Abstract
BACKGROUND
Nivolumab, a programmed death-1 checkpoint inhibitor, demonstrated encouraging overall survival in uncontrolled studies in previously treated patients with advanced renal cell carcinoma. This randomized, open-label, phase 3 study compared nivolumab with everolimus in renal cell carcinoma after prior treatment.
METHODS
Eight hundred twenty-one patients with advanced clear-cell renal cell carcinoma previously treated with one or two antiangiogenic therapies were randomized (1:1) to receive nivolumab 3 mg/kg intravenously every 2 weeks or everolimus 10-mg tablet orally once daily. Primary end point was overall survival. Secondary end points included objective response rate and safety.
RESULTS
Median (95% confidence interval [CI]) overall survival was 25.0 months (21.8 to not estimable) with nivolumab and 19.6 months (17.6 to 23.1) with everolimus. The hazard ratio for risk of death with nivolumab versus everolimus was 0.73 (98.5% CI, 0.57 to 0.93; P=0.0018), meeting the predefined criterion for superiority (P≤0.0148). Objective response rate was greater with nivolumab (25%) than everolimus (5%; odds ratio 5.98; 95% CI, 3.68 to 9.72; P<0.001). Median (95% CI) progression-free survival was 4.6 months (3.7 to 5.4) with nivolumab and 4.4 months (3.7 to 5.5) with everolimus (hazard ratio, 0.88; 95% CI, 0.75 to 1.03; P=0.11). Grade 3 or 4 treatment-related adverse events occurred in 19% (nivolumab) and 37% (everolimus) of patients; most common was fatigue (3%) with nivolumab and anemia (8%) with everolimus.
CONCLUSIONS
Overall survival was longer and fewer grade 3 or 4 adverse events occurred for nivolumab versus everolimus in treatment-experienced patients with advanced renal cell carcinoma.
ClinicalTrials.gov Identifier: NCT01668784
INTRODUCTION
Each year there are an estimated 338,000 new cases of renal cell carcinoma worldwide,1 and approximately 30% of patients present with metastatic disease at diagnosis.2 A number of targeted therapies have been approved for the treatment of advanced or metastatic renal cell carcinoma. These agents include vascular endothelial growth factor pathway inhibitors and mammalian target of rapamycin (mTOR) inhibitors.3,4 Everolimus is an mTOR inhibitor recommended for the treatment of advanced renal cell carcinoma after failure with sorafenib or sunitinib.3–6 Although everolimus and other agents have changed the therapeutic landscape for this disease, these treatments are associated with limited overall survival following resistance to therapy.
Nivolumab is a fully human IgG4 programmed death-1 (PD-1) immune checkpoint inhibitor antibody that selectively blocks the interaction between PD-1, expressed on activated T cells, and PD-1 ligands 1 and 2 (PD-L1/L2), expressed on immune cells and tumor cells. Interaction between PD-1 and PD-L1/L2 normally results in inhibition of the cellular immune response.7–9 Prior reports have demonstrated that PD-L1 is associated with poor prognosis in renal cell carcinoma, presumably attributed to its immunosuppressive function.10–12. It has been postulated that PD-L1 expression would be associated with improved overall survival to nivolumab as disruption of PD-1:PD-L1 signaling mediated by nivolumab leads to restored antitumor immunity.13,14
In a phase 2 dose-ranging trial in previously treated patients with metastatic renal cell carcinoma, nivolumab demonstrated objective responses of 20% to 22% and overall survival ranging from 18.2 to 25.5 months.15 Here, we report results from a phase 3 study comparing nivolumab with everolimus in patients with previously treated advanced renal cell carcinoma (clinicaltrials.gov identifier: NCT01668784).
PATIENTS AND METHODS
PATIENTS
Eligible patients were 18 years of age or older, had histological confirmation of advanced or metastatic renal cell carcinoma with a clear-cell component, measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST v1.1),16 and previous treatment with one or two antiangiogenic therapies. Patients must have had three or fewer total prior systemic therapies, including cytokines and cytotoxic chemotherapy drugs, and had progression on or after the last therapy received and within 6 months before study enrollment. All had a Karnofsky performance status of ≥70%.17 Key exclusion criteria included central nervous system metastases, previous treatment with an mTOR inhibitor, or a condition requiring glucocorticoids (>10 mg daily prednisone equivalent).
STUDY DESIGN
This was a randomized, open-label, phase 3 study of nivolumab compared with everolimus. Stratified randomization (1:1 ratio) with block size of 4 was implemented. Stratification factors were region (US/Canada or Western Europe or rest of world), Memorial Sloan Kettering Cancer Center (MSKCC) prognostic risk group (favorable, intermediate, or poor risk based on the presence of 0, 1 or 2, or 3 prognostic factors, respectively [anemia, hypercalcemia, poor performance status]),18 and number of prior antiangiogenic therapy regimens (one or two) for advanced renal cell carcinoma.
