Abstract
Anthrax is caused by Bacillus anthracis, a zoonotic bacterial pathogen affecting humans and livestock worldwide. The current human anthrax vaccine, anthrax vaccine adsorbed (AVA), is an injected vaccine with a cumbersome administration schedule and fails to promote mucosal immunity. Bacterial enterotoxins, which stimulate production of the cyclic nucleotide cAMP are effective experimental mucosal vaccine adjuvants, but their inherent toxicity has precluded their use in humans. We investigated whether cyclic dinucleotides that target Stimulator of Interferon Gamma Genes (STING) in mammalian cells could represent an alternative to bacterial enterotoxins as adjuvant for sublingual immunization and promotion of mucosal immunity and secretory IgA responses in addition to systemic immunity. We found that sublingual immunization of mice with Bacillus anthracis protective antigen (PA) and the STING ligand 3′3′-cGAMP promotes PA-specific serum IgG Ab responses of the same magnitude as those induced after immunization with PA and the experimental adjuvants cholera toxin (CT). Interestingly, this STING ligand also promoted serum anti-PA IgA and IgA-producing cells in the bone marrow. Furthermore, the saliva of mice immunized with the STING ligand exhibited similar levels of PA-specific IgA Abs as groups immunized with CT as adjuvant. The adjuvant activity of 3′3′-cGAMP was associated with mixed Th1, Th2, and Th17 responses. This STING ligand also induced rapid IFN-β and IL-10 responses in sublingual tissues and cervical lymph nodes, and TGF-β responses in the cervical lymph nodes, which could contribute to promoting IgA responses after sublingual immunization.
Introduction
Mucosal delivery of vaccines is a well-accepted strategy for eliciting systemic immune responses mediated by IgG, but also mucosal IgA [1–4] for protection at the points of entry for most pathogens [4–8]. Subunit mucosal vaccines require addition of adjuvants capable of breaking tolerance and promoting innate responses necessary for mucosal homing receptors expression and IgA class switching [4–6, 8]. Bacterial toxins including cholera toxin (CT), the closely related heat labile toxin of E. coli (LT-I), or Bacillus anthracis edema toxin, induce cyclic nucleotide cyclic AMP (cAMP), which is crucial for mucosal adjuvant activity [9–12]. Unfortunately, ganglioside targeting by CT or LT-I and the high magnitudes of cAMP they induce in mammalian cells can lead to unacceptable complications such as diarrhea or CNS inflammation after oral or nasal administration in humans [13, 14].
The cytoplasmic DNA detection protein Stimulator of Interferon Gamma genes (STING) is a trans-membrane protein intercalated into the endoplasmic reticulum of cells including macrophages, dendritic cells, and fibroblasts to detect senses cytosolic cyclic di-nucleotides (CDNs [15, 16]. STING senses DNA and directly responds to CDNs produced by pathogens or indirectly through scyclic GMP-AMP synthase (cGAS), which can bind non-cyclic DNA produced by pathogens or released from damaged host cells and produce the non-canonical CDN 2′3′-cGAMP [15–21]. This pathway has been shown to be essential for activation of IRF3 and induction of IFNβ, which improve antibody responses during infections [16, 21–25]. Structural similarities between cAMP and cytosolic CDNs suggest that STING ligands may exhibit mucosal adjuvant activity and promote antibody and T cell responses, which share characteristics with those induced by bacterial enterotoxins. Furthermore, because STING ligands lack the ganglioside-targeting characteristic of bacterial enterotoxins, they may be safer for mucosal delivery.
