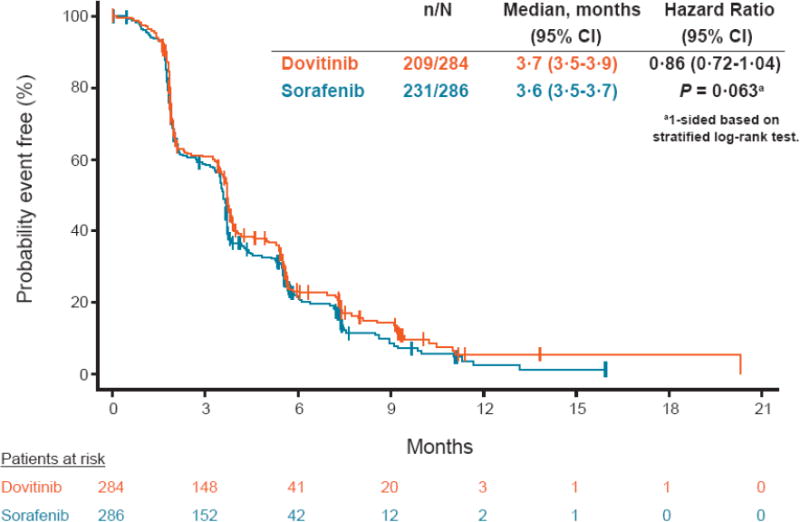
Kaplan-Meier estimates of progression-free survival (by central analysis).
Figure 3.
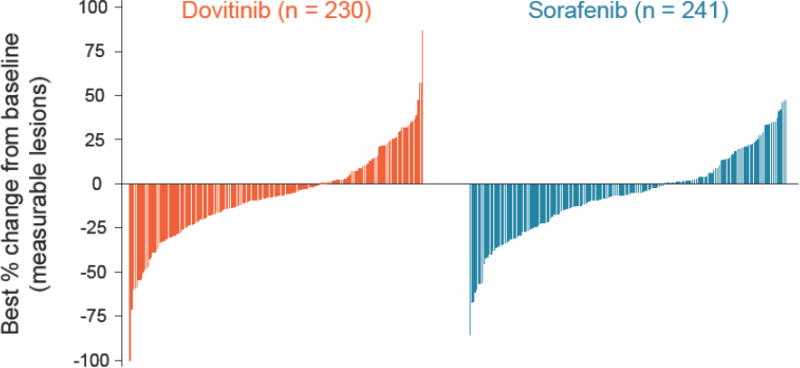
Best percentage change from baseline.
Excludes one dovitinib patient who had 170% increase from baseline and 48 dovitinib and 60 sorafenib patients who had percentage changes in target lesions that were contradicted by overall lesion response of disease progression.
The median OS was 11·1 and 11·0 months in the dovitinib and sorafenib arms, respectively (HR, 0·96; 95% CI, 0·75-1·22; Figure 4). The median time to definitive worsening of Karnofsky performance status was 5·1 months in the dovitinib arm and 5·7 months in the sorafenib arm (HR, 1·12; 95% CI, 0·87-1·45; appendix Figure 3).
Figure 4.
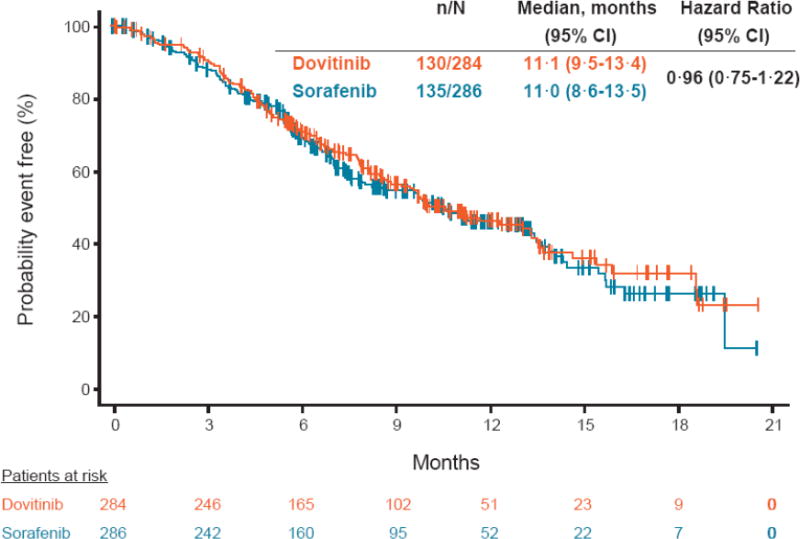
Kaplan-Meier estimates of overall survival.
