Abstract
Study Objectives
The maternal postpartum period is characterized by sleep fragmentation, which is associated with daytime impairment, mental health disturbances, and changes in melatonin patterns. In addition to sleep fragmentation, women undergo a complex set of physiological and environmental changes upon entering the postpartum period, confounding our understanding of effects of postpartum sleep disturbance. The primary study aim was to understand the basic impact of a single night of postpartum-like sleep fragmentation on sleep architecture, nocturnal melatonin levels, mood, daytime sleepiness, and neurobehavioral performance.
Measurements and Results
For one week prior to entry into the laboratory, eleven healthy nulliparous women kept a stable sleep-wake schedule (verified via actigraphy). Participants contributed three consecutive nights of laboratory overnight polysomnography: (1) a habituation/sleep disorder screening night; (2) a baseline night; and (3) a sleep fragmentation night, when participants were awakened three times for ~30 min each. Self-reported sleep quality and mood (Profile of Mood States Survey) both decreased significantly after sleep fragmentation compared to baseline measurements. Unexpectedly, daytime sleepiness (Multiple Sleep Latency Test) decreased significantly after sleep fragmentation. Experimental fragmentation had no significant effect on time spent in nocturnal sleep stages, urinary 6-sulphatoxymelatonin concentration, or psychomotor vigilance test performance. Participants continued to provide actigraphy data, and daily PVTs and self-reported sleep quality assessments at home for one week following sleep fragmentation; these assessments did not differ from baseline values.
Conclusions
While there were no changes in measured physiological components of a single night of postpartum-like experimental sleep fragmentation, there were decreases in self-reported measures of mood and sleep quality. Future research should examine the effects of multiple nights of modeling postpartum-like sleep fragmentation on objective measures of sleep and daytime functioning.
Keywords: Maternal, sleep fragmentation, mood, daytime sleepiness, sleep quality, polysomnography
1. Introduction
The early maternal postpartum period is characterized by fragmented sleep [1,2], during which new mothers obtain an average of 7.2 hr of total sleep time (TST), despite spending about 9 hr dedicated to sleep [3]. In addition to fragmented sleep, postpartum women experience alterations in sleep architecture [4,5] and nocturnal melatonin patterns [6–8], daytime sleepiness [9], impaired neurobehavioral performance [10], and mood disturbances [11–13]. Yet, new mothers vary in the severity to which they experience these outcomes, likely because of a complex process of individual physiological and environmental differences. The primary aim of the current study was to understand the relative contribution of sleep fragmentation to each of these outcomes. The current study imposed a postpartum sleep schedule based on nocturnal infant caretaking, the primary mechanism for postpartum sleep fragmentation [14,15], on childless women in a tightly controlled laboratory environment. This model allows causal testing of the effects of infant-driven postpartum sleep fragmentation independent of the physiological and environmental changes that co-exist during the typical postpartum period and also have the potential to disrupt sleep.
1.1 Postpartum Physiological and Environmental Changes
New mothers experience physiological changes throughout pregnancy and the postpartum period that may impact their sleep and mental health above and beyond nocturnal infant demands. For instance, postpartum women undergo a host of hormonal changes as they progress through pregnancy and into the postpartum period. Steroid hormones such as estrogens, progesterone, and cortisol increase during pregnancy and abruptly decrease after childbirth [16,17]; this abrupt transition is posited to be associated with mood disturbances [18,19]. Furthermore, high cortisol levels are associated with stress and depressive symptoms [20]. Estrogens and progesterone have sleep-promoting effects [21]. Estrogens decrease sleep onset latency and nocturnal awakenings, while increasing TST and time spent in REM sleep [20]. Progesterone is sedative, decreasing wakefulness and latency to NREM sleep as well as decreasing REM sleep [20]. Finally, decreased melatonin peak values [22] and circadian phase shifts [6] have been reported during the postpartum period. These hormonal changes may contribute to postpartum sleep quality and confound the unique impact of postpartum sleep fragmentation. The current study’s model of nulliparous women who do not experience these hormonal changes can, therefore, more closely approximate the basic impact of postpartum sleep fragmentation, independent of the hormonal consequences of the perinatal period.
Physiological changes during the postpartum period that may impact sleep are not limited to hormonal changes. Most new mothers report at least one negative, physical health symptom during the early postpartum period [23], such as general pain, headaches, and breast soreness [24,25]. While there is a lack of specific literature on postpartum physical pain and sleep, more than half of adults with chronic pain also experience sleep disturbances [26–28]. Further, postpartum physical health conditions are associated with emotional well-being and depressive symptoms [25], which is bidirectionally associated with sleep [21,29–31]. The participants in the current study were healthy without major medical conditions which may otherwise impact their sleep and mental health, allowing for an ideal model to test the impact of an infant-driven postpartum sleep fragmentation schedule.
Finally, the postpartum period is also characterized by adjustment to a new parenting role that includes caring for an infant as well as emotional and social changes [32]. Child-care responsibilities and lack of knowledge related to parenting are common sources of frustration and fatigue for new mothers [33]. Postpartum fatigue is indirectly associated with stress, via depressive symptoms and sleep quality [34,35]. New mothers may vary substantially in their adjustment to the postpartum period as a result of available social support. New mothers report social networks as their primary source of support [36], and social support is a recognized buffer for stressful life events and predictor of emotional and physical well-being [37]. Thus, the impact of the postpartum period on sleep and mental health is not limited to physiological changes, but also includes environmental stimuli. As nulliparous women, the current study’s participants did not undergo these life changes, strengthening the use of this model to isolate the effect of infant-driven postpartum sleep fragmentation.
1.2 Effects of Experimental Sleep Disturbance
Laboratory-based experimental sleep deprivation conducted in non-postpartum populations causes melatonin suppression [38], despite using illuminance levels lower that what is expected to suppress melatonin [39]. Further, there is evidence of a rebound in nocturnal peak melatonin values following a night of sleep deprivation [40]. These studies suggest nocturnal melatonin levels may be influenced by sleep loss, but the effects of sleep fragmentation on melatonin are unknown.
Laboratory-based experimental sleep fragmentation conducted in non-postpartum populations causes decreases in deep sleep and REM and subsequent increases in lighter stages of sleep [41,42], daytime sleepiness [42], and degraded mood [41]. However, these experiments have focused on the effects of sleep fragmentation as manifested by sleep disorders such as sleep apnea and periodic limb movement disorders, thus inducing forced awakenings every 1–2 min across the night. The sleep fragmentation postpartum women experience is unique, in that they have much longer periods of consolidated sleep, but also relatively longer periods of wakefulness [43].
1.3 Development of Study Protocol and Hypotheses
In order to model postpartum sleep fragmentation, we chose to awaken participants three times, equally spaced throughout the night. This schedule was chosen based on data from our lab indicating women with infants 0–6 months old awaken an average of 2.9 times each night and that this is stable across this 6-month time period [43], as well as recommendations from the American Academy of Pediatrics that breastfed newborns should be fed at intervals of 2–3 hr [44]. Previous literature on newborns within the first three days after birth indicate that feeding time is approximately 13.8–16 min [45,46], but that mothers spend an additional 12 min engaged with the infant in non-feeding activities [46]. Based on these cumulative data and our previous finding that mothers of infants 0–6 months spend an average of 33.9 min during each nocturnal awakening on infant caregiving [43], the protocol for the current study was 3 awakenings of ~30 min each, spaced equally throughout the night.
Prior to the current study, no study had controlled for the physiological and environmental factors associated with the postpartum period to isolate the impact of the sleep fragmentation, likely due to the difficulties of studying this population in a laboratory and the logistic and ethical barriers to changing and manipulating their routine. This study attempted to overcome these barriers and provide a basic understanding of the effects of a single night of postpartum-like sleep fragmentation by manipulating the sleep of healthy women without children to resemble what is observed during the early postpartum period. Isolating the impacts of this unique sleep fragmentation on sleep and daytime outcomes is important in order to understand the magnitude of impact interventions directed at improving postpartum sleep have in mitigating the consequences of this vulnerable period.
Based on previous sleep fragmentation studies [41,42,47] and their reported occurrences among postpartum women [4–6,9–13], it was hypothesized that modeling postpartum sleep fragmentation among nulliparous women would cause: (1) a decrease in slow wave sleep and REM sleep at the expense of increases in Stages N1 and N2; (2) an increase in daytime sleepiness; (3) a decrease in subjective sleep quality; (4) a decrease in neurobehavioral performance; (5) a decrease in mood. Based on experimental sleep deprivation work [38] and reported melatonin suppression among populations experiencing sleep fragmentation (i.e., postpartum women [7] and patients with obstructive sleep apnea [48]), it was hypothesized that our protocol would cause: (6) a suppression of nocturnal 6-sulphatoxymelatonin.
2. Material and methods
The study was approved by the Office of Research Compliance (IRB) at West Virginia University. Women were recruited from March through November, 2013 on the basis of being of child-bearing age and generally healthy. A telephone screening was conducted prior to administration of informed consent and Health Information Portability and Accountability Act authorization. Participants who signed informed consent were explained in detail the purpose of the research and all aspects of the study. A priori hypotheses were not shared with participants.