Nivolumab and everolimus were provided by the Sponsor, except in cases when everolimus was procured as a local commercial product in certain countries. Nivolumab was administered at a dose of 3 mg/kg as a 60-minute intravenous infusion every 2 weeks. Everolimus was administered as a daily oral dose of 10 mg. Dose modifications were not permitted for nivolumab but were permitted for everolimus.
This study was approved by the institutional review board/independent ethics committee for each center and conducted in accordance with Good Clinical Practice guidelines defined by the International Conference on Harmonisation. All patients provided written informed consent to participate based on the principles of the Declaration of Helsinki. A data monitoring committee reviewed efficacy and safety during the study.
The authors vouch for the accuracy and completeness of analyses reported and for the fidelity of the study to the protocol. Development of the manuscript first draft was led by the lead author. All authors contributed to drafting the manuscript and provided final approval to submit for publication. Medical writing support, funded by the sponsor, was provided by PPSI. The study protocol is available with the full text of this article at www.nejm.org.
END POINTS AND ASSESSMENTS
The primary end point was overall survival, defined as time from randomization to date of death. Secondary end points included objective response rate, progression-free survival, association of overall survival with PD-L1 tumor expression, and incidence of adverse events. Disease assessments were performed using computed tomography or magnetic resonance imaging at baseline, every 8 weeks for the first year, then every 12 weeks until disease progression or treatment discontinuation. Imaging data were evaluated by the investigator to assess tumor response (per RECIST v1.1). Patients were allowed to continue study therapy after initial progression if investigator-assessed clinical benefit was noted and the patient tolerated study drug. Safety assessments were conducted at each clinic visit. After treatment discontinuation, patients were followed every 3 months for survival and subsequent anticancer therapy.
Objective response rate (investigator-assessed) was defined as the number of patients with complete response or partial response divided by the number of randomized patients. Best overall response was defined as the best response (investigator-assessed) from randomization to objectively documented progression or subsequent therapy, whichever occurred first. Progression-free survival was defined as the time from randomization to first documented RECIST tumor progression/death from any cause. Tumor PD-L1 membrane expression (≥1% vs <1% and ≥5% vs <5%) was assessed by a central laboratory in sections with ≥100 evaluable tumor cells and stained with the Dako PD-L1 immunohistochemistry assay.19
Adverse events were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.20 Quality of life was assessed using the functional assessment of cancer therapy - kidney symptom index - disease-related symptoms (FKSI-DRS) scoring algorithm.21 The FKSI-DRS questionnaire comprises nine symptom-specific questions that address lack of energy, pain, weight loss, bone pain, fatigue, dyspnea, cough, fevers, and hematuria. A summary score ranging from 0 to 36 was produced, with 36 being the best possible score (no symptoms) and 0 being the worst possible score (all the worst symptoms).21 See Supplementary Appendix for additional details.
STATISTICAL ANALYSES
This planned interim analysis was conducted after 398 of 569 deaths (70%) required for the final analysis had occurred; the stopping boundary was derived based on the number of deaths using an O’Brien–Fleming α spending function that provides 90% power to detect a hazard ratio of 0.76 with an overall type 1 error of 0.05 (two-sided).22 Interim overall survival was projected at a 0.0148 nominal significance level at which the study could be stopped at the recommendation of the data monitoring committee and declared positive for efficacy. Interim analysis would then be considered the final analysis. In July 2015, the study was stopped early because an assessment conducted by the independent data monitoring committee concluded that the study met its endpoint.
All randomized patients were included for efficacy analyses; patients who received one or more doses of study drug were included in safety analyses. Overall survival, progression-free survival, and duration of response were estimated using Kaplan–Meier methodology.16 Median and corresponding 95% confidence intervals (CIs) were provided using Brookmeyer and Crowley methodology23; 95% CIs were constructed using log-log transformation. A stratified log-rank test was performed to compare overall survival and progression-free survival between nivolumab and everolimus. A stratified hazard ratio and CI between nivolumab and everolimus was obtained by fitting a stratified Cox model with the group variable as single covariate. The difference in response rates between nivolumab and everolimus along with the two-sided 95% CI were estimated using the Cochran–Mantel–Haenszel method of weighting, adjusting for the stratification factors.24 Survival was compared between nivolumab and everolimus using the interim analysis monitoring feature of East software based on the Lan–DeMets error spending function approach using an O’Brien–Fleming stopping boundary to reject the null hypothesis (no treatment difference), controlling for a two-sided overall α of 5.0%.22 If superiority of the primary end point was demonstrated, a hierarchical statistical testing procedure was followed for objective response rate (estimated along with the exact 95% CI using the Clopper–Pearson method25) and progression-free survival at an α of 5.0%. For quality-of-life assessments, descriptive statistics were used to assess completion rates and changes in quality of life.
