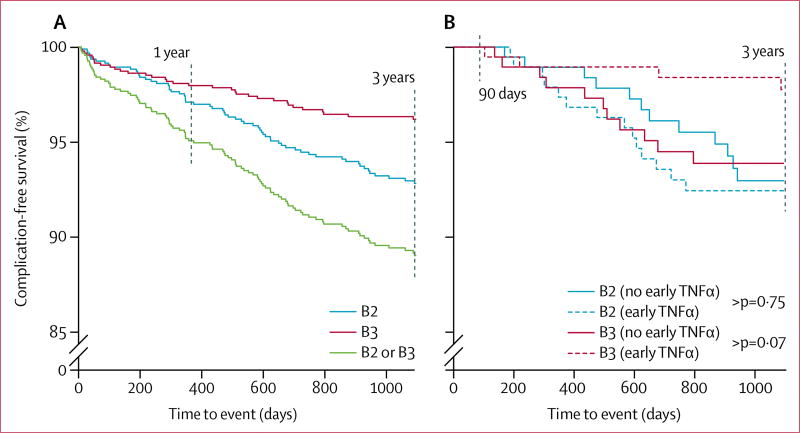
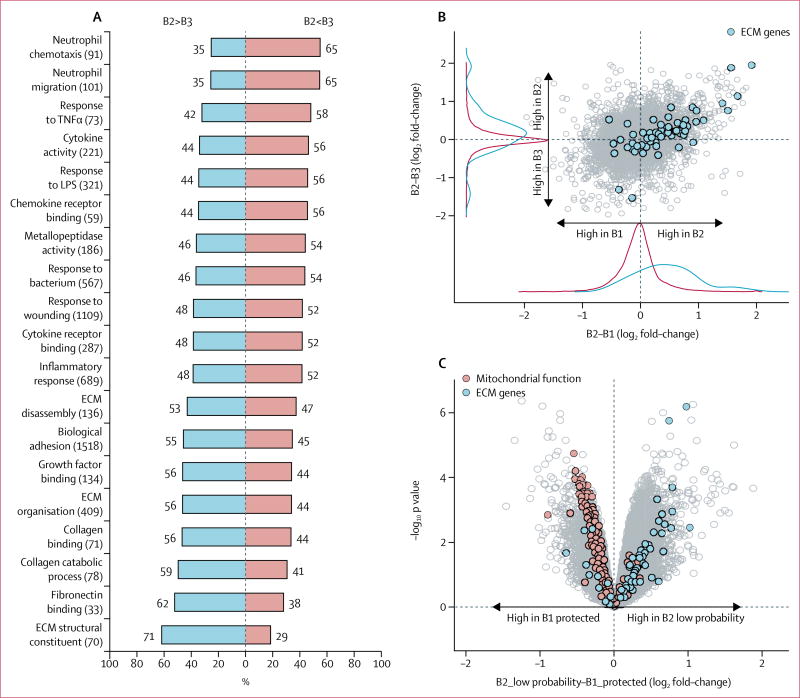
Subra Kugathasan
Subra Kugathasan
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,*,
Lee A Denson
Lee A Denson
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,*,
Thomas D Walters
Thomas D Walters
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,*,
Mi-Ok Kim
Mi-Ok Kim
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Urko M Marigorta
Urko M Marigorta
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Melanie Schirmer
Melanie Schirmer
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Kajari Mondal
Kajari Mondal
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Chunyan Liu
Chunyan Liu
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Anne Griffiths
Anne Griffiths
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Joshua D Noe
Joshua D Noe
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Wallace V Crandall
Wallace V Crandall
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Scott Snapper
Scott Snapper
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Shervin Rabizadeh
Shervin Rabizadeh
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Joel R Rosh
Joel R Rosh
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Jason M Shapiro
Jason M Shapiro
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Stephen Guthery
Stephen Guthery
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
David R Mack
David R Mack
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Richard Kellermayer
Richard Kellermayer
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Michael D Kappelman
Michael D Kappelman
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Steven Steiner
Steven Steiner
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Dedrick E Moulton
Dedrick E Moulton
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
David Keljo
David Keljo
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Stanley Cohen
Stanley Cohen
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Maria Oliva-Hemker
Maria Oliva-Hemker
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Melvin B Heyman
Melvin B Heyman
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Anthony R Otley
Anthony R Otley
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Susan S Baker
Susan S Baker
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Jonathan S Evans
Jonathan S Evans
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Barbara S Kirschner
Barbara S Kirschner
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Ashish S Patel
Ashish S Patel
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
David Ziring
David Ziring
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Bruce C Trapnell
Bruce C Trapnell
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Francisco A Sylvester
Francisco A Sylvester
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Michael C Stephens
Michael C Stephens
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Robert N Baldassano
Robert N Baldassano
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
James F Markowitz
James F Markowitz
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Judy Cho
Judy Cho
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Ramnik J Xavier
Ramnik J Xavier
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Curtis Huttenhower
Curtis Huttenhower
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Bruce J Aronow
Bruce J Aronow
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,
Greg Gibson
Greg Gibson
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,†,
Jeffrey S Hyams
Jeffrey S Hyams
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,†,
Marla C Dubinsky
Marla C Dubinsky