2.1 Procedure
The 17-day study protocol is illustrated in Figure 1. For the duration of the study, participants wore an actigraph on their non-dominant wrist and completed a watch diary, daily sleep diary, and the psychomotor vigilance test (PVT) each morning within 2 hr after awakening. Between overnight laboratory stays, participants followed their usual routine.
Figure 1.
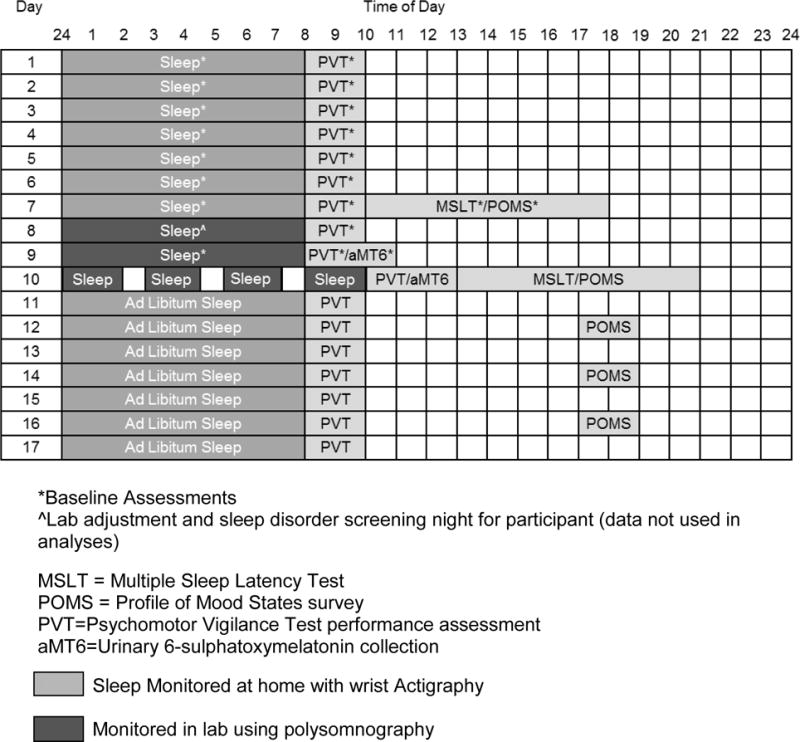
Study protocol for a participant with a habitual sleep period from 12–8am
A week of baseline actigraphy was used to normalize sleep and ensure participants were well rested. Participants kept a consistent sleep schedule (sleep and wake times <1 hr deviation from habitually reported times) and spent at least 8 hr in bed. Immediately following the first week of actigraphy, participants came into the lab for a multiple sleep latency test (MSLT). Between the third and fourth nap opportunities, participants completed the Profile of Mood States (POMS) survey. At the end of the MSLT day, participants whose average sleep onset latency was 8 min or less were disqualified from the study. Otherwise, they were discharged from the laboratory for a few hours, until the first overnight polysomnography (PSG). During this first in-lab night, participants slept according to their habitual sleep periods (determined by the baseline week of actigraphy data). This first night served as a habituation night to account for a potential first-night effect [49] and to ensure no participant had a sleep disorder. The second in-lab night was conducted in the same manner as the preceding night but was used as a baseline PSG night in analyses. The third night was the experimental sleep fragmentation night. Participants were awakened 3 times throughout the night for ~30 min each, spaced equally throughout the night. Each participant’s bedtime and habitual time spent in bed were preserved; scheduled awakenings were added to the sleep period, resulting in a rise time 90 – 105 min later than typical for each participant. Actigraphy data were also collected from participants during each of the PSG nights.
The current protocol for the ~30 min awakenings was standardized. Posture and dim lighting levels (1 lux in the direction of gaze, verified by a light meter) during each awakening were kept consistent because both changes in posture and lighting levels can impact melatonin levels and sleepiness [50,51]. Each participant was awakened three times throughout the night by a prerecorded audio clip of an infant crying via an intercom next to the participant’s bed. During each awakening, participants were given standard instructions in real time over the intercom (see Table 1).
Table 1.
Participant instructions during awakenings on sleep fragmentation night.
| 1. | “Pick up the baby doll, remove her onesie, change her diaper with the diaper provided, and then put her onesie back on.” (5 min - participant was standing during the procedure). |
| 2. | “Sit in the rocking chair and pretend to feed the baby doll with the bottle provided until I instruct you to stop.” (15 min – participant was seated) |
| 3. | “Pretend to burp the baby while remaining in the rocking chair.” (5 min – participant was seated) |
| 4. | “Stand up and gently place the baby doll back down on the spare bed.” At this point, the participant used the bathroom (if needed) and the researcher replaced any PSG sensors that had fallen off. The participant was then helped back into bed. |
After the experimental night, participants remained in the laboratory for the second MSLT, and completed a second POMS survey between nap opportunities three and four. During the final study week, participants followed their usual routine and adhered to the same protocol as the first 7 nights of the study, except that sleep was ad libitum and participants self-administered the POMS every other day.
2.2 Measures
2.2.1 Actigraphy
To ensure that participants maintained a regular sleep schedule during the baseline period, sleep/wake periods were monitored at home using Mini Mitter’s Actiwatch-64 (AW-64) actigraphs (Phillips Respironics, Bend, Oregon), and periods of nocturnal sleep and daytime naps were participant-identified using a handheld computer. Sleep diaries were used to behaviorally corroborate actigraphy data and identify sleep periods for analysis [52]. Actigraphy has been well-validated for detecting sleep/wake patterns among adults [53–56]. Consistent with our previous work [3,57], the actigraph was programmed at the most sensitive 15-epoch setting. Sleep and rise times were manually identified by researchers as the first 2 min of immobility preceeding diary-reported bedtime and last 2 min of immobility preceding diary-reported rise time, respectively. Actigraph software was used to calculate total sleep time and sleep efficiency during this sleep period.
2.2.2 Psychomotor Vigilance Test (PVT)
The PVT was self-administered using customized software (Bruner Consulting Co., Longmont, CO) on the handheld computer each morning within two hours after awakening and prior to consuming any caffeine [58]. Each test lasted 5 min, during which stimuli were presented at random inter-stimulus intervals. The use of this 5-min test is supported by a validation of PVTs less than 10 min in duration [59,60].
2.2.3 Subjective Sleep Quality
Subjective sleep quality was assessed within two hours after awakening each morning using the handheld computer. Participants were asked: “Where 100 is fully rested, please indicate your quality of sleep:” (0–100 visual analog scale).
2.2.4 Profile of Mood States (POMS)
The Profile of Mood States (POMS) was administered in the laboratory prior to and after experimental sleep fragmentation and was given to each subject to self-administer at home each day during the recovery week (automated text messages were sent as a reminder at the same time each day). The POMS consists of 65 adjectives rated by participants based on how they are feeling using a 5-point Likert scale (range: 0 “Not at all” – 4 “Extremely”). Six subscales with different ranges are derived from the POMS: tension-anxiety, depression-dejection, anger-hostility, fatigue-inertia, confusion-bewilderment, and vigor-activity. A POMS Total Mood Disturbance (TMD) score is derived from the sum of the six subscale scores, with a range from −32 to 200, and is an indicator of a global dimension of mood disturbance. POMS TMD scores and all but the vigor-activity subscale are negative dimensions, so high scores represent worse mood. Survey responses reflected the participant’s mood during the past day. Internal consistency of the POMS in our sample at baseline was α=0.71.
2.2.5 Overnight Polysomnography (PSG)
The established 10–20 system [61] was used for full PSG including F3/F4, C3/C4, O1/O2, bilateral electro-oculogram (EOG) and submental electromyogram (EMG), electrocardiogram (EKG), abdominal and thoracic respiratory belts, an oral/nasal airflow thermistor, a snore sensor, a pulse oximeter, and leg EMG sensors. When the first night of screening indicated no sleep disorders, the participant continued in the study and the next two nights (baseline and sleep fragmentation) included a minimal montage of EEG, EOG, EMG, and EKG. High resolution video monitoring was used for behavioral observation and video recording. Recordings were made with the Embla N7000 system (Natus Neurology, Middleton, WI) and data were analyzed using Rembrandt software (Natus Neurology, Middleton, WI).
2.2.6 Multiple Sleep Latency Test (MSLT)
Daytime sleepiness was objectively measured using standardized guidelines for the laboratory-based four-nap MSLT with the minimum montage described above. [62] Participants’ sleep was monitored for one week prior to the test via actigraphy. Participants reported that they did not consume caffeine or alcohol on days the MSLT was conducted. Sleep latency time was defined as the time it took from lights out to the first 30-second epoch of any sleep stage using the American Academy of Sleep Medicine’s rules for PSG sleep stage scoring. [61] Average sleep onset latency scores ≤5 min across the four naps indicated pathological level of daytime sleepiness and scores between 10 and 20 min were considered normal levels of sleepiness. Scores between 5 and 10 min fall into a diagnostic „grey area’ [63]. The use of a baseline MSLT score of ≤8 min as exclusion criteria in the current study was based on the definition of sleepiness for diagnostic purposes, as identified by the International Classification of Sleep Disorders. [64]
2.2.7 Urinary 6-sulphatoxymelatonin (aMT6s)
To assess aMT6s during in-lab baseline and experimental nights, participants were instructed to void immediately before lights out, then each overnight void (if any) and first morning void were collected using a commode hat (Kendall™, Covidien, Minneapolis, Minnesota). The bedroom was completely dark during sleep periods (0 lux). A red light manufactured for use in a photographic was used in the bathroom for participants who awakened in the middle of the night to void in order to minimize the impact of light on aMT6s secretion [65]. Urine was homogenized and divided into two aliquots. One aliquot was centrifuged at 2000 × g for five min, and creatinine was analyzed within 24 hr by the Alkaline Picrate method (at West Virginia University Hospital Laboratories). The other aliquot was centrifuged the same day as collection, and the supernatant was stored at −80°C until assay. Once all samples were collected, they were thawed once and run in duplicate using an aMT6 enzyme-linked immunosorbent assay (ELISA) kit, per manufacturer’s instructions (ALPCO, Salem, New Hampshire). The plate was read at 450 nm using a VMax Kinetic ELISA Microplate Reader (Molecular Devices, Sunnyvale, California). After subtracting background, the optical density of each well was plotted along a standard curve of known aMT6 samples that were run within the same assay plate.