RESULTS
PATIENTS
Between October 2012 and March 2014, 821 patients were randomized at 146 sites in 24 countries in North America, Europe, Australia, South America, and Asia; 803/821 randomized patients were treated; 406 and 397 patients in the nivolumab and everolimus arms, respectively. At data cut-off (June 2015), 67/406 patients (17%) in the nivolumab arm and 28/397 patients (7%) in the everolimus arm remained on treatment (Figure S1 in Supplementary Appendix). Minimum follow-up was 15 months. The primary reason for discontinuation was disease progression (285/406 [70%] with nivolumab; 273/397 [69%] with everolimus; Figure S1 in Supplementary Appendix). Demographic and clinical characteristics were balanced between treatment arms; the majority of patients (72%) received one prior antiangiogenic therapy for advanced renal cell carcinoma (Table 1).
Table 1.
Baseline Demographics and Clinical Characteristics (All Randomized Patients).
| Characteristic | Nivolumab N=410 |
Everolimus N=411 |
Total N=821 |
|---|---|---|---|
|
| |||
| Median age (range), years | 62 (23–88) | 62 (18–86) | 62 (18–88) |
|
| |||
| Sex, n (%) | |||
| Male | 315 (77) | 304 (74) | 619 (75) |
| Female | 95 (23) | 107 (26) | 202 (25) |
|
| |||
| Race, n (%) | |||
| White | 353 (86) | 367 (89) | 720 (88) |
| Asian | 42 (10) | 32 (8) | 74 (9) |
| Black | 1 (0.2) | 4 (1) | 5 (1) |
| Other | 14 (3) | 8 (2) | 22 (3) |
|
| |||
| MSKCC risk group, n (%) | |||
| Favorable | 145 (35) | 148 (36) | 293 (36) |
| Intermediate | 201 (49) | 203 (49) | 404 (49) |
| Poor | 64 (16) | 60 (15) | 124 (15) |
|
| |||
| Karnofsky performance status, n (%) | |||
| <70* | 2 (1) | 1 (0.2) | 3 (0.4) |
| 70 | 22 (5) | 30 (7) | 52 (6) |
| 80 | 110 (27) | 116 (28) | 226 (28) |
| 90 | 150 (37) | 130 (32) | 280 (34) |
| 100 | 126 (31) | 134 (33) | 260 (32) |
|
| |||
| Number of evaluable disease sites, n (%) | |||
| 1 | 68 (17) | 71 (17) | 139 (17) |
| ≥2 | 341 (83) | 338 (82) | 679 (83) |
|
| |||
| Common sites of metastasis | |||
| Lung | 278 (68) | 273 (66) | 551 (67) |
| Liver | 100 (24) | 87 (21) | 187 (23) |
| Bone | 76 (19) | 70 (17) | 146 (18) |
|
| |||
| Prior nephrectomy | |||
| Yes | 364 (89) | 359 (87) | 723 (88) |
| No | 46 (11) | 52 (13) | 98 (12) |
|
| |||
| Median time from initial diagnosis to randomization (range), months | 31 (1–392) | 31 (2–372) | 31 (1–392) |
|
| |||
| Number of prior antiangiogenic regimens in advanced setting, n (%) | |||
| 1 | 294 (72) | 297 (72) | 591 (72) |
| 2 | 116 (28) | 114 (28) | 230 (28) |
|
| |||
| Previous systemic cancer therapy in the metastatic setting,† n (%) | |||
| Sunitinib | 246 (60) | 242 (59) | 488 (59) |
| Pazopanib | 119 (29) | 131 (32) | 250 (31) |
| Axitinib | 51 (12) | 50 (12) | 101 (12) |
|
| |||
| Patients with quantifiable PD-L1 expression, n (%) | 370 (90) | 386 (94) | 756 (92) |
| PD-L1 expression levels,‡ n (%) | |||
| ≥1% | 94 (25) | 87 (23) | 181 (24) |
| <1% | 276 (75) | 299 (78) | 575 (76) |
| ≥5% | 44 (12) | 41 (11) | 85 (11) |
| <5% | 326 (88) | 345 (89) | 671 (89) |
| Patients without quantifiable PD-L1 expression,* n (%) | 40 (10) | 25 (6) | 65 (8) |
All patients had KPS ≥70 at time of study entry which may have decreased at randomization.