1Division of Pediatric Gastroenterology, Emory University School of Medicine, Atlanta, GA, USA (S Kugathasan MD, K Mondal PhD); Children’s Healthcare of Atlanta, Atlanta, GA, USA (S Kugathasan, S Cohen MD); Division of Pediatric Gastroenterology, Hepatology, and Nutrition (L A Denson MD), Division of Biostatistics and Epidemiology (M-O Kim PhD, C Liu MS), Division of Pulmonary Biology (B C Trapnell MD), and Division of Biomedical Informatics (B J Aronow PhD), Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Division of Pediatric Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada (T D Walters MD, A Griffiths MD); Center for Integrative Genomics, Georgia Institute of Technology, Atlanta, GA, USA (U M Marigorta PhD, G Gibson PhD); The Broad Institute of MIT and Harvard, Cambridge, MA, USA (M Schirmer PhD, R J Xavier MD, C Huttenhower PhD); Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, USA (M Schirmer, C Huttenhower); Department of Pediatric Gastroenterology, Hepatology, and Nutrition, Medical College of Wisconsin, Milwaukee, WI, USA (J D Noe MD); Department of Pediatric Gastroenterology, Nationwide Children’s Hospital, Ohio State University College of Medicine, Columbus, OH, USA (W V Crandall MD); Department of Gastroenterology and Nutrition, Boston Children’s Hospital, Boston, MA, USA (S Snapper MD); Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, USA (S Rabizadeh MD); Department of Pediatrics, Goryeb Children’s Hospital, Morristown, NJ, USA (J R Rosh MD); Department of Pediatrics, Hasbro Children’s Hospital, Providence, RI, USA (J M Shapiro MD); Department of Pediatrics, University of Utah, Salt Lake City, UT, USA (S Guthery MD); Department of Pediatrics, Children’s Hospital of Eastern Ontario IBD Centre and University of Ottawa, ON, Canada (D R Mack MD); Section of Pediatric Gastroenterology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX, USA (R Kellermayer MD); Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA (M D Kappelman MD, F A Sylvester MD); Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA (S Steiner MD); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA (D E Moulton MD); Department of Gastroenterology, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA (D Keljo MD); Children’s Center for Digestive Health Care, Atlanta, GA, USA (S Cohen); Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA (M Oliva-Hemker MD); Department of Pediatrics, University of California, San Francisco, San Francisco, CA, USA (M B Heyman MD); Department of Pediatrics, Dalhousie University, Halifax, NS, Canada (A R Otley MD); Department of Digestive Diseases and Nutrition Center, University at Buffalo, Buffalo, NY, USA (S S Baker MD); Department of Pediatrics, Nemours Children’s Specialty Care, Jacksonville, FL, USA (J S Evans MD); Department of Pediatrics, University of Chicago, Chicago, IL, USA (B S Kirschner MD); Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA (A S Patel MD); Department of Pediatrics, UCLA David Geffen School of Medicine, Los Angeles, CA, USA (D Ziring MD); Department of Pediatric Gastroenterology, Mayo Clinic, Rochester, MN, USA (M C Stephens MD); Department of Pediatrics, University of Pennsylvania, Philadelphia, PA, USA (R N Baldassano MD); Department of Pediatrics, Northwell Health, New York, NY, USA (J F Markowitz MD); Department of Pediatrics, Mount Sinai Hospital, New York, NY (J Cho MD, M C Dubinsky MD); Center for Computational and Integrative Biology, Gastrointestinal Unit and Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital, Boston, MA, USA (R J Xavier); Center for Microbiome Informatics and Therapeutics, Massachusetts Institute of Technology, Cambridge, MA, USA (R J Xavier); and Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA (J S Hyams MD)
1,†