2.3 Statistical Analyses
The sample size of 11 participants was determined by a priori power analyses based on previous work on the effects of sleep fragmentation and sleep restriction [41,63,66], using a 2-tailed alpha level =.05 and sigma =.80. SPSS version 21.0 was used for data analyses (IBM Corp, Armonk, NY). A p<.05 was considered statistically significant, except in noted cases when a Bonferroni correction was used. Analysis of Variance (ANOVA) and chi-square tests were used to test for differences in demographics among participants who completed the study versus those who did not. Paired samples t-tests were used, where appropriate, to determine whether dependent variables differed between baseline and fragmentation nights in the laboratory, and to assess changes in dependent variables from baseline to post-fragmentation. Wilcoxon’s signed rank test was used when variables did not meet normality assumptions. Repeated measures ANOVA was used to assess changes in variables across the baseline week, to determine differences in the time spent in each sleep stage on baseline compared to fragmentation nights, and to test for differences in sleep onset latencies across MSLT nap opportunities between the baseline and post-fragmentation MSLTs. ANOVA was also used for polynomial and linear trend analyses on sleep onset latencies after forced awakenings across the night, and for within-subject changes in TST, self-reported sleep quality, PVT lapses, and POMS scores across the at-home recovery week. Partial eta squared (η2p) (small=.01, medium=.06, large=.14), Cohen’s d (small=.2, medium=.5, large=.8), Cramer’s V (small=.1, medium=.3, large=.5), and r (small=.1, medium=.3, large=.5) were used to calculate effect sizes where appropriate [67].
Pairwise deletion was used to deal with missing PVT data (one trial for one participant) and actigraphy of nocturnal sleep data (one night for one participant). One overnight PSG had 40 min of unscoreable data; % sleep stages for this participant were based on the total sleep scored. PSG equipment malfunction during one MSLT nap precluded scoring of SOL; the MSLT score for this participant was based on the three usable naps.
3. Results
3.1 Participants
Women who completed the study (n=11) were all nulliparous with no reported history or current symptoms of depression, as defined by a score of <16 on the Center for Epidemiologic Studies Depression Scale [68]; they did not report symptoms of premenstrual dysphoric disorder (DSM-IV) [69] and all had regular menstrual cycles (defined as being confident in their ability to predict when their period would start, to the week); they did not work night shifts or travel through >1 time zone during the month prior to participating; did not have a clinically significant sleep disorder (verified with polysomnography); did not report blindness or visual impairments that would interfere with the production of melatonin, did not have a major medical illness for which they were under the care of a physician, were not current smokers, consumed no more caffeine per day than the amount in two 6-oz cups of coffee, and consumed on average <3 alcoholic drinks per week. Figure 2 illustrates how many women expressed initial interest in and then declined to begin or continue participation, or were excluded at various points throughout the study.
Figure 2. Participant inclusion and exclusion throughout the study.
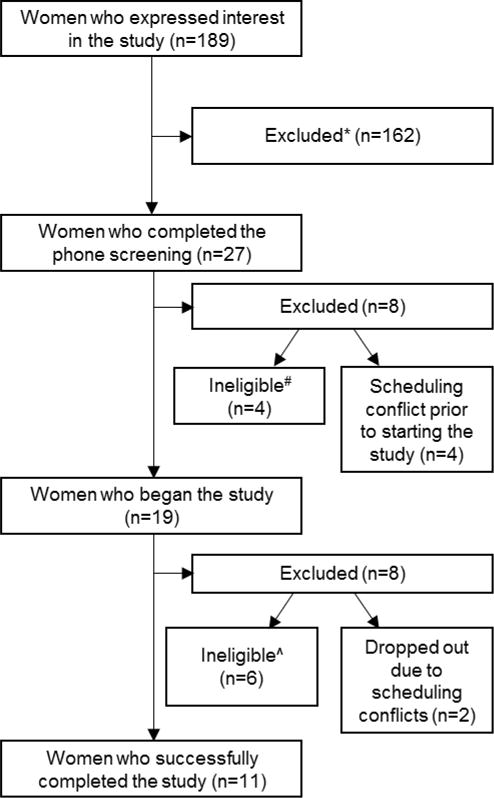
*Were unable to participate due to scheduling difficulties or were no longer interested after the details of the study were described.
#Reasons for ineligibility to begin study: on antidepressant medication (n=1), symptoms of a sleep disorder (n=2), major medical condition (n=1).
^Reasons for ineligibility to continue study: sleep/wake times differed across baseline week by >3 hr (n=1), scored <8 min on baseline MSLT (n=4), sleep disorder symptoms on screening night (n=1).
Demographic characteristics of the final sample of 11 women are in Table 2. Between-groups one-way ANOVA and chi-square tests were used to determine whether the participants who began but did not complete the study (i.e. signed consent, but either dropped out or were excluded part-way through) differed from those who completed the study. No differences were found between groups on any demographic variable or baseline actigraphically-recorded TST. Based on the PSG screening night, no participant had any respiratory events. Three of the completed participants had periodic limb movements in sleep (PLMS), with PLMS/hr indices of 8.3 (PLMS with arousals [PLMA]/hr=1.0), 2.6 (PLMA/hr=0.8), and 1.6 (PLMA/hr=0), respectively. These participants were retained because none had clinically significant levels of PLMS (>15 PLMS/hr) [70], complaints of daytime fatigue, or showed signs of excessive daytime sleepiness (based on recorded MSLT scores).
Table 2.
Participant Demographics (n=11)
| Mean/Percentage ± Standard Deviation | Range | |
|---|---|---|
| Age | 25.3 ± 2.3 | 23.0–29.8 |
|
| ||
| Income | $23,000 ± $11,000 | $12,000–$50,000 |
|
| ||
| Years of Education | 17.7 ± 1.1 | 16–20 |
|
| ||
| Race/Ethnicity | ||
| White | 72.7% | |
| Asian | 18.2% | |
| Biracial | 9.1% | |
|
| ||
| Marital Status | ||
| Single | 63.6% | |
| Married/Cohabitating | 36.4% | |
|
| ||
| Work Status | ||
| Full-time Student | 81.8% | |
| Full-time Employed | 9.1% | |
| Unemployed | 9.1% | |
|
| ||
| Currently using a Hormonal Birth Control Method | 54.6% | |
|
| ||
| BMIa | 23.3 ± 4.9 | 16.9–35.6 |
|
| ||
| Average Baseline TST | 7hr 41min ± 33.2 min | 6hr 53min – 8hr 24min |
Calculated in laboratory: height with a stadiometer, weight with a digital scale, BMI calculator used was from www.nhlbi.nih.gov
3.2 Baseline Polysomnography Night
Analyses were first conducted to determine whether sleep, and next-morning self-reported sleep quality and PVT lapses differed between the at-home baseline week, in-lab screening night, or in-lab baseline night. All sleep variables in these analyses were obtained using actigraphy recordings to avoid making comparisons across different measurement techniques, (i.e., comparing actigraphy to PSG). There was a significant difference in sleep efficiency between nights, and post-hoc tests with a Bonferroni correction confirmed greater sleep efficiency on the in-lab baseline night compared to the at-home baseline week. There were no statistically significant differences between any of the other measures (see Table 3), confirming that neither polysomnography night disrupted participants’ sleep.
Table 3.
Differences between baseline week and screening and baseline polysomnography nights
| At-home Baseline Week | In-lab Screening Night | In-lab Baseline Night | p value | η2p | |
|---|---|---|---|---|---|
| Total Sleep Timeˆ | 461.2 ± 33.2 | 460.2 ± 36.4 | 474.1 ± 23.0 | .24 | .13 |
| Sleep Efficiencyˆ | 90.4 ± 3.6 | 92.4 ± 4.4 | 93.1 ± 3.0 | .03 | .29 |
| Self-report Sleep Quality | 86.1 ± 5.7 | 81.6 ± 13.9 | 89.1 ± 7.2 | .13 | .20 |
| PVT Lapses | 1.6 ± 1.8 | 2.27 ± 2.2 | 2.0 ± 2.4 | .65 | .04 |
Measured via actigraphy
3.3 Postpartum Sleep Fragmentation Simulation
Analyses were conducted to determine whether the protocol was successful at simulating postpartum sleep disturbance. Participants were awakened three times equally spaced throughout the night for approximately the same duration of time spent out of bed (no sleep opportunity permitted) during each awakening (see Table 4). As intended, there was a significant decrease in sleep efficiency from in-lab baseline to sleep fragmentation night, without a significant change in total sleep time (see Table 5). These data indicate that the planned sleep fragmentation protocol was successful in simulating postpartum sleep disturbance and that participants returned to sleep quickly after each awakening.