The sublingual route is used for delivery of medication and immune therapy in humans and animals [26, 27]. Studies in mice showed that cAMP-inducing bacterial toxins differ in their ability to induce mucosal and systemic responses after sublingual immunization (SI) [28], with CT promoting both systemic immunity and mucosal SIgA, and edema toxin failing to induce serum or mucosal IgA [28]. We used Bacillus anthracis protective antigen (PA) as a model antigen to address the regulatory effect of the STING ligand 3′3′-cGAMP on immune responses to a sublingually co-administered vaccine antigen. Our results show that 3′3′-cGAMP is an effective adjuvant for sublingual vaccination capable of promoting broad immunity including serum anti-PA neutralizing and anti-PA SIgA responses in airway secretions.
Materials and methods
Animals
Female C57BL/6J mice (The Jackson Labs, Bar Harbor, ME) were use at 9–12 weeks of age. Mice were specific pathogen-free and all procedures were approved by The Ohio State University’s Institutional Animal Care and Use Committee.
Sublingual immunization
SI was performed as previously described [28]. Mice received 10–13 μL of PBS containing 10μg protective antigen of Bacillus anthracis (PA, BEI Resources, Manassas, VA) alone, 10μg PA and 2μg cholera toxin (CT, List Biological Laboratories, Campbell, CA), 10μg PA and 10μg CpG ODN 1826 (CpG, Integrated DNA Technologies, Coralville, IA), or 10μg PA and 10μg 3′3′-cGAMP (InvivoGen, San Diego, CA). Groups of six animals were immunized at weekly intervals for 3 consecutive weeks (days 0, 7, and 14). Blood samples and vaginal wash samples were collected weekly, and saliva was collected on day 28.
Histologic analysis of sublingual tissue
Sublingual tissues and tongues were collected either 2 hours (n = 3 per group) or 42 hours (n = 2 per group) after SI. After decalcification and formalin fixation, thin sagittal sections were stained with hematoxylin and eosin (OSU, Comparative Pathology and Mouse Phenotyping Shared Resource) and images were scanned and analyzed using an Aperio Imagescope (Leica Biosystems Inc, Buffalo Grove, IL).
Flow cytomety analysis
Cell suspensions were stained with the following antibodies: B220, CD11b (Miltenyi Biotec, Auburn, CA), Gr-1, F4/80 (Abd Serotec, Raleigh, NC), Ly6G, Ckit (Biolegend, San Diego, CA), CD19, CD3e (BD Biosciences, San Jose, CA), IgG, IgA (Southern Biotech, Birmingham, AL), GL7, α4β7 (LPAM) (BD Biosciences, San Jose, CA), CCR9 (eBiosciences, San Diego, CA), and B220 (Miltenyi Biotec, Auburn, CA). Stained cells were analyzed with an Accuri C6 flow cytometer (BD Biosciences, San Jose, CA)
ELISA
PA-specific Ab responses in serum and mucosal secretions were assessed by ELISA as previously reported [10, 28, 29]. Titers were expressed as Log2 of sample dilutions that yielded an absorbance (OD405) > 0.1 above non-immunized control samples.
ELISPOT assays
Spleen and CLN samples were collected 2 weeks after the last immunization (day 35) and the frequency of antibody-secreting cells analyzed by ELISPOT as previously described [28, 30].
Analysis of cytokine responses by antigen-specific T helper cells
To analyze T helper cell cytokine responses, CLN cells and splenocytes were collected 35 days after immunization, and stimulated with PA in vitro. Cytokines secreted in culture supernatant were analyzed as previously described [10, 28, 29].
Toxin neutralization assay
Toxin neutralization assay was performed as previously described [28]. Toxin neutralizing antibody titers were calculated as the lowest concentration (highest dilution) of serum that protected macrophages from cytotoxicity caused by LeTx, and results were expressed as Log2 titers.
Analysis of innate cytokine responses in organ cultures of sublingual tissues and cervical lymph nodes
Sublingual tissue cells were isolated from naïve mice and cultured overnight at 37°C in a 5% CO2 atmosphere in cRPMI alone or stimulated with 5μg/mL 3′3′-cGAMP or 5μg/mL poly I:C (InvivoGen, San Diego, CA). Innate immune responses were analyzed by real-time quantitative RT-PCR as previously described [31].