Safety and exposure
In each arm, the median duration of exposure was 3·7 months and the median relative dose intensity was 99%. The geometric mean trough concentration of dovitinib was 93·8, 82·7, and 83·7 ng/mL in the 205, 202, and 170 patients with pharmacokinetic assessments on day 5 of weeks 2, 4, and 6, respectively. These trough concentrations, which were similar to those predicted,23 were not predictive of the safety and efficacy of dovitinib in the current study (data not shown).
Dose changes (mostly reductions) took place in 96 (34%) and 119 (42%) patients in the dovitinib and sorafenib arms, respectively. Dose interruptions were reported in 134 patients (48%) in the dovitinib arm and 99 patients (35%) in the sorafenib arm. AEs led to dose changes/interruptions in 144 patients (51%) in the dovitinib arm, most commonly due to fatigue (11%), diarrhea (9%), nausea (6%), and vomiting (5%). In the sorafenib arm, 138 patients (49%) had AEs leading to dose change/interruption, most commonly due to palmar-plantar erythrodysesthesia (14%), fatigue (7%), diarrhea (5%), and decreased appetite (4%).
Treatment-emergent AEs of any grade reported with a frequency of ≥ 10% in the dovitinib arm are shown in Table 2. Treatment-emergent grade 3/4 AEs with a frequency of ≥ 5% were hypertriglyceridemia (14%), fatigue (10%), hypertension (8%), diarrhea (7%), dyspnea (6%), anemia (5%), and γ-glutamyltransferase increase (5%) in the dovitinib arm, and hypertension (17%), fatigue (8%), dyspnea (7%), palmar-plantar erythrodysesthesia (6%), and anemia (6%) in the sorafenib arm. Treatment-emergent acne-like rashes (including rash, rash maculopapular, rash pruritic, dermatitis, dermatitis acneiform, and acne) were reported in 85 (30% any grade, 1% grade 3) and 66 (23% any grade, 2% grade 3) patients treated with dovitinib and sorafenib, respectively. Treatment-emergent serious AEs were reported in 133 (48%) and 112 (39%) patients in the dovitinib and sorafenib arms, respectively. The frequency of individual serious AEs was < 6% (any grade).
Table 2. Treatment-emergent adverse events (≥ 10%) and selected laboratory abnormalities.
| Dovitinib (n = 280) | Sorafenib (n = 284) | |||||
| Any grade n (%) | Grade 3 n (%) | Grade 4 n (%) | Any grade n (%) | Grade 3 n (%) | Grade 4 n (%) | |
| Adverse events | ||||||
| Diarrhea | 189 (68) | 18 (6) | 2 (1) | 128 (45) | 10 (4) | 1 (< 1) |
| Nausea | 147 (53) | 9 (3) | 0 | 83 (29) | 7 (2) | 0 |
| Vomiting | 123 (44) | 10 (4) | 0 | 46 (16) | 3 (1) | 0 |
| Fatigue | 115 (41) | 28 (10) | 0 | 97 (34) | 23 (8) | 1 (< 1) |
| Decreased appetite | 92 (33) | 5 (2) | 0 | 99 (35) | 13 (5) | 1 (< 1) |
| Rash* | 85 (30) | 4 (1) | 0 | 66 (23) | 6 (2) | 0 |