Table 4.
Descriptive Information on Sleep Fragmentation Night
| Mean (min) |
SD (min) |
Range (min) |
|
|---|---|---|---|
| Duration of Time Spent Out of Bed | |||
| Awakening 1 | 32.9 | 3.2 | 29.8 – 38.7 |
| Awakening 2 | 31.8 | 1.8 | 29.6 – 34.7 |
| Awakening 3 | 32.4 | 2.5 | 29.9 – 37.1 |
|
| |||
| Mean (hh:mm) |
SD (hh:mm) |
Range (hh:mm) |
|
|
| |||
| Duration Between: | |||
| Lights Out & Awakening 1 | 2:06 | 0:09 | 1:48–2:21 |
| End of 1st & Beginning of 2nd Awakening | 2:06 | 0:07 | 1:59–2:20 |
| End of 2nd & Beginning of 3rd Awakening | 2:06 | 0:07 | 2:00–2:19 |
| End of 3rd Awakening & Lights On | 2:06 | 0:07 | 1:56–2:18 |
Table 5.
Time spent in each sleep stage between baseline and sleep fragmentation nights
| Baseline | Sleep Fragmentation | P value | Cohen’s d | |
|---|---|---|---|---|
| % N1 | 6.7 ± 2.5 | 4.3 ± 2.4 | .02 | .97 |
| % N2 | 43.5 ± 8.3 | 39.7 ± 5.4 | .10 | .55 |
| % N3 | 28.8 ± 6.3 | 30.2 ± 4.8 | .54 | .24 |
| % REM | 23.3 ± 7.0 | 23.5 ± 6.5 | .66 | – |
| TST (min) | 461 ± 28 | 448 ± 34 | .17 | .42 |
| % SE | 90.9 ± 6.1 | 74.4 ± 3.9 | <.001 | 3.2 |
3.4 Subjective Measures
3.4.1 Self-Reported Sleep Quality
As sleep quality the morning after sleep fragmentation was negatively skewed and leptokurtic, Wilcoxon’s signed rank test was used to test for differences in self-reported sleep quality between the morning after baseline and sleep fragmentation nights in the lab. There was a significant decrease in self-reported sleep quality after sleep fragmentation (Mdn=73.0) compared to baseline (Mdn=90.0; Z=2.8, p=.005, r=.84). A trend analysis revealed a significant increasing linear trend across the in-lab sleep fragmentation night and at-home recovery week, F(1,9)=16.3, p=.003, η2p=.64 (see Figure 3). This trend was not significant when the in-lab sleep fragmentation night was removed from the analysis (F[1,9]=0.39, p=.55, η2p=.04) suggesting the low sleep quality reported the morning after sleep fragmentation was driving the linear trend. However, the assumption of normality of all variables used in the trend analysis was not met even after transformation of variables, so results should be interpreted with caution.
Figure 3. Self-reported sleep quality the morning after baseline and sleep fragmentation nights, and across the post-fragmentation week (Error bars represent SE; **=p<.01).
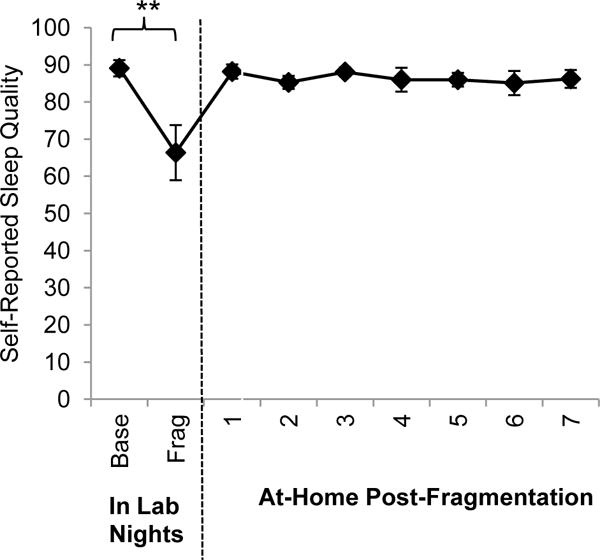
Base = Baseline polysomnography night in the lab; Frag = Experimental sleep fragmentation night.
To estimate the association between participants’ self-report of their sleep and PSG-defined measures, Pearson’s correlations were performed on the number of awakenings and duration of awakenings on the adaptation and baseline nights in the laboratory. On the adaptation night, participants’ reports of the number of awakenings were not significantly associated with the PSG-measured number of awakenings, r=−0.03, p=0.94, however, on the baseline night, this relation was a medium effect size, r=0.45, p=0.17. On both the adaptation night and baseline night, participants’ reports of the duration of awakenings were strongly related to the PSG-calculated WASO, r=0.68, p=0.02 and r=0.90, p<0.001, respectively. These data support that the participants self-reports of their sleep experiences were accurate when compared to objective data.
3.4.2 Mood
A paired samples t-test was used to test for differences in POMS TMD scores between baseline and the day after sleep fragmentation. There was a statistically significant increase from baseline POMS TMD scores (M=−1.0, SD=7.1) compared to the day after sleep fragmentation (M=9.6, SD=8.9; t[10]=2.6, p=.03, d=.1.3). A linear trend analysis revealed no trend across the at-home recovery week, F(1,10)=.05, p=.82, η2p=.01 (see Figure 4).
Figure 4.
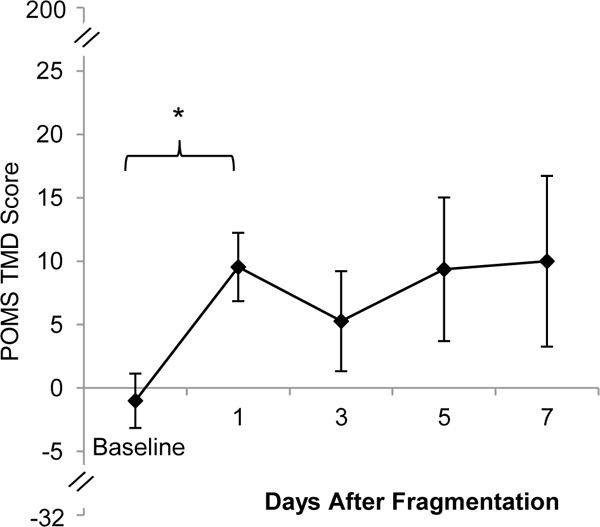
POMS TMD scores during baseline and days after sleep fragmentation (Error bars represent SE; *=p<.05).
Given significant differences on the POMS TMD scale between baseline and the day after sleep fragmentation, paired samples t-tests were conducted on each of the seven POMS subscales to determine which components of mood were affected by the sleep fragmentation. After a Bonferroni correction (p=.007) there was a significant decrease in the vigor subscale after sleep fragmentation (M=12.5, SD=6.5) compared to baseline (M=21.9, SD=1.6; t[10]=5.2, p<.001, d=2.0). There was no significant change on the tension, depression, anger, fatigue, or confusion subscales (see Figure 5).
Figure 5.
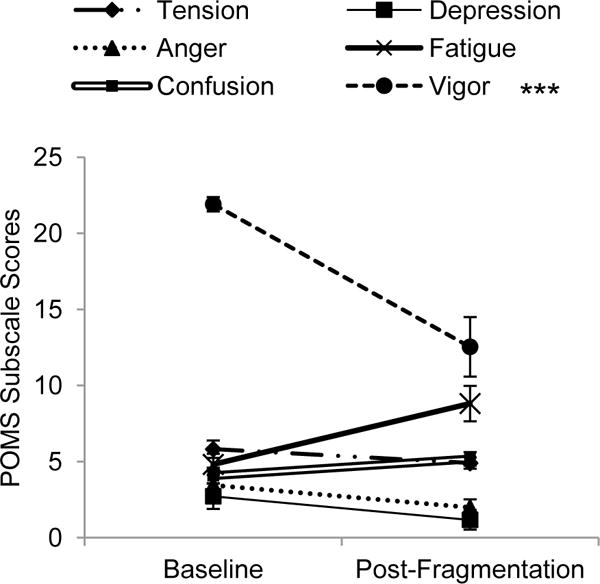
POMS subscale scores at baseline and the day after sleep fragmentation (Error bars represent SE; ***p<.001).
3.5 Objective Measures
3.5.1. Sleep Architecture
With the use of a Bonferroni correction for analyses between 4 variables (p=.012), there were no significant differences in %N1, %N2 or %N3 sleep during the sleep fragmentation night compared to baseline (see Table 5). Wilcoxon’s signed rank test was used for comparisons between %REM because this measure was negatively skewed on the sleep fragmentation night. There was no difference in %REM sleep on the sleep fragmentation (Mdn=25.9%) night compared to baseline (Mdn =22.9%; Z=.44, p=.66, r=.13) (mean values are reported in Table 4).