Therapies received in more than 10% of all randomized patients.
Percent membrane staining in ≥100 tumor cells.
EFFICACY
Overall Survival
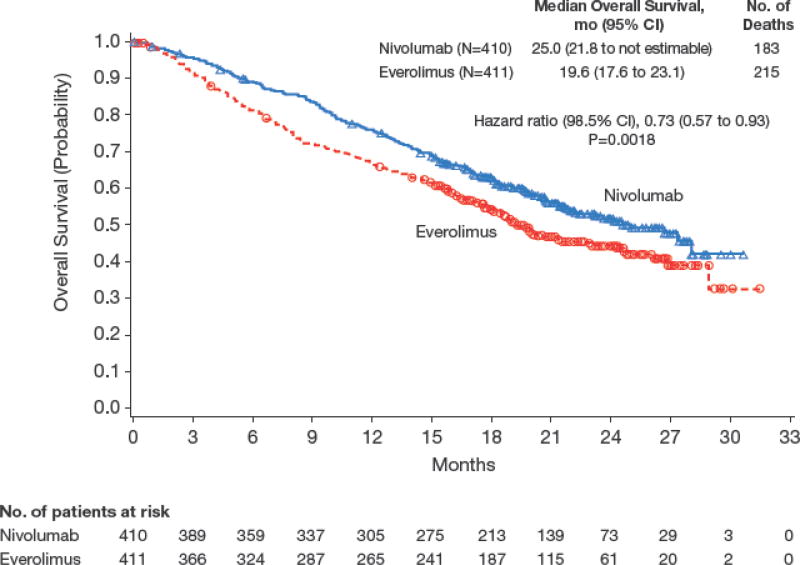
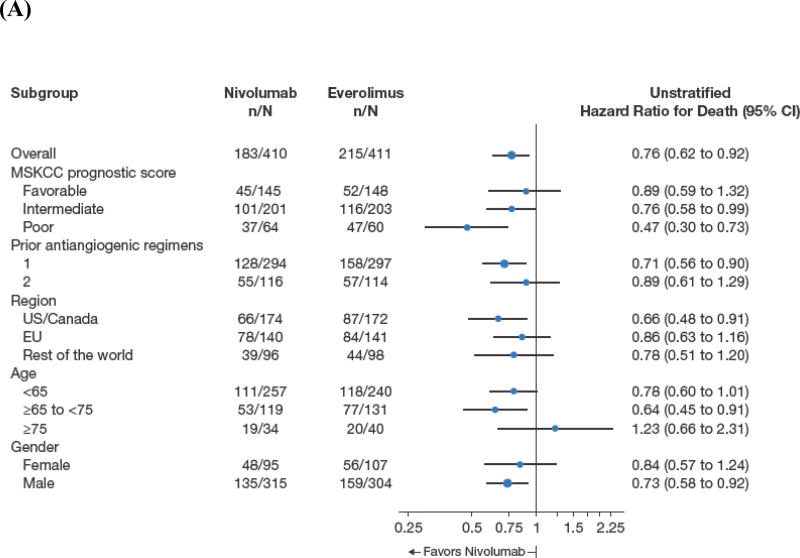
Median overall survival was 25.0 months (95% CI, 21.8 to not estimable) with nivolumab and 19.6 months (95% CI, 17.6 to 23.1) with everolimus (Figure 1). Deaths occurred in 181/406 patients (45%) treated with nivolumab and in 213/397 patients (54%) treated with everolimus. The hazard ratio for risk of death (any cause) with nivolumab versus everolimus was 0.73 (98.5% CI, 0.57 to 0.93; P=0.0018), meeting the predefined criterion for superiority. Overall survival benefit with nivolumab was observed across predefined subgroups including region, MSKCC prognostic score, and number of prior antiangiogenic therapies (Figure 2A). The heterogeneity of treatment effect within each subgroup shown in Figure 2a was tested through an interaction test in a Cox proportional hazards model with treatment, subgroup, and treatment by subgroup interaction as covariates. None of the interaction terms was statistically significant at the 5% level.
Figure 1.
Kaplan–Meier Curve for Overall Survival.