| Asthenia | 64 (23) | 13 (5) | 0 | 47 (17) | 11 (4) | 0 |
| Weight decrease | 61 (22) | 4 (1) | 0 | 87 (31) | 1 (< 1) | 0 |
| Dyspnea | 61 (22) | 14 (5) | 2 (1) | 57 (20) | 18 (6) | 3 (1) |
| Hypertriglyceridemia | 55 (20) | 27 (10) | 11 (4) | 2 (1) | 1 (< 1) | 0 |
| Hypertension | 54 (19) | 22 (8) | 0 | 79 (28) | 47 (17) | 0 |
| Constipation | 50 (18) | 0 | 0 | 69 (24) | 3 (1) | 0 |
| Cough | 50 (18) | 4 (1) | 0 | 48 (17) | 2 (1) | 0 |
| Pyrexia | 46 (16) | 2 (1) | 0 | 42 (15) | 2 (1) | 1 (< 1) |
| Back pain | 41 (15) | 7 (3) | 0 | 35 (12) | 7 (2) | 1 (< 1) |
| Abdominal pain | 38 (14) | 10 (4) | 0 | 38 (13) | 4 (1) | 0 |
| Pain in extremity | 35 (13) | 5 (2) | 0 | 29 (10) | 4 (1) | 0 |
| Palmar-plantar erythrodysesthesia | 32 (11) | 3 (1) | 0 | 115 (40) | 17 (6) | 1 (< 1) |
| Anemia | 32 (11) | 14 (5) | 1 (< 1) | 29 (10) | 16 (6) | 1 (< 1) |
| Stomatitis | 31 (11) | 1 (< 1) | 0 | 55 (19) | 5 (2) | 1 (< 1) |
| Dyspepsia | 31 (11) | 0 | 0 | 14 (5) | 1 (< 1) | 0 |
| Dysgeusia | 31 (11) | 0 | 0 | 8 (3) | 0 | 0 |
| Abdominal pain upper | 30 (11) | 3 (1) | 0 | 24 (8) | 3 (< 1) | 0 |
| Myalgia | 28 (10) | 3 (1) | 0 | 17 (6) | 0 | 0 |
| Dizziness | 28 (10) | 3 (1) | 0 | 7 (2) | 0 | 0 |
| Hematologic laboratory abnormalities | ||||||
| Anemia | 207 (74) | 18 (6) | 0 | 193 (68) | 16 (6) | 0 |
| Lymphopenia | 116 (41) | 41 (15) | 1 (< 1) | 123 (43) | 36 (13) | 4 (1) |
| Leukopenia | 85 (30) | 6 (2) | 0 | 28 (10) | 2 (1) | 0 |
| Thrombocytopenia | 47 (17) | 3 (1) | 3 (1) | 30 (11) | 0 | 1 (< 1) |
| Neutropenia | 45 (16) | 5 (2) | 2 (1) | 20 (7) | 6 (2) | 0 |
| Elevated biochemistry laboratory levels | ||||||
| Aspartate aminotransferase | 93 (33) | 4 (1) | 0 | 63 (22) | 4 (1) | 1 (< 1) |
| Alanine aminotransferase | 81 (29) | 3 (1) | 0 | 51 (18) | 2 (1) | 2 (1) |
| Total bilirubin | 13 (5) | 0 | 0 | 22 (8) | 3 (1) | 0 |
Includes rash, rash maculopapular, rash pruritic, dermatitis, dermatitis acneiform, and acne.
Selected hematologic and laboratory abnormalities are shown in Table 2. Notable changes (≤ 20%) from baseline in cardiac ejection fraction (evaluated by echocardiogram or multiple gated acquisition scan) were reported in 16 patients in each arm. Hypothyroidism was reported in 14 (5%) and 8 (3%) patients (all grade 1/2 events) in the dovitinib and sorafenib arms, respectively.
Deaths on study or within 30 days after last dose were reported in 39 (14%) and 42 (15%) patients, most commonly due to RCC (n = 33 and n = 36) in the dovitinib and sorafenib arms, respectively. Four deaths were suspected to be related to study drug (large intestine perforation, pulmonary embolism, and death not otherwise specified in the dovitinib arm and toxic epidermal necrolysis in the sorafenib arm).
Biomarkers
Plasma samples were available from 281 patients in the dovitinib arm and 280 patients in the sorafenib arm. Prolonged OS in the dovitinib and sorafenib arms was observed in the subgroups of patients with low baseline levels of FGF2, HGF, PLGF, and VEGFA (Figure 5). A similar trend was observed for PFS; however, P values were less significant for all biomarkers except HGF (appendix Figure 4). Significant changes from baseline including increases in PLGF and VEGFA levels were observed following treatment with dovitinib and sorafenib, consistent with VEGFR inhibitory effects (Figure 5). HGF and FGF2 levels increased following treatment with dovitinib and decreased following treatment with sorafenib (Figure 5).
Figure 5.
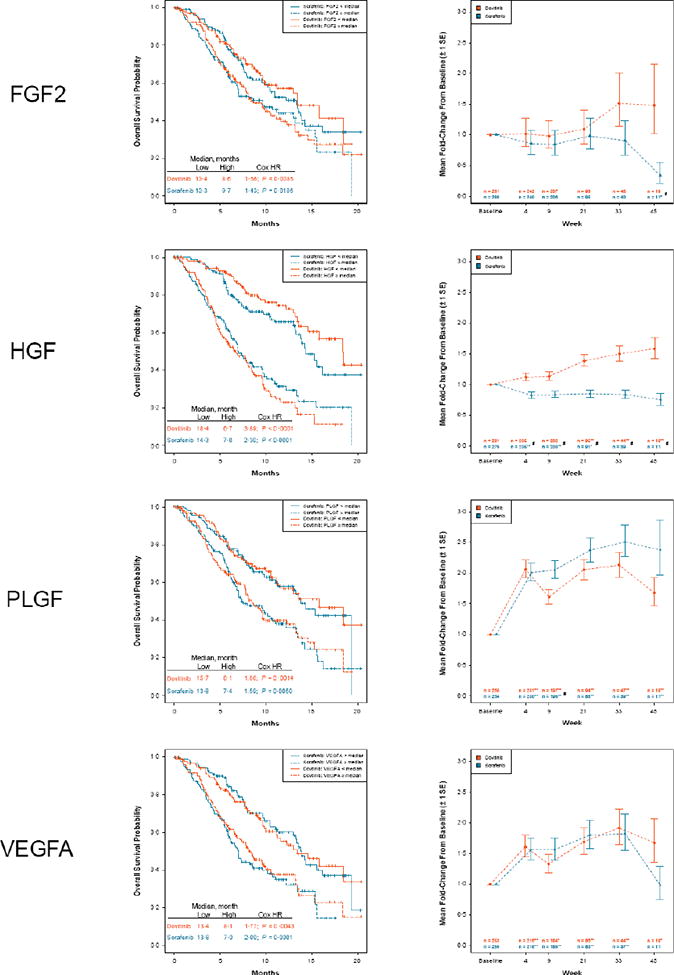
Kaplan-Meier estimates of overall survival by treatment and baseline plasma biomarker groups and model-adjusted average fold-change from baseline ± 1 standard error.
FGF2, fibroblast growth factor 2; HGF, hepatocyte growth factor; HR, hazard ratio; PLGF, placental growth factor; VEGFA, vascular endothelial growth factor A. Low biomarker is defined as < median, and high biomarker is defined as ≥ median. P values are adjusted for the false discovery rate. *0.05 ≥ P > 0.01. **P ≤ 0.01 (change from baseline). #P ≤ 0.05 (dovitinib to sorafenib).
Discussion
GOLD RCC is the first phase 3 trial comparing two TKIs in the third-line setting following both VEGF and mTOR inhibitor drugs. Nearly all patients treated with these therapies in the front line eventually progress; although the mechanism for acquired resistance is not clearly understood, activation of the FGF pathway is suspected to play a role in antiangiogenic escape from VEGF-targeted therapies.8,9,12 Dovitinib was selected for comparison with sorafenib based on its targeting profile which includes FGFR, in addition to the VEGFR and PDGFR inhibition that is shared with sorafenib. Historical cross-study comparisons suggested superior antitumor activity for dovitinib compared with sorafenib in patients who progressed on prior anti-VEGF and mTOR inhibitor therapies.12,14
Although both TKIs showed similar antitumor activity, the study did not meet its primary endpoint of demonstrating PFS superiority for dovitinib over sorafenib. An objective response rate of 4% was observed in each arm, and a relatively high proportion of patients had measurable lesions decreased by waterfall assessment. Comparison with historical control data suggests that both dovitinib and sorafenib may prolong PFS in the third-line setting. For example, in the everolimus pivotal phase 3 study (Renal Cell Cancer Treatment With Oral RAD001 Given Daily [RECORD-1]), patients in the placebo arm who had previously progressed on sunitinib and sorafenib had a median PFS of 1 ·8 months.24 In GOLD RCC, patients had a median PFS nearly twice that despite receiving both prior VEGF-targeted therapy and mTOR inhibitors. Thus, the results of this study support the use of a VEGFR TKI (ie, sorafenib) in patients who have already received prior VEGF and mTOR inhibitors. The OS results of ≈ 11 months highlight the continued need for study and identification of novel drugs for RCC and provide landmark outcome data for clinical trial design.
Comparable efficacy of dovitinib and sorafenib challenges the hypothesis that FGF activation is the fundamental mechanism of resistance to VEGF-targeted therapy. However, multiple factors could be responsible, including alternative mechanisms of resistance,10 reactivation of previously suppressed pathways, drug dosing, and pharmacodynamic effects at the tumor site. Dovitinib, as an FGF and VEGF pathway inhibitor, had demonstrated activity in preclinical models of RCC and in a phase 1/2 trial in patients who failed prior anti-VEGF and mTOR therapies.12,14 Moreover, up-regulation of FGF signaling may cause escape from VEGF-targeted therapies, as was suggested by relative high plasma FGF2 levels found in patients who previously received anti-VEGF and mTOR therapies in the prior study.12 In GOLD RCC, however, the median baseline plasma FGF2 levels were comparable to those observed in the RCC patient population regardless of prior therapies and were not particularly elevated (data not shown). However, the role of the FGF pathway in disease prognosis cannot be discounted because prolonged OS was observed in patients with low baseline FGF2 levels (Figure 5).
Increased FGF levels following dovitinib treatment are consistent with FGFR inhibition,12,25 and both dovitinib and sorafenib had similar anti-VEGF target inhibition (as demonstrated by VEGFA and PLGF induction). The activity of these agents in the third-line setting suggests that VEGF pathway inhibition might offer an effective treatment option for patients previously treated with anti-VEGF and mTOR inhibitors. HGF levels also increased following treatment with dovitinib, which has not been reported before. Further analyses are required to determine the significance of this finding. Nevertheless, the biomarker results are hypothesis generating and should be interpreted with caution.
GOLD RCC provides the first comparison of the AE profile for dovitinib with that of a VEGFR TKI. Gastrointestinal side effects were the most common AEs reported in the dovitinib arm. As with other VEGFR TKIs, mild elevation of liver enzymes was observed, although no patients were reported to have met Hy's law criteria: the presence of concurrent alanine or aspartate aminotransferase levels > 3 × upper limit of normal (ULN), serum total bilirubin > 2 × ULN, and serum alkaline phosphatase < 2 × ULN.26,27 Rash was observed in 30% of patients treated with dovitinib and was characterized as a distinct follicular acneiform rash predominantly on the face and chest. Overall, dovitinib demonstrated a tolerable safety profile with no unexpected toxicities noted beyond those in phase 1/2 dovitinib studies.12,14
In summary, dovitinib showed clinical activity in the treatment of advanced RCC, following VEGF-targeted and mTOR inhibitor therapy, but was not superior to sorafenib. Results highlight the continued need for more effective drugs for RCC and provide landmark outcome data for studies of novel third-line agents.
Panel: Research in context
Systematic review
We searched PubMed using the MeSH terms “enal cell carcinoma, ” “receptors, vascular endothelial growth factor,” and “TOR serine-threonine kinases” for English-language articles. Prior to this study, there had been no large, prospective, randomized phase 3 clinical trials comparing treatment options in patients with RCC who had previously received one prior VEGF-targeted therapy and one prior mTOR inhibitor.