3.5.2 6-sulphatoxymelatonin
The intra-assay coefficient of variation of the ELISA was 4.4%. There was not a significant difference between aMT6 concentrations following the baseline (M=23.3, SD=13.5) and sleep fragmentation nights (M=25.0, SD=12.2; t[10]=.69, p=.51, d=.09).
3.5.3 Total Sleep Time
Six participants took daytime naps during the study; for these participants, 24-hr sleep time was used. Specifically, one participant napped on two days during the baseline week, two participants napped on two days during the recovery week, and three participants napped on one day during the recovery week.
A paired samples t-test was used to test for differences in total sleep time between the baseline night in the lab and the first night after sleep fragmentation night. There was no difference in total sleep time the night after sleep fragmentation (M=463.4, SD=83.4) compared to baseline (M=460.6, SD=27.8; t[10]=.11, p=.91, d=.05). A linear trend analysis revealed no trend across the at-home recovery week, F(1,9)=.09, p=.77, η2p=.01.
3.5.4 Objective Daytime Sleepiness
The baseline week of actigraphy simultaneously served as monitoring to ensure sufficient sleep prior to the MSLT, specifically that the night prior to the MSLT was representative of the participants’ typical sleep per standard protocol [62]. There was no significant change in total sleep time between the average total sleep time of the baseline week (M=461, SD=33) and the seventh day (the night before the MSLT) (M=454, SD=53; t[10]=.65, p=.53, d=.16).
There was a significant decrease in objective sleepiness between the baseline MSLT (M=13.1, SD=3.54) and the post-fragmentation MSLT (M=16.3, SD=3.51; t[10]=2.8, p=.02, d=.92).
In order to determine whether a time of day change in sleepiness across the day was driving the overall decrease in sleepiness, the MSLT scores of the four individual naps were compared from baseline to post-sleep fragmentation. A 2 (condition: baseline vs. post-fragmentation) × 4 (each individual nap) repeated-measures ANOVA was conducted. A main effect of condition was qualified by a significant condition × nap interaction, F(3)=5.6, p=.004, η2p =.39. Post-hoc paired samples t-tests using a Bonferroni correction for analyses between 4 variables (p=.012) determined that there was a significantly shorter sleep onset latency during the baseline MSLT for the fourth nap compared to post-fragmentation, (t[10]=3.4, p=.006, d=1.6) (see Figure 6). More specifically, 81.8% of participants did not fall asleep on the fourth nap post-fragmentation. This compares to 27.3% who did not fall asleep on the fourth nap of the baseline MSLT.
Figure 6.
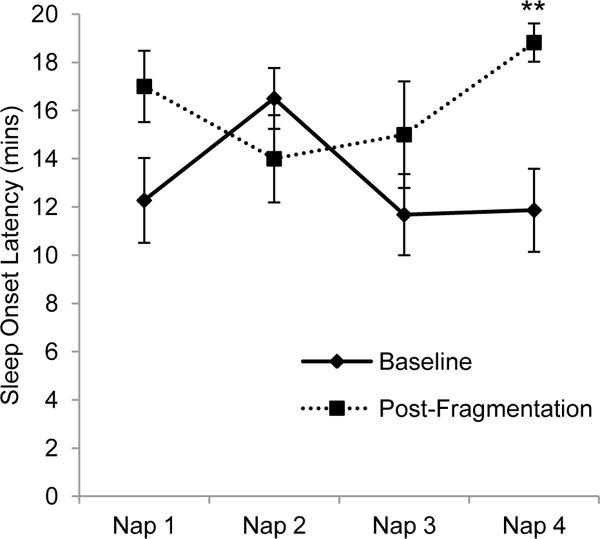
MSLT sleep onset latencies for individual nap opportunities between baseline and the day after experimental sleep fragmentation (Error bars represent SE; **=p<.01)
3.5.5 Daytime Performance
A paired samples t-test was used to test for differences in the frequency of PVT lapses between the morning after baseline and sleep fragmentation nights in the lab. There was no difference in average frequency of PVT lapses post-fragmentation (M=2.9, SD=2.8) compared to baseline (M=2.0, SD=2.4; t[10]=.98, p=.35, d=.35). A linear trend analysis revealed no trend across the at-home recovery week, F(1,10)=.14, p=.72, η2p=.01. Nor were there significant differences between other PVT outcomes including the mean reaction time (baseline: M=330.6, SD=46.5; post-fragmentation: M=343.4, SD=42.4; t[10]=1.08, p=.31, d=.29) and slowest 10% of reaction times (baseline: M=506.4, SD=115.7; post-fragmentation: M=508.7, SD=110.5; t[10]=.06, p=.95, d=.02). Samples sizes of roughly 10–15 subjects are commonly reported in the literature when examining recovery sleep and performance from sleep disturbances [41,66,71]. Based on the large effect sizes (range: Cohen’s d=0.87–2.24) reported in these studies, a priori power analyses with 80% power at an alpha=0.05 were calculated to determine our sample size of N=11.
4. Discussion
The primary goal of this study was to understand the discrete impacts of postpartum sleep disturbance driven by infant nocturnal caretaking and independent of postpartum physiological and environmental changes. A functional protocol to model postpartum sleep fragmentation was established among childless women; women had significantly lower sleep efficiency on the sleep fragmentation night compared to baseline. The sample’s PSG-recorded average sleep efficiency (74.4%) was slightly lower than the actigraphically-recorded sleep efficiency of women in the second week postpartum (79.7%) [3]. In an attempt to isolate the impacts of sleep fragmentation from total sleep time, the time in bed of each participant from baseline to sleep fragmentation night was preserved. This resulted in a total sleep time that was only slightly higher (M=7.46hr) than the duration we found using actigraphy data during postpartum weeks 2–12 (M=7.2hr) [3], that Thomas and Foreman found using self-reported sleep diaries during postpartum weeks 4–10 (M=7.18hr) [72], and that Filtness et al. found from sleep diary self-reports at 6, 12, and 18 weeks postpartum (M=7.33hr) [73]. However, others have reported lower total sleep times for postpartum women, especially in the immediate postpartum period. For instance, during the week of birth, actigraphically-measured total sleep time gradually increased from 5.46 hr the day after birth to 6.73 hr on the 6th day postpartum [74], and within the first month average actigraphically-determined nocturnal sleep time was 6.38 hr [75].
4.1 Subjective Measures
Women self-reported decreased sleep quality and mood states after only one night of sleep fragmentation when compared to baseline. Analyses performed on the POMS subscales suggest it may not be the obvious dimensions of mood (e.g. depression, anxiety) that are impacted, at least not immediately. Rather, there was a significant decrease in vigor (e.g. adjectives include: “Cheerful” and “Alert”) post-fragmentation, and a non-significant increase in fatigue. No changes were noted in tension, depression, anxiety, or confusion. This is consistent with previous work that found chronic sleep restriction impacts POMS total mood disturbance scores, and specifically subscales of fatigue, vigor, confusion, and tension [76]. However, it is inconsistent with the postpartum depression literature; based on the occurrence of postpartum depression, there would be an expected increase in the depression subscale after one night of sleep fragmentation. This inconsistency with the postpartum depression literature is not surprising given our study design is incapable of explaining the complex milieu of interactions between physiological and environmental factors during the postpartum period. In fact, that we even observed changes in subjective measures of sleep and overall mood and vigor levels after just a single night of sleep fragmentation is surprising. However, as participants knew the design of the study upfront, we cannot rule out that they were hypothesis guessing with respect to the self-reported measures. Nonetheless, the moderate relation found on the baseline night and very strong effects between self-report and PSG-determined time spent awake at night suggest the participants were accurately reporting their sleep experience.
4.2 Objective Measures
The current study’s sleep fragmentation protocol resulted in no changes in any of the nocturnal sleep stages. This finding counters the literature on experimental sleep fragmentation among healthy subjects. Previous studies have reliably demonstrated sleep fragmentation-induced increases in stage N1 and decreases in stage N3 and/or REM [41,42]. However, these studies have used sleep fragmentation protocols that simulate disorders such as obstructive sleep apnea, in which awakenings are induced every couple of min throughout the night. Studies on the effects of infrequent sleep fragmentation are scarce. As far as we are aware, the study protocol most similar to ours is that by Gonnissen et al. [77], who interrupted the sleep of 12 healthy males about every 90 min throughout the night for approximately 2 min each awakening. The results showed a decrease in REM sleep at the expense of an increase in stage N2 sleep. These conflicting findings compared to our own may be explained by two salient differences in the study designs: (1) our awakenings were 35 min in duration as opposed to 2 min; (2) we used an entirely female population as opposed to an entirely male population.
The concentration of aMT6 among the current sample did not change between baseline and sleep fragmentation nights. This is inconsistent with previously-found differences in melatonin levels among postpartum women [7] and other populations who experience fragmented sleep [48,78]. However, these studies were done in naturalistic settings, not a highly controlled laboratory. Our results were consistent with the recent work by Gonnissen and colleagues mentioned above who found no changes in plasma melatonin concentration with sleep fragmentation with no postural or light changes during awakenings [77]. Indeed, our laboratory setting limited light to 1 lux and excluded phone and television screens, which are commonly used during nocturnal feedings in the home [43]. Our findings, combined with the current literature, suggest the associations previously found between fragmented sleep and reduction in concentration of melatonin may be due to environment influences such as light at night, postpartum hormonal changes, or an interaction between these physiological and environmental factors.
Contrary to our expectations and the experimental sleep fragmentation literature [41,42,79], neither total sleep time nor PVT lapses changed post-fragmentation, as compared to baseline. Moreover, we found a decrease in daytime sleepiness. It is possible this inconsistency results from the lack of change in sleep architecture in the current study. Previous work has found positive associations between the amount of slow wave sleep and daytime sleepiness and performance [80–82]. However, conflicting evidence supports that daytime sleepiness within a sleep fragmentation protocol is related to sleep continuity, and not sleep architecture [83,84]. Therefore, interpretation of our current results suggest either alteration to sleep stages is necessary to reduce daytime functioning or else multiple nights of sleep fragmentation are required before detecting a decrease in daytime functioning. We believe the latter is more likely, based on our previous work that found postpartum women do not differ from controls on PVT performance during the earliest studied period (the second postpartum week), after which they perform more poorly than controls across the remaining of the first three postpartum months [10].
However, this does not explain the decrease in sleepiness we found. There was an increase in sleep onset latency and decrease in number of participants who fell asleep on the fourth and final nap opportunity after sleep fragmentation compared to baseline. After spending three consecutive nights in the laboratory, the post-fragmentation MSLT was the final laboratory-based procedure in the protocol. It cannot be discounted that participants may have been less likely to fall asleep because they were motivated by the near-end of the study, driving the perplexing difference in MSLT scores found. Regardless, both the average baseline and post-fragmentation MSLT scores were >10, a score that is considered normal among a healthy population [63]. Thus, the meaning of the change in scores from a clinical perspective for diagnosing daytime sleepiness is negligible.
4.3 Subjective vs. Objective Sleep
The worsened self-reported sleep quality, despite no differences found in objective sleep measures, is not entirely surprising given previously-reported inconsistencies between subjective estimates and objective polysomnographic measurements of sleep [85]. Furthermore, the number of nocturnal awakenings was the most frequently cited parameter used as a basis for daily sleep quality assessments among healthy sleepers [86]. Thus, the forced awakenings in the current study may have been the driving force behind the poorer subjective sleep quality ratings by participants.
4.4 Limitations
The study had a number of limitations, including the small sample size. Although the sample size was consistent with previous literature that have similar experimental sleep fragmentation protocols [41,77,87], it limited analyses such as comparing individuals who had changes in dependent variables from baseline to sleep fragmentation nights vs. those who had no changes in dependent variables on demographic measures. Individual differences such as these may give insight into why some women were more affected by the sleep fragmentation protocol than others.
Another limitation may have been the rigorous selection criteria. While these criteria were put in place to reduce confounding factors on the outcome variables of interest, a relatively large proportion of women were excluded from continued participation because they did not meet minimum scores on the MSLT. However, their baseline actigraphy sleep appeared healthy. Participants who were more resistant to the effects of sleep disturbances may have been over selected. Those who were excluded may have been more sensitive to the effects of sleep disturbances, and may have been impacted more by the sleep fragmentation.
Phase of the menstrual cycle or use of hormonal contraceptives was not controlled in the current study. Participants were enrolled in the study at times of convenience, which corresponded to various points in their menstrual cycle, and 54.6% used hormonal contraceptives. Differences in the sleep EEG and subjective sleep quality have been reported across the menstrual cycle and there may also be changes in melatonin concentrations [88]. Increases in nocturnal melatonin levels and decreases in slow wave sleep are also reported in women taking oral contraceptives compared to naturally cycling women [88].
There are obviously potential differences in motivation and emotional investment in caring for a doll compared to one’s own infant that may have impacted these outcomes. In particular, relatively reduced activation of the alertness circuits may have caused our participants to be less fully alert at night. This may have contributed to their ability to fall back to sleep after awakenings quickly and remain asleep without interfering thoughts such as worrying about their newborn. Similarly, the emotional investment of caring for one’s own child compared to a doll may lead to differences in self-reported mood following postpartum sleep disturbance.
Finally, it is important to keep in mind that maternal sleep is both highly variable between postpartum women and is not stagnant within women across the postpartum period [89,90]. As such, our use of averages to model the number and duration of awakenings cannot be broadly applied to all postpartum sleep experiences.
4.5 Conclusions
Results from the current study provide novel information regarding the acute effects of a simulated postpartum sleep fragmentation schedule on aspects of both physiology and behavior in a controlled laboratory setting. Results suggested no changes in objective measures of sleep and sleepiness, with the exception of a non-meaningful increase in daytime sleepiness, but significant deficits in mood and self-reported sleep quality. However, some of the limitations of the model may have contributed to the lack of change in objective measures. While the current study’s protocol was an initial step in understanding the effects of postpartum sleep fragmentation, future work should improve upon this overly simplified model to more thoroughly capture the complexities of perinatal sleep disturbance. For instance, longer durations of nocturnal awakenings [43] and shorter total sleep times [74,75] than those in the current protocol have been reported in the immediate postpartum period and should be considered in future work. Additionally, the single night modeled in this study is inadequate to represent the chronic postpartum sleep disruption experienced, nor did it take into account that women often enter the postpartum period with sleep debt built up from disturbed sleep during pregnancy and sleep deprivation during labor [74,91]. As more recent work has indicated there may be circadian phase shifts associated with the postpartum period [6], future work should also consider modeling the combination of this phase shift with the sleep fragmentation.
Furthermore, variations in nocturnal activities and lighting levels could be assessed to determine their impact on physiological measures and performance outcomes. The current protocol was carefully designed to kept participants out of light levels that could disrupt their melatonin levels and the focus of the awakenings was solely on infant caregiving. The limiting of light levels was performed in order to isolate the impact of the sleep fragmentation, per se. Future studies should work to understand how the differences in activities and behaviors postpartum women engage in may be negatively impacting their sleep. Data from our lab indicate 81.5% of postpartum women reported using an electronic device (television, computer, cell phone, backlit tablet, or a combination of these) during nocturnal awakenings and 89% of women are using at least one extra light source during caregiving, but their light sources vary widely in intensity [43]. Examining the impact of these behaviors, both in field-based and laboratory settings, is important for understanding maternal sleep disturbances.
Highlights.
A single night of postpartum-like sleep fragmentation decreased mood and subjective sleep quality
A single night of postpartum-like sleep fragmentation did not alter sleep stages or melatonin levels
A single night of postpartum-like sleep fragmentation did not alter next-morning performance
The effects of chronic postpartum sleep fragmentation need to be elucidated
Acknowledgments
The authors thank the research participants. Data collection and processing were carried out with the help of Margeaux Schade, MS; Kelsey Meekins, MS; Hannah Ritchie, BS; Caleb Rhodes, BS; Emma Platt, BS; Kristen Johnson, BS; and John Artimez. The authors also thank Melissa Blank, Ph.D., Helen Burgess, Ph.D., and Barry Edelstein, Ph.D. for their intellectual contributions.
Financial Support: This work was supported by the following:
NIGMS Training Grant T32 GM081741 & Department of Psychiatry, West Virginia University; William E. Vehse Endowment Award, Department of Psychology, West Virginia University; Funding for Doctoral Student Research, Department of Psychology and Office of Academic Affairs, West Virginia University
Abbreviations
- ANOVA
Analysis of Variance
- aMT6
6-sulphatoxymelatonin
- ELISA
Enzyme-linked Immunosorbent
- Assay MSLT
Multiple Sleep Latency Test
- POMS
Profile of Mood States Survey
- PSG
Polysomnography
- PVT
Psychomotor Vigilance Test
- SE
Sleep Efficiency
- TMD
Total Mood Disturbance
- TST
Total Sleep Time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gay CL, Lee KA, Lee S-Y. Sleep patterns and fatigue in new mothers and fathers. Biol Res Nurs. 2004;5:311–8. doi: 10.1177/1099800403262142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter LP, Rychnovsky JD, Yount SM. A selective review of maternal sleep characteristics in the postpartum period. J Obstet Gynecol Neonatal Nurs. 2009;38:60–8. doi: 10.1111/j.1552-6909.2008.00309.x. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery-Downs HE, Insana SP, Clegg-Kraynok MM, Mancini LM. Normative longitudinal maternal sleep: the first 4 postpartum months. Am J Obstet Gynecol. 2010;203:465.e1–7. doi: 10.1016/j.ajog.2010.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driver HS, Shapiro CM. Clinical Research A Longitudinal Study of Sleep Stages in Young Women During Pregnancy and Postpartum. 1992;15:449–53. doi: 10.1093/sleep/15.5.449. [DOI] [PubMed] [Google Scholar]
- 5.Nishihara K, Horiuchi S, Eto H, Uchida S, Honda M. Delta and theta power spectra of night sleep EEG are higher in breast-feeding mothers than in non-pregnant women. Neurosci Lett. 2004;368:216–20. doi: 10.1016/j.neulet.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Sharkey KM, Pearlstein TB, Carskadon MA. Circadian phase shifts and mood across the perinatal period in women with a history of major depressive disorder: a preliminary communication. J Affect Disord. 2013;150:1103–8. doi: 10.1016/j.jad.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas KA, Burr RL. Melatonin Level and Pattern in Postpartum Versus Nonpregnant Nulliparous Women. J Obstet Gynecol Neonatal Nurs. 2006;35:608–15. doi: 10.1111/j.1552-6909.2006.00082.x. [DOI] [PubMed] [Google Scholar]
- 8.Wierrani F, Grin W, Hlawka B, Kroiss A, Grünberger R. Elevated serum melatonin levels during human late pregnancy and labour. J Obstet Gynaecol (Lahore) 1997;17:449–51. doi: 10.1080/01443619750112411. [DOI] [PubMed] [Google Scholar]
- 9.Insana SP, Montgomery-Downs HE. Sleep and sleepiness among first-time postpartum parents: A field- and laboratory-based multimethod assessment. Dev Psychobiol. 2012 doi: 10.1002/dev.21040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Insana SP, Williams KB, Montgomery-Downs HE. Sleep disturbance and neurobehavioral performance among postpartum women. Sleep. 2013;36:73–81. doi: 10.5665/sleep.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milgrom J, Gemmill AW, Bilszta JL, Hayes B, Barnett B, Brooks J, et al. Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord. 2008;108:147–57. doi: 10.1016/j.jad.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Dennis C-L, Ross L. Relationships among infant sleep patterns, maternal fatigue, and development of depressive symptomatology. Birth. 2005;32:187–93. doi: 10.1111/j.0730-7659.2005.00368.x. [DOI] [PubMed] [Google Scholar]
- 13.Pearlstein T, Howard M, Salisbury A, Zlotnick C. Postpartum depression. Am J Obstet Gynecol. 2009;200:357–64. doi: 10.1016/j.ajog.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishihara K, Horiuchi S, Eto H, Uchida S. Mothers’ wakefulness at night in the postpartum period is related to their infants’ circadian sleep-wake rhythm. Psychiatry Clin Neurosci. 2000;54:305–6. doi: 10.1046/j.1440-1819.2000.00689.x. [DOI] [PubMed] [Google Scholar]
- 15.Santiago J, Nollego M, Kinzler W, Santiago T. Sleep and sleep disorders in pregnancy. Ann Intern Med. 2001;134:396–408. doi: 10.7326/0003-4819-134-5-200103060-00012. [DOI] [PubMed] [Google Scholar]
- 16.Bonnar J, Franklin M, Nott P, Mcneilly A. Effect of breast-feeding after childbirth. Br Med J. 1975;4:82–4. doi: 10.1136/bmj.4.5988.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone, adn 17-hydroxyprogesterone in human pregnancy. Am J Obstet Gynecol. 1972;112:1095–100. doi: 10.1016/0002-9378(72)90185-8. [DOI] [PubMed] [Google Scholar]
- 18.Bloch M, Rubinow DR, Schmidt PJ, Lotsikas A, Chrousos GP, Cizza G. Cortisol response to ovine corticotropin-releasing hormone in a model of pregnancy and parturition in euthymic women with and without a history of postpartum depression. J Clin Endocrinol Metab. 2005;90:695–9. doi: 10.1210/jc.2004-1388. [DOI] [PubMed] [Google Scholar]
- 19.Doornbos B, Fokkema DS, Molhoek M, Tanke MAC, Postema F, Korf J. Abrupt rather than gradual hormonal changes induce postpartum blues-like behavior in rats. Life Sci. 2009;84:69–74. doi: 10.1016/j.lfs.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Manber R, Armitage R. Sex, Steroids, and Sleep : A Review. Sleep. 1999;22:540–55. [PubMed] [Google Scholar]
- 21.Ross LE, Murray BJ, Steiner M. Sleep and perinatal mood disorders : a critical review. J Psychiatry Neurosci. 2005;30:247–57. [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas KA, Burr RL. Melatonin level and pattern in postpartum versus nonpregnant nulliparous women. J Obstet Gynecol Neonatal Nurs. 2006;35:608–15. doi: 10.1111/j.1552-6909.2006.00082.x. [DOI] [PubMed] [Google Scholar]
- 23.Ansara D, Cohen MM, Gallop R, Kung R, Schei B. Predictors of women’s physical health problems after childbirth. J Psychosom Obstet Gynecol. 2005;26:115–25. doi: 10.1080/01443610400023064. [DOI] [PubMed] [Google Scholar]
- 24.Cheng C-Y, Li Q. Integrative review of research on general health status and prevalence of common physical health conditions of women after childbirth. Womens Health Issues. 2008;18:267–80. doi: 10.1016/j.whi.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Webb D, Bloch J, Coyne J, Chung E, Bennett I, Culhane JF. Postpartum Physical Symptoms in New Mothers: Their Relationship to Functional Limitations and Emotional Well-being. Birth. 2008;35:179–88. doi: 10.1111/j.1523-536X.2008.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marty M, Rozenberg S, Duplan B, Thomas P, Duquesnoy B, Allaert F. Quality of sleep in patients with chronic low back pain: a case-control study. Eur Spine J. 2008;17:839–44. doi: 10.1007/s00586-008-0660-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCracken L, Iverson G. Disrupted sleep patterns and daily functioning in patients with chronic pain. Pain Res Manag. 2002;7:75–9. doi: 10.1155/2002/579425. [DOI] [PubMed] [Google Scholar]
- 28.Rohrbeck J, Jordan K, Croft P. The frequency and characteristics of chronic widespread pain in general practice: a case–control study. Br J Gen Pract. 2007;57:109–15. [PMC free article] [PubMed] [Google Scholar]
- 29.Dørheim SK, Bondevik GT, Eberhard-Gran M, Bjorvatn B. Sleep and depression in postpartum women: a population-based study. Sleep. 2009;32:847–55. doi: 10.1093/sleep/32.7.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breese McCoy SJ. Postpartum depression: an essential overview for the practitioner. South Med J. 2011;104:128–32. doi: 10.1097/SMJ.0b013e318200c221. [DOI] [PubMed] [Google Scholar]
- 31.Posmontier B. Sleep quality in women with and without postpartum depression. J Obstet Gynecol Neonatal Nurs. 2008;37:722–35. doi: 10.1111/j.1552-6909.2008.00298.x. quiz 735–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brage Hudson D, Elek SM, Ofe Fleck M. First-time mothers’ and fathers’ transition to parenthood: Infant care, self-efficacy, parenting satisfaction, and infant sex. Issues Compr Pediatr Nurs. 2001;24:31–43. doi: 10.1080/014608601300035580. [DOI] [PubMed] [Google Scholar]
- 33.Kanotra S, D’Angelo D, Phares TM, Morrow B, Barfield WD, Lansky A. Challenges faced by new mothers in the early postpartum period: an analysis of comment data from the 2000 Pregnancy Risk Assessment Monitoring System (PRAMS) survey. Matern Child Health J. 2007;11:549–58. doi: 10.1007/s10995-007-0206-3. [DOI] [PubMed] [Google Scholar]
- 34.Groër M, Davis M, Casey K. Neuroendocrine and immune relationships in postpartum fatigue. MCN Am J Matern Nurs. 2005;30:133–8. doi: 10.1097/00005721-200503000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Song J-E, Chang S-B, Park S-M, Kim S, Nam C-M. Empirical test of an explanatory theory of postpartum fatigue in Korea. J Adv Nurs. 2010;66:2627–39. doi: 10.1111/j.1365-2648.2010.05380.x. [DOI] [PubMed] [Google Scholar]
- 36.Leahy-Warren P. Social support for first-time mothers: An Irish study. MCN Am J Matern Nurs. 2007;32:368–74. doi: 10.1097/01.NMC.0000298133.39785.a2. [DOI] [PubMed] [Google Scholar]
- 37.Hung C-H. Predictors of Postpartum Women’s Health Status. J Nurs Scholarsh. 2004;36:345–51. doi: 10.1111/j.1547-5069.2004.04062.x. [DOI] [PubMed] [Google Scholar]
- 38.Kavčič P, Rojc B, Dolenc-Grošelj L, Claustrat B, Fujs K, Poljak M. The impact of sleep deprivation and nighttime light exposure on clock gene expression in humans. Croat Med J. 2011;52:594–603. doi: 10.3325/cmj.2011.52.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526(Pt 3):695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goh V, Tong T, Lim C, Low E, Lee L. Effects of one night of sleep deprivation on hormone profiles and performance efficiency. Mil Med. 2001;166:427–31. [PubMed] [Google Scholar]
- 41.Bonnet MH. Effect of sleep disruption on sleep, performance, and mood. Sleep. 1985;8:11–9. doi: 10.1093/sleep/8.1.11. [DOI] [PubMed] [Google Scholar]
- 42.Roehrs T, Merlotti L, Petrucelli N, Stepanski E, Roth T. Experimental sleep fragmentation. Sleep. 1994;17:438–43. doi: 10.1093/sleep/17.5.438. [DOI] [PubMed] [Google Scholar]
- 43.McBean AL, Montgomery-Downs HE. What are postpartum women doing while the rest of the world is asleep? J Sleep Res. 2014 doi: 10.1111/jsr.12265. [DOI] [PubMed] [Google Scholar]
- 44.American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–41. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 45.De Carvalho M, Klaus MH, Merkatz RB. Frequency of Breast-feeding and Serum Bilirubin Concentration. Am J Dis Child. 1982;136:737–8. doi: 10.1001/archpedi.1982.03970440081024. [DOI] [PubMed] [Google Scholar]
- 46.Thoman EB, Barnett CR, Leiderman PH. Feeding Behaviors of Newborn Infants as a Function of Parity of the Mother. Child Dev. 1971;42:1471–83. [PubMed] [Google Scholar]
- 47.Ko C-H, Fang Y-W, Tsai L-L, Hsieh S. The effect of experimental sleep fragmentation on error monitoring. Biol Psychol. 2015;104:163–72. doi: 10.1016/j.biopsycho.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 48.Hernández C, Abreu J, Abreu P, Castro A, Jiménez A. Nocturnal melatonin plasma levels in patients with OSAS: the effect of CPAP. Eur Respir J. 2007;30:496–500. doi: 10.1183/09031936.00051906. [DOI] [PubMed] [Google Scholar]
- 49.Agnew HW, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2:263–6. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 50.Cajochen C, Münch M, Kobialka S, Kräuchi K, Steiner R, Oelhafen P, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–6. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 51.Deacon S, Arendt J. Posture influences melatonin concentrations in plasma and saliva in humans. Neurosci Lett. 1994;167:191–4. doi: 10.1016/0304-3940(94)91059-6. [DOI] [PubMed] [Google Scholar]
- 52.Acebo C, LeBourgeois M. Actigraphy. Respir Care Clin N Am. 2006;12:23–30. doi: 10.1016/j.rcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 53.Benson K, Friedman L, Noda A, Wicks D, Wakabayashi E, Yesavage J. The measurement of sleep by actigraphy: direct comparison of 2 commercially available actigraphs in a nonclinical population. Sleep. 2004;27:986–9. doi: 10.1093/sleep/27.5.986. [DOI] [PubMed] [Google Scholar]
- 54.Edinger JD, Means MK, Stechuchak KM, Olsen MK. A Pilot Study of Inexpensive Sleep-Assessment Devices A Pilot Study of Inexpensive Sleep-Assessment Devices. Behav Sleep Med. 2004;2:41–9. doi: 10.1207/s15402010bsm0201_4. [DOI] [PubMed] [Google Scholar]
- 55.Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–29. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 56.Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15:259–67. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 57.Montgomery-Downs HE, Clawges HM, Santy EE. Infant feeding methods and maternal sleep and daytime functioning. Pediatrics. 2010;126:e1562–8. doi: 10.1542/peds.2010-1269. [DOI] [PubMed] [Google Scholar]
- 58.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 59.Basner M, Dinges DF. An adaptive-duration version of the PVT accurately tracks changes in psychomotor vigilance induced by sleep restriction. Sleep. 2012;35:193–202. doi: 10.5665/sleep.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loh S, Lamond N, Dorrian J, Roach G, Dawson D. The validity of psychomotor vigilance tasks of less than 10-minute duration. Behav Res Methods Instrum Comput. 2004;36:339–46. doi: 10.3758/bf03195580. [DOI] [PubMed] [Google Scholar]
- 61.Medicine AA of S. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specification. 1st. Westchester, IL: 2007. [Google Scholar]
- 62.Littner MR, Kushida C, Wise M, Davila DG, Morgenthaler T, Lee-Chiong T, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–21. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 63.Afifi L, Kushida C, Carskadon M. Multiple sleep latency test. In: Kushida C, editor. Sleep deprivation Clin issues, Pharmacol sleep loss. 2005. pp. 11–24. [Google Scholar]
- 64.Medicine AA of S. The International Classification of Sleep Disorders. Westchester, IL: 2005. [Google Scholar]
- 65.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–12. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Axelsson J, Kecklund G, Åkerstedt T, Donofrio P, Lekander M, Ingre M. Sleepiness and Performance in Response to Repeated Sleep Restriction and Subsequent Recovery during Semi‐Laboratory Conditions. Chronobiol Int. 2008;25:297–308. doi: 10.1080/07420520802107031. [DOI] [PubMed] [Google Scholar]
- 67.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 68.Radloff L. Center for Epidemiological Studies Depression Scale. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 69.Association AP. Diagnostic and statistical manual of mental disorders. 4th. 2000. text rev. [DOI] [Google Scholar]
- 70.Medicine AA of S. The International Classification of Sleep Disorders. 2nd. Westchester, IL: 2005. [Google Scholar]
- 71.Wehrens SMT, Hampton SM, Kerkhofs M, Skene DJ. Mood, alertness, and performance in response to sleep deprivation and recovery sleep in experienced shiftworkers versus non-shiftworkers. Chronobiol Int. 2012;29:537–48. doi: 10.3109/07420528.2012.675258. [DOI] [PubMed] [Google Scholar]
- 72.Thomas KA, Foreman SW. Infant sleep and feeding pattern: Effects on maternal sleep. J Midwifery Women’s Heal. 2005;50:399–404. doi: 10.1016/j.jmwh.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 73.Filtness AJ, MacKenzie J, Armstrong K. Longitudinal change in sleep and daytime sleepiness in postpartum women. PLoS One. 2014;9:e103513. doi: 10.1371/journal.pone.0103513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bei B, Coo Calcagni S, Milgrom J, Trinder J. Day-to-day alteration of 24-hour sleep pattern immediately before and after giving birth. Sleep Biol Rhythms. 2012;10:212–21. [Google Scholar]
- 75.Gay CL, Lee KA, Lee S-Y. Sleep patterns and fatigue in new mothers and fathers. Biol Res Nurs. 2004;5:311–8. doi: 10.1177/1099800403262142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 77.Gonnissen HKJ, Hursel R, Rutters F, Martens EAP, Westerterp-Plantenga MS. Effects of sleep fragmentation on appetite and related hormone concentrations over 24 h in healthy men. Br J Nutr. 2013;109:748–56. doi: 10.1017/S0007114512001894. [DOI] [PubMed] [Google Scholar]
- 78.Sack R, Auckley D, Auger R, Carskadon M, Wright K, Vitiello M, et al. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. Sleep. 2007;30:1484–501. doi: 10.1093/sleep/30.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Benbadis SR. Questionnaires and rating scales. In: Kushida C, editor. Lung Biol Heal Dis. New York: Marcel Dekker; 2005. pp. 1–9. [Google Scholar]
- 80.Martin S, Wraith P. The effect of nonvisible sleep fragmentation on daytime function. Am J …. 1997;155:1596–601. doi: 10.1164/ajrccm.155.5.9154863. [DOI] [PubMed] [Google Scholar]
- 81.Walsh J, Snyder E, Hall J, Randazzo A, Griffin K, Groeger J, et al. Slow wave sleep enhancement with gaboxadol reduces daytime sleepiness during sleep restriction. Sleep. 2008;31:659–72. doi: 10.1093/sleep/31.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walsh J, Hall-Porter J, Griffin K, Dodson ER, Forst EH, Curry DT, et al. Enhancing slow wave sleep with sodium oxybate reduces the behavioral and physiological impact of sleep loss. Sleep. 2010;33:1217–25. doi: 10.1093/sleep/33.9.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martin SE, Brander PE, Deary IJ, Douglas NJ. The effect of clustered versus regular sleep fragmentation on daytime function. J Sleep Res. 1999;8:305–11. doi: 10.1046/j.1365-2869.1999.00169.x. [DOI] [PubMed] [Google Scholar]
- 84.Stepanski E, Lamphere J, Roehrs T, Zorick F, Roth T. Experimental sleep fragmentation in normal subjects. Int J Neurosci. 1987;33:207–14. doi: 10.3109/00207458708987405. [DOI] [PubMed] [Google Scholar]
- 85.Krystal AD, Edinger JD. Measuring sleep quality. Sleep Med. 2008;9(Suppl 1):S10–7. doi: 10.1016/S1389-9457(08)70011-X. [DOI] [PubMed] [Google Scholar]
- 86.Harvey AG, Stinson K, Whitaker KL, Moskovitz D, Virk H. The subjective meaning of sleep quality: a comparison of individuals with and without insomnia. Sleep. 2008;31:383–93. doi: 10.1093/sleep/31.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nykamp K, Rosenthal L, Helmus T, Gerhardstein R, Day R, Roehrs T, et al. Repeated nocturnal sleep latencies in narcoleptic, sleepy and alert subjects. Clin Neurophysiol. 1999;110:1531–4. doi: 10.1016/s1388-2457(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 88.Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8:613–22. doi: 10.1016/j.sleep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 89.Armstrong K, MacKenzie J, Smith S. Postpartum sleepiness and sleepy driving in Australian mothers. Int J Heal Promot Educ. 2015;53:76–86. [Google Scholar]
- 90.Volkovich E, Ben Zion H, Karny D, Meiri G, Tikotzky L. Sleep patterns of co-sleeping and solitary sleeping infants and mothers: a longitudinal study. Sleep Med. 2015;16:1305–12. doi: 10.1016/j.sleep.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 91.Beebe KR, Lee KA. Sleep Disturbance in Late Pregnancy and Early Labor. J Perinat Neonatal Nurs. 2007;23:103–8. doi: 10.1097/01.JPN.0000270626.66369.26. [DOI] [PubMed] [Google Scholar]