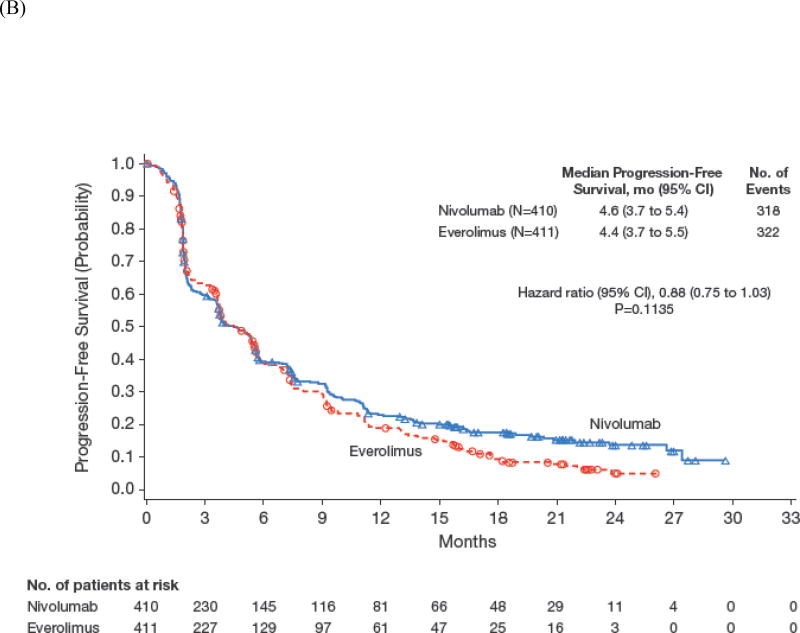
Figure 2.
Overall Survival According to Subgroup Analyses (A) and Kaplan–Meier Curve for Progression-Free Survival (B).
Tumor Response and Progression-Free Survival
Objective response rate was higher with nivolumab than with everolimus (25% vs. 5%; Table S1; odds ratio 5.98; 95% CI, 3.68 to 9.72; P<0.001). Partial responses were observed in 99 patients (24%) treated with nivolumab and 20 patients (5%) treated with everolimus. Complete responses were observed in four patients treated with nivolumab (1%) and two patients treated with everolimus (<1%). The median time to response was 3.5 months (range 1.4 to 24.8; n=103) with nivolumab and 3.7 months (range 1.5 to 11.2; n=22) with everolimus; median (range) duration of response was 12.0 months (0 to 27.6+) and 12.0 months (0 to 22.2+), respectively. In patients with a response, 45/103 (44%) and 8/22 (36%) had an ongoing response; 32/103 (31%) and 6/22 (27%) patients with a response had an ongoing response ≥12 months with nivolumab and everolimus, respectively (Figure S2 in Supplementary Appendix).
Median progression-free survival was 4.6 months (95% CI, 3.7 to 5.4) with nivolumab and 4.4 months (95% CI, 3.7 to 5.5) with everolimus (hazard ratio, 0.88; 95% CI, 0.75 to 1.03; P=0.11; Figure 2B). To explore the apparent delayed separation of the curves, we performed an ad hoc sensitivity analysis of progression-free survival in patients who had not progressed/died at 6 months (n=145 [35%], nivolumab; n=129 [31%], everolimus). The analysis of this subset of patients yielded a median (95% CI) progression-free survival of 15.6 months (11.8 to 19.6) with nivolumab and 11.7 months (10.9 to 14.7) with everolimus (hazard ratio, 0.64; 95% CI, 0.47 to 0.88).
PD-L1 Expression
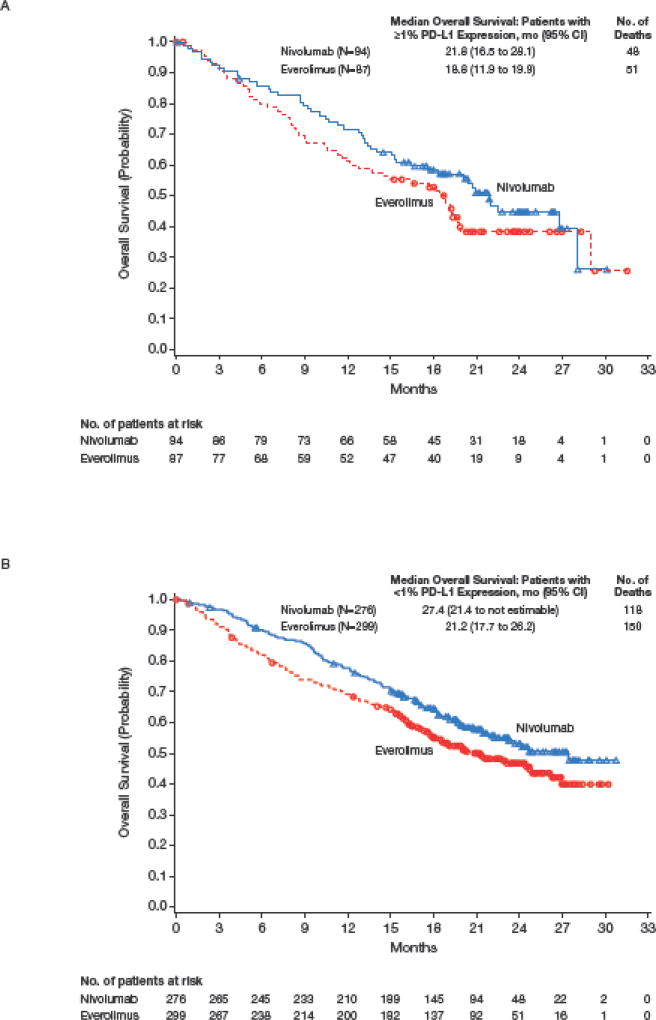
Of the 821 patients who were randomized, 756 (92%) had quantifiable tumor PD-L1 expression in pretreatment samples; 370/410 (90%) treated with nivolumab and 386/411 (94%) treated with everolimus (Table 1). In total, 181/756 patients (24%) with quantifiable PD-L1 expression had ≥1% PD-L1 expression and 575/756 patients (76%) had <1% PD-L1 expression (Table 1). In patients with ≥1% PD-L1 expression, median (95% CI) overall survival was 21.8 months (16.5 to 28.1) with nivolumab and 18.8 months (11.9 to 19.9) with everolimus (hazard ratio 0.78; 95% CI, 0.53 to 1.16; Figure 3A). In patients with <1%, PD-L1 expression, median (95% CI) overall survival was 27.4 months (21.4 to not estimable) with nivolumab and 21.2 months (17.7 to 26.2) with everolimus (hazard ratio, 0.76; 95% CI, 0.60 to 0.97; Figure 3B). Similar results were observed in patients with ≥5% PD-L1 expression versus patients with <5% PD-L1 expression, although interpretation is limited by small numbers of patients with ≥5% expression (Figure S3 in Supplementary Appendix).