Therefore, GOLD RCC is the first study of its kind in this patient population, comparing the efficacy and safety of dovitinib vs sorafenib in 570 patients.
Interpretation
The study did not meet its primary endpoint (PFS by central review) because dovitinib and sorafenib had similar efficacy in this patient population. Biomarker data from this study suggest that VEGFR and FGFR inhibition can be achieved in the third-line setting. Data from GOLD RCC provide landmark efficacy data for future study of third-line agents. Indeed, the 11-month median OS highlights the need for novel agents in this setting.
Supplementary Material
Appendix Figure 1: Kaplan-Meier analysis of progression-free survival by investigator review.
Appendix Figure 2: Forest plots of progression-free survival.
MSKCC, Memorial Sloan-Kettering Cancer Center.
Appendix Figure 3: Definitive worsening of Karnofsky performance status.
Appendix Figure 4: Kaplan-Meier estimates of progression-free survival by treatment and baseline plasma biomarker groups.
FGF2, fibroblast growth factor 2; HGF, hepatocyte growth factor; HR, hazard ratio; PLGF, placental growth factor; VEGFA, vascular endothelial growth factor A. Low biomarker is defined as < median, and high biomarker is defined as ≥ median.
Acknowledgments
The study was sponsored by Novartis Pharmaceuticals Corporation. The authors thank the patients, families, and research teams for their involvement with the study. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. We thank Peter J. Simon, PhD, for medical editorial assistance with this manuscript.
Appendix Table 1
Best overall response.
| Dovitinib (n = 284) | Sorafenib (n = 286) | |
|---|---|---|
| Central review, n (%) | ||
| Complete response | 0 | 0 |
| Partial response | 11 (4) | 11 (4) |
| Stable disease | 147 (52) | 149 (52) |
| Progressive disease | 82 (29) | 90 (31) |
| No measurable disease at baseline per central review | 3 (1) | 1 (< 1) |
| Unknown* | 41 (14) | 35 (12) |
| Investigator review, n (%) | ||
| Complete response | 0 | 0 |
| Partial response | 18 (6) | 11 (4) |
| Stable disease | 164 (58) | 173 (60) |
| Progressive disease | 60 (21) | 66 (23) |
| No measurable disease at baseline per central review | 0 | 0 |
| Unknown* | 42 (15) | 36 (13) |
Includes patients with no valid postbaseline assessment, all postbaseline assessments having overall response of unknown, new antineoplastic therapy started before first postbaseline assessment, stable disease < 6 weeks after randomization, and progressive disease > 17 weeks after randomization.
Footnotes
Conflicts of Interest: RJM has received research funding from Novartis, Pfizer, and GlaxoSmithKline, has consulted for Bayer and Pfizer, and has provided paid expert testimony for Pfizer. CP has acted as a speaker and/or consultant for Novartis, GlaxoSmithKline, Bayer Schering, Astellas, AVEO, and Boehringer-Ingelheim and has received research grants from Novartis and Bayer Schering. NJV has consulted for Novartis and Bayer. CNS has received honoraria from Novartis, Pfizer, and GlaxoSmithKline. CS has been an advisory board member for GlaxoSmithKline, Astellas, Pfizer, and Novartis. CK has received honoraria for presentations from and has consulted and been an advisory board member for Novartis. GAB has received honoraria for CME lectures on RCC and funding for travel to cancer meetings from Novartis. BM has received honoraria for speeches and advisory board meetings from Novartis, Bayer, Pfizer, Roche, and Astellas. UDG has received honoraria as a speaker and/or consultant for GlaxoSmithKline, Pfizer, Bayer, and Novartis. VG has received honoraria from Novartis, Pfizer, Astellas, Bayer, Roche, and GlaxoSmithKline and has consulted for Novartis, Pfizer, Astellas, Bayer, and GSK. IDD has chaired GlaxoSmithKline pazopanib (RCC) advisory board (2009), Janssen abiraterone advisory board (2010), and Bayer Asia-Pacific radium-223 dichloride advisory board (2012) and has been an advisory board member for Pfizer RCC advisory board, Novartis RAD001 advisory board, Medivation MDV3100 international advisory board, Bristol-Myers Squibb ipilimumab advisory board, Sanofi Jevtana advisory board, Bristol-Myers Squibb anti-PD1 advisory board, Astellas oncology portfolio advisory board, and Ipsen tasquinimod advisory board. IDD received no renumeration for any of this activity; all payments and honoraria were invoiced by and paid to ANZUP Cancer Trials Group. J-LL has received research funding from Bayer and has consulted for Pfizer, Novartis, Bayer, Sanofi-Aventis, and Astellas. EE has been an advisory board member for GlaxoSmithKline, Pfizer, and Novartis with all honoraria directly donated to Fundeso (Fundacion for Cancer Research). GU, CC, MS, MM and MShi are employees of Novartis Pharmaceuticals Corporation. BE has received honoraria for advisory board meetings from Bayer, Pfizer, Novartis, GlaxoSmithKline, and AVEO. All other authors report no conflicts of interest.
Contributors: RJM, NJV, GU, CC, and MMS participated in study design. SYR performed a literature search. RJM, CP, NJV, CNS, CS, JZ, CK, SYR, GAB, BM UDG, VG, IDD, J-LL, EE, and BE recruited patients. RJM, NJV, CK, GAB, BM, UDG, VG, MMS, and BE wrote the manuscript. MS designed and implemented the biomarker strategy, contributed to biomarker data analysis and interpretation and contributed to the biomarker sections of the manuscript. MM performed all the statistical analyses for the biomarker data, which included the longitudinal analysis for plasma biomarkers and association between PFS/OS and baseline biomarkers (plasma and tissue), and contributed toward the correct interpretation of the results for these models. All authors participated in data collection and/or interpretation/analysis, contributed to drafting/revising the manuscript, and reviewed and approved the final version of the manuscript.
References
- 1.Escudier B, Szczylik C, Porta C, Gore M. Treatment selection in metastatic renal cell carcinoma: expert consensus. Nat Rev Clin Oncol. 2012;9:327–37. doi: 10.1038/nrclinonc.2012.59. [DOI] [PubMed] [Google Scholar]
- 2.Bukowski RM. Temsirolimus: a safety and efficacy review. Expert Opin Drug Saf. 2012;11:861–79. doi: 10.1517/14740338.2012.713344. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. Clinical practice guidelines in oncology. [accessed December 10, 2013];Kidney cancer version 2.2014. 2013 http://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf.
- 4.European Association of Urology. Ljungberg B, Bensalah K, Bex A, et al. [accessed May 7, 2013];Guidelines on renal cell carcinoma. 2013 http://www.uroweb.org/gls/pdf/10_Renal_Cell_Carcinoma_LRV2.pdf.
- 5.Presta M, Dell'Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–78. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Granato AM, Nanni O, Falcini F, et al. Basic fibroblast growth factor and vascular endothelial growth factor serum levels in breast cancer patients and healthy women: useful as diagnostic tools? Breast Cancer Res. 2004;6:R38–45. doi: 10.1186/bcr745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korc M, Friesel RE. The role of fibroblast growth factors in tumor growth. Curr Cancer Drug Targets. 2009;9:639–51. doi: 10.2174/156800909789057006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Tsimafeyeu I, Demidov H, Ta H, Stepanova E, Wynn N. Fibroblast growth factor pathway in renal cell carcinoma. J Clin Oncol. 2010;28(suppl) doi: 10.3109/00365599.2011.552436. abstr 4621. [DOI] [PubMed] [Google Scholar]
- 10.Porta C, Paglino C, Imarisio I, et al. Changes in circulating pro-angiogenic cytokines, other than VEGF, before progression to sunitinib therapy in advanced renal cell carcinoma patients. Oncology. 2013;84:115–22. doi: 10.1159/000342099. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Lopes de Menezes D, Vora J, et al. In vivo target modulation and biological activity of CHIR-258, a multitargeted growth factor receptor kinase inhibitor, in colon cancer models. Clin Cancer Res. 2005;11:3633–41. doi: 10.1158/1078-0432.CCR-04-2129. [DOI] [PubMed] [Google Scholar]
- 12.Angevin E, Lopez-Martin J, Lin CC, et al. Phase I study of dovitinib (TKI258), an oral FGFR, VEGFR, and PDGFR inhibitor, in advanced or metastatic renal cell carcinoma. Clin Cancer Res. 2013;19:1257–68. doi: 10.1158/1078-0432.CCR-12-2885. [DOI] [PubMed] [Google Scholar]
- 13.Sivanand S, Peña-Llopis S, Zhao H, et al. A validated tumorgraft model reveals activity of dovitinib against renal cell carcinoma. Sci Transl Med. 2012;4:137ra75. doi: 10.1126/scitranslmed.3003643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angevin E, Grünwald V, Ravaud A, et al. A phase II study of dovitinib (TKI258), an FGFR- and VEGFR-inhibitor, in patients with advanced or metastatic renal cell cancer (mRCC) J Clin Oncol. 2011;29(suppl) abstr 4551. [Google Scholar]
- 15.Garcia JA, Hutson TE, Elson P, et al. Sorafenib in patients with metastatic renal cell carcinoma refractory to either sunitinib or bevacizumab. Cancer. 2010;116:5383–90. doi: 10.1002/cncr.25327. [DOI] [PubMed] [Google Scholar]
- 16.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–9. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 17.Di Lorenzo G, Buonerba C, Federico P, et al. Third-line sorafenib after sequential therapy with sunitinib and mTOR inhibitors in metastatic renal cell carcinoma. Eur Urol. 2010;58:906–11. doi: 10.1016/j.eururo.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Di Lorenzo G, Carteni G, Autorino R, et al. Phase II study of sorafenib in patients with sunitinib-refractory metastatic renal cell cancer. J Clin Oncol. 2009;27:4469–74. doi: 10.1200/JCO.2009.22.6480. [DOI] [PubMed] [Google Scholar]
- 19.Hutson T, Escudier B, Esteban E, et al. Temsirolimus vs sorafenib as second line therapy in metastatic renal cell carcinoma: results from the INTORSECT trial. Ann Oncol. 2012;23(suppl 9) abstr LBA22. [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:454–63. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 22.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Kay A, Anak O, et al. Population pharmacokinetic/pharmacodynamic modeling to assist dosing schedule selection for dovitinib. J Clin Pharmacol. 2013;53:14–20. doi: 10.1177/0091270011433330. [DOI] [PubMed] [Google Scholar]
- 24.Calvo E, Escudier B, Motzer RJ, et al. Everolimus in metastatic renal cell carcinoma: subgroup analysis of patients with 1 or 2 previous vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapies enrolled in the phase III RECORD-1 study. Eur J Cancer. 2012;48:333–9. doi: 10.1016/j.ejca.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 25.Kim KB, Chesney J, Robinson D, Gardner H, Shi MM, Kirkwood JM. Phase I/II and pharmacodynamic study of dovitinib (TKI258)—an inhibitor of fibroblast growth factor receptors and VEGF receptors—in patients with advanced melanoma. Clin Cancer Res. 2011;17:7451–61. doi: 10.1158/1078-0432.CCR-11-1747. [DOI] [PubMed] [Google Scholar]
- 26.Björnsson E. Drug-induced liver injury: Hy's rule revisited. Clin Pharmacol Ther. 2006;79:521–8. doi: 10.1016/j.clpt.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 27.US Food and Drug Administration. Guidance for industry. Drug-induced liver injury: premarketing clinical evaluation. 2009 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Figure 1: Kaplan-Meier analysis of progression-free survival by investigator review.
Appendix Figure 2: Forest plots of progression-free survival.
MSKCC, Memorial Sloan-Kettering Cancer Center.
Appendix Figure 3: Definitive worsening of Karnofsky performance status.
Appendix Figure 4: Kaplan-Meier estimates of progression-free survival by treatment and baseline plasma biomarker groups.
FGF2, fibroblast growth factor 2; HGF, hepatocyte growth factor; HR, hazard ratio; PLGF, placental growth factor; VEGFA, vascular endothelial growth factor A. Low biomarker is defined as < median, and high biomarker is defined as ≥ median.