Statistical nalysis
Results were expressed as mean ± one standard deviation. Statistical significance was determined by ANOVA followed by Dunnett’s post-test for significance versus the PA group. For multiplex analysis, Tukeys post-test was performed to assess significance. Results were considered significant at p < 0.05. Statistical tests were performed using GraphPad Prism 6 (GraphPad Software Inc, La Jolla, CA).
Results
Sublingual immunization with the STING ligand 3′3′-cGAMP promotes serum anti-PA antibody responses
Analysis of serum Ab responses in mice immunized with PA only, PA plus CT, PA plus CpG, or PA plus 3′3′-cGAMP, showed that all the adjuvants enhanced serum IgG Ab responses (Figure 1A) The profile of PA-specific serum IgG subclasses in mice immunized with 3′3′-cGAMP was similar to that induced by CT, and consisted of IgG1, IgG2a, and IgG2b (Figure 1B). However, 3′3′-cGAMP promoted a greater IgG2a/c:IgG1 ratio (close to 1), which indicated stronger induction of the Th1component than observed after SI with CT or CpG (Figure 1C). This was consistent with a lack of IgE responses in the 3′3′-cGAMP group (Figure 1D). The STING ligand also induced neutralizing antibody titers, which were significantly higher than those induced by SI with PA alone and slightly higher in magnitude than those induced by CT (Figure 1E). It is worth noting that CpG showed no significantly adjuvant effect on neutralizing anti-PA antibody titers (Figure 1E).
FIGURE 1.
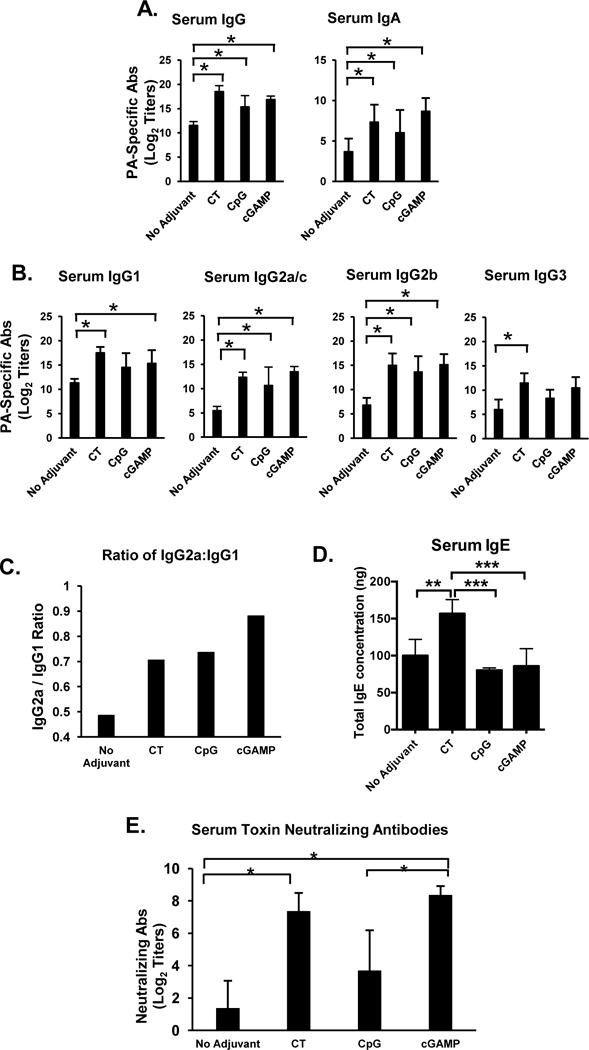
Sublingual immunization with the STING ligand 3′3′-cGAMP as adjuvant enhances systemic antibody responses. Mice were immunized by sublingual application of PA alone, PA plus cholera toxin (CT), PA plus CpG ODN 1826 (CpG), or PA plus the STING ligand 3′3′-cGAMP (3′3′-cGAMP), three times at weekly intervals. Serum samples were collected 1 week (Day 21) after the last immunization and PA-specific Ab responses were analyzed by ELISA. (A) PA-specific serum IgG and IgA Ab responses; (B) PA-specific serum IgG subclass (IgG1, IgG2a, IgG2b and IgG3) responses; (C) Ratio of IgG2a/IgG1 Ab responses. Data are expressed as mean Ab titers ± SD (n=6 per group). (D) IgE Ab responses. Data are expressed as mean concentration of total IgE ± SD (n=6 per group). (E) PA-specific neutralizing Ab titers were determined by the in vitro toxicity assay. Neutralizing Ab titers are expressed as mean ± SD (n=3 per group). *p ≤ 0.05 compared to the PA only (no adjuvant) group (Dunnett’s post-test).
Sublingual immunization with the STING ligand 3′3′-cGAMP promotes broad anti-PA IgA antibody responses
We next examined the ability of 3′3′-cGAMP to induce antigen-specific IgA responses. The STING ligand 3′3′-cGAMP successfully induced serum IgA (Figure 2A). It also promoted SIgA production in the saliva at levels comparable to those induced by CT (Figure 2B). Although all adjuvants increased PA-specific IgA responses in vaginal washes (Figure 2B), the titers were not significantly different from the PA-only group (no adjuvant). Only CT induced a trend towards increased SIgA in the feces (p = 0.0597), which suggested that neither CpG nor 3′3′-cGAMP induced homing of effector B cells to the gastrointestinal tract (Figure 2B). Bone marrow collected from mice immunized with PA plus CT contained higher frequencies of PA-specific IgG ASCs when compared with those from mice immunized sublingually with CpG or 3′3′-cGAMP as adjuvant (Figure 2C). Interestingly, only 3′3′-cGAMP significant increases PA-specific IgA ASCs in the bone marrow, which suggested that this adjuvant had a greater potential to induce memory IgA responses following SI.
FIGURE 2.

Sublingual immunization with the STING ligand 3′3′-cGAMP as adjuvant induces PA-specific IgA Abs in the saliva and PA-specific IgA ASCs in bone marrow. Mice were immunized by sublingual application of PA alone, PA plus cholera toxin (CT), PA plus CpG ODN 1826 (CpG), or PA plus 3′3′-cGAMP (3′3′-cGAMP), three times at weekly intervals. (A) Vaginal washes and fecal samples were collected 1 week (Day 21), and saliva samples, two weeks (Day 28) after the last immunization. PA-specific Ab responses were analyzed by ELISA. Data are expressed as mean Ab titers ± SD (n=6 per group). (B) Bone marrow cells were collected 2 weeks (Day 28) after the last immunization and the frequencies of PA-specific antibody secreting cells (ASCs) were determined by ELISPOT assay. Results are expressed as mean ASCs ± SD (n=6–7 per group). * p ≤ 0.05 compared with the PA only (no adjuvant) group (Dunnett’s post-test).
3′3′-cGAMP adjuvant activity involves mixed Th1/Th2/Th17 responses
Analysis of cytokines secreted by CLNs and spleen cells after in vitro re-stimulation with PA confirmed that CT induces Th2 and Th17 cytokine responses and a weak Th1 response (Figure 3A–3C). We also found high levels IFN-γ responses in the group immunized with 3′3′-cGAMP (Figure 3A.). This adjuvant failed to induce the Th2 cytokines IL-4 and IL-13 (Figure 3B–3C), but supported IL-6 responses. The STING ligand 3′3′-cGAMP also induced Th-17 associated cytokine IL-17A (Figure 3D). Culture supernatants of cells from the group immunized with 3′3′-cGAMP also contained higher levels of IL-1β, CCL5, GM-CSF (Figure 3E), which are cytokines and chemokines known to support mucosal immunity and the production of SIgA (34, 35). Thus, 3′3′-cGAMP induces a balanced antigen-specific Th1 and Th17 response.