Figure 3.
Kaplan–Meier Curve for Overall Survival by PD-L1 Expression ≥1% (A) and PD-L1 Expression <1% (B).
TREATMENT ADMINISTRATION AND SAFETY
The median (range) duration of treatment was 5.5 months (<0.1 to 29.6) with nivolumab and 3.7 months (0.2 to 25.7) with everolimus. In total, 207/406 patients (51%) had dose delays with nivolumab and 262/397 patients (66%) had dose delays (including interruptions) with everolimus. Dose reductions were not allowed with nivolumab and 102/397 patients (26%) had at least one dose reduction with everolimus.
Any-grade treatment-related adverse events occurred in 319/406 (79%) and 349/397 (88%) of patients treated with nivolumab and everolimus, respectively (Table 2). The most common treatment-related adverse events were fatigue (134/406; 33%), nausea (57/406; 14%), and pruritus (57/406; 14%) with nivolumab and fatigue (134/397; 34%), stomatitis (117/397; 30%), and anemia (94/397; 24%) with everolimus. Grade 3 or 4 treatment-related adverse events occurred in 76/406 and 145/397 patients (19% and 37%) treated with nivolumab and everolimus, respectively; the most common was fatigue (10/406; 3%) with nivolumab and anemia (31/397; 8%) with everolimus.
Table 2.
Treatment-Related Adverse Events Reported in >10% of Patients (All Treated Patients).*
| Event | Nivolumab N=406 |
Everolimus N=397 |
||
|---|---|---|---|---|
| Any Grade | Grade 3 or 4 | Any Grade | Grade 3 or 4 | |
| All treatment-related adverse events, n (%) | 319 (79) | 76 (19) | 349 (88) | 145 (37) |
| Fatigue | 134 (33) | 10 (3) | 134 (34) | 11 (3) |
| Nausea | 57 (14) | 1 (0) | 66 (17) | 3 (1) |
| Pruritus | 57 (14) | 0 | 39 (10) | 0 |
| Diarrhea | 50 (12) | 5 (1) | 84 (21) | 5 (1) |
| Decreased appetite | 48 (12) | 2 (1) | 82 (21) | 4 (1) |
| Rash | 41 (10) | 2 (1) | 79 (20) | 3 (1) |
| Cough | 36 (9) | 0 | 77 (19) | 0 |
| Anemia | 32 (8) | 7 (2) | 94 (24) | 31 (8) |
| Dyspnea | 30 (7) | 3 (1) | 51 (13) | 2 (1) |
| Edema peripheral | 17 (4) | 0 | 56 (14) | 2 (1) |
| Pneumonitis | 16 (4) | 6 (2) | 58 (15) | 11 (3) |
| Mucosal inflammation | 11 (3) | 0 | 75 (19) | 12 (3) |
| Dysgeusia | 11 (3) | 0 | 51 (13) | 0 |
| Hyperglycemia | 9 (2) | 5 (1) | 46 (12) | 15 (4) |
| Stomatitis | 8 (2) | 0 | 117 (30) | 17 (4) |
| Hypertriglyceridemia | 5 (1) | 0 | 64 (16) | 20 (5) |
| Epistaxis | 3 (1) | 0 | 41 (10) | 0 |
Treatment-related adverse events leading to discontinuation occurred in 31/406 (8%) and 52/397 (13%) of patients treated with nivolumab and everolimus, respectively. No deaths from study drug toxicity were reported with nivolumab and two deaths were reported with everolimus (septic shock; acute bowel ischemia). Treatment beyond initial RECIST v1.1-defined progression with investigator-assessed clinical benefit occurred in 44% (n=179) of patients treated with nivolumab and 45% (n=183) of patients treated with everolimus.
QUALITY OF LIFE
The FKSI-DRS questionnaire completion rate was ≥80% throughout the study. Quality-of-life scores were similar in both arms at baseline (Table S2 in Supplementary Appendix). From the first assessment after baseline (week 4) through week 104, median quality-of-life scores were numerically higher than baseline in 24/26 assessments (92%) with nivolumab and 2/26 assessments (8%) with everolimus (Table S2 in Supplementary Appendix).
SUBSEQUENT THERAPIES
Two hundred twenty-seven (55%) of 410 patients treated with nivolumab and 260/411 patients (63%) treated with everolimus received subsequent systemic therapy. The most common subsequent therapies following nivolumab included everolimus (105/410; 26%), axitinib (99/410; 24%), and pazopanib (37/410; 9%); the most common following everolimus were axitinib (150/411; 37%), pazopanib (64/411; 16%), and sorafenib (38/411; 9%). Anti-PD-1 therapy was given as subsequent therapy to six patients in the everolimus arm.
DISCUSSION
This phase 3 randomized study demonstrated that patients with advanced renal cell carcinoma experienced longer survival with nivolumab treatment than with everolimus treatment after prior antiangiogenic treatment. The separation of the overall survival curves occurred early in the study and median overall survival was 5.4 months longer with nivolumab (25.0 months) compared with everolimus (19.6 months), a difference that crossed the prespecified boundary for statistical significance at the time of the interim analysis.
This study also demonstrated a higher number of objective responses with nivolumab versus everolimus, many of which were durable. Median progression-free survival was similar between treatments and consistent with that reported previously in an uncontrolled study in patients previously treated with antiangiogenic therapies.15 Moreover, comparison of progression-free survival between nivolumab and everolimus arms was not a surrogate for overall survival in this study. Late separation of the curves suggested a potential delayed benefit in progression-free survival with nivolumab. This delayed progression-free survival benefit was subsequently quantified using a sensitivity analysis in patients who had not progressed or died at 6 months; median progression-free survival was longer with nivolumab versus everolimus in this subset of patients. These patients likely contributed to the overall survival benefit observed with nivolumab in this study.
We observed consistent prolonged survival with nivolumab versus everolimus irrespective of MSKCC prognostic score, number of prior antiangiogenic therapies, and region. Benefit was observed with nivolumab irrespective of PD-L1 expression. Nivolumab has been reported to be associated with pharmacodynamic changes in blood and tumor markers consistent with PD-1 inhibition.12 Our data corroborate previous reports indicating that higher levels of PD-L1 expression are associated with poorer survival in renal cell carcinoma,10,11 but do not support PD-L1 as a marker of treatment benefit in renal cell carcinoma. The relationship between PD-L1 expression and nivolumab outcomes appears to depend on tumor type and histology and may also depend on treatment setting. An association between PD-L1 expression and improved outcomes with nivolumab treatment has been observed for metastatic melanoma and only some types of lung cancer26,27 .28
Nivolumab had a safety profile consistent with findings in other studies.13–15 Grade 3 or 4 treatment-related adverse events were less frequent with nivolumab than with everolimus and treatment-related adverse events leading to discontinuation were experienced by fewer patients treated with nivolumab. Differences in the frequency of specific adverse events between treatments were reflective of drug class. Quality-of-life, as assessed by the FKSI-DRS, improved during nivolumab treatment but remained stable with everolimus. Additional analyses will further characterize the quality-of-life observed with nivolumab compared with everolimus treatment in this study.