FIGURE 3.
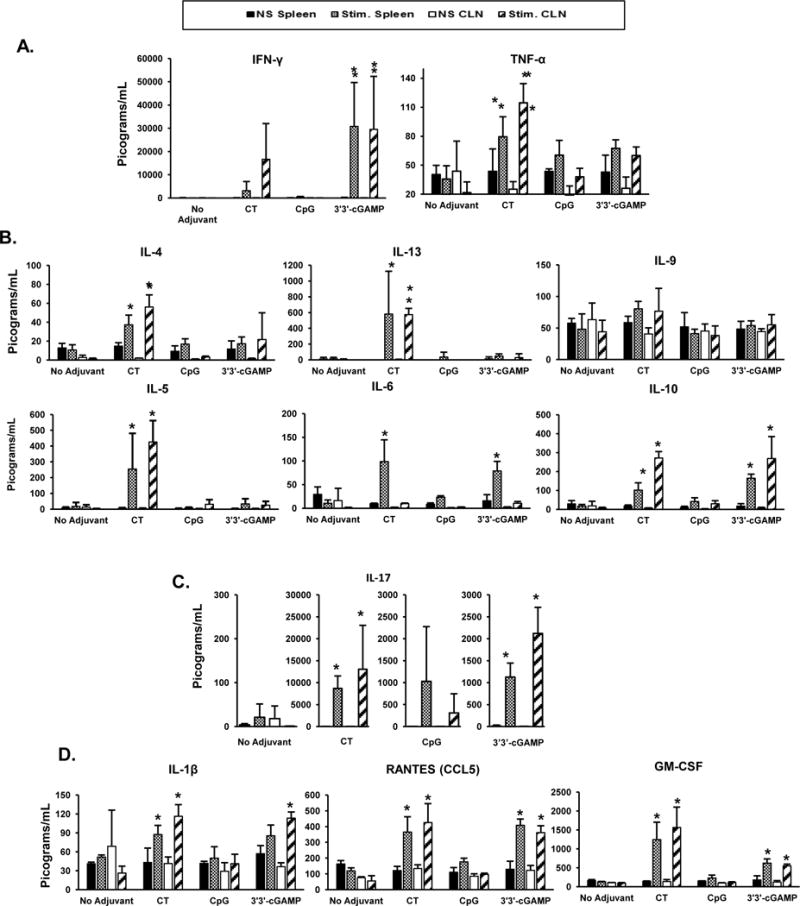
T helper cytokine responses induced by sublingual immunization with 3′3′-cGAMP as adjuvant. Cervical lymph nodes (CLNs) and spleen were collected 3 weeks after the last immunization and single cell suspensions were cultured for 5 days in the presence of recombinant PA (10 μg/ml). Th1 (A); Th2 (B); and IL-17A (Th17) (C) cytokines secreted in culture supernatant were measured using a multiplex mouse cytokine assay (Bio-Plex Pro Mouse Cytokine kit, BioRad, Hercules, CA). Other relevant cytokines and chemokines (D) were also measured from culture supernatants of non-stimulated spleens (NS spleen: black bars), PA-stimulated spleens (Stim. Spleen: grey bars), non-stimulated CLN (NS CLN: white bars), or PA-stimulated CLN (Stim. CLN: stripped bars). Data are expressed as mean ± SD (n=6 per group). * p ≤ 0.05 compared to non-stimulated cells (Tukeys test).
SI with 3′3′-cGAMP does not induce acute local inflammatory responses
It has been shown that the frequency of APCs including neutrophils and dendritic cells transiently increases in the sublingual tissues within 2–3 hours of SI [28, 32]. Analysis of immune cell populations in the sublingual