There has been considerable progress in the treatment of this disease since 2005, with five VEGF pathway inhibitors (sorafenib, sunitinib, bevacizumab, pazopanib, and axitinib) and two mTOR inhibitors (everolimus, temsirolimus) showing benefit in pivotal phase 3 trials, leading to regulatory approval. Prior to this era, infrequent but occasionally long-standing responses were observed with cytokines, including high doses of interleukin-2.29 With one exception,30 the benefit for approved targeted drugs has been established by phase 3 studies showing improvement in progression-free survival, but not overall survival compared with standard treatment, which included interferon-α, placebo, or an approved antiangiogenic drug.3 In patients previously treated with sunitinib in the phase 3 AXIS trial, no benefit in overall survival was detected (median overall survival: 15.2 months with axitinib vs. 16.5 months with sorafenib).31 The median overall survival of 25.0 months and the survival improvement for the immune checkpoint inhibitor nivolumab compared with everolimus provides evidence of benefit in treatment-experienced patients with advanced renal cell carcinoma.
Supplementary Material
Acknowledgments
We thank the patients and their families; research colleagues and clinical teams; and Bristol-Myers Squibb (Lawrenceville, NJ)/Ono Pharmaceutical Company Limited (Osaka City, Japan) for supporting the work. We also thank Jennifer McCarthy, CheckMate 025 protocol manager; Justin Doan for involvement with quality-of-life interpretation; and Dako, for collaborative development of the automated PD-L1 immunohistochemistry assay. Writing and editorial assistance was provided by Jennifer Granit, PhD, of PPSI, funded by Bristol-Myers Squibb.
Research support: This study was sponsored by Bristol-Myers Squibb. Authors received no financial support or compensation for publication of this manuscript.
Footnotes
Presented, in part, at the European Cancer Congress, Vienna, Austria, September 25–29, 2015.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Fisher R, Gore M, Larkin J. Current and future systemic treatments for renal cell carcinoma. Semin Cancer Biol. 2013;23:38–45. doi: 10.1016/j.semcancer.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Kidney cancer. Fort Washington, PA: National Comprehensive Cancer Network; 2015. Version 3.2015. [DOI] [PubMed] [Google Scholar]
- 4.Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii49–iii56. doi: 10.1093/annonc/mdu259. [DOI] [PubMed] [Google Scholar]
- 5.Afinitor (everolimus) [prescribing information] East Hanover, NJ: Novartis Pharmaceuticals Corp.; 2015. [Google Scholar]
- 6.Dabney R, Devine R, Sein N, George B. New agents in renal cell carcinoma. Target Oncol. 2014;9:183–93. doi: 10.1007/s11523-013-0303-8. [DOI] [PubMed] [Google Scholar]
- 7.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–5. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamid O, Carvajal RD. Anti-programmed death-1 and anti-programmed death-ligand 1 antibodies in cancer therapy. Expert Opin Biol Ther. 2013;13:847–61. doi: 10.1517/14712598.2013.770836. [DOI] [PubMed] [Google Scholar]
- 9.Nurieva RI, Liu X, Dong C. Molecular mechanisms of T-cell tolerance. Immunol Rev. 2011;241:133–44. doi: 10.1111/j.1600-065X.2011.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–5. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 11.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–9. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choueiri T, Fishman MN, Escudier B. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma (mRCC): Association of biomarkers with clinical outcomes. J Clin Oncol. 2015;33(suppl) doi: 10.1158/1078-0432.CCR-15-2839. abstr 4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015;33:1430–7. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2:187–93. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:454–63. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 19.Cogswell JP, Goldberg SM, Gupta AK, Jure-Kunkel M, Wang XT, Wigginton JM. Cancer immunotherapy by disrupting pd-1/pd-l1 signaling. [Accessed July 31, 2015]; FreePatentsOnline.com Website. at http://www.freepatentsonline.com/20130309250.pdf.
- 20.National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. 2015 [Google Scholar]
- 21.Cella D, Yount S, Brucker PS, et al. Development and validation of a scale to measure disease-related symptoms of kidney cancer. Value Health. 2007;10:285–93. doi: 10.1111/j.1524-4733.2007.00183.x. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–56. [PubMed] [Google Scholar]
- 23.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 24.Berry DA, editor. Categorical data analysis. Statistical methodology in the pharmaceutical sciences. New York, NY: Marcel Dekker; 1990. [Google Scholar]
- 25.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–14. [Google Scholar]
- 26.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 27.Paz-Ares L, Horn L, Borghaei H, et al. Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC) [abstract] J Clin Oncol. 2015;33 abstr LBA109. [Google Scholar]
- 28.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S, Margolin K. Cytokines in cancer immunotherapy. Cancers (Basel) 2011;3:3856–93. doi: 10.3390/cancers3043856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 31.Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14:552–62. doi: 10.1016/S1470-2045(13)70093-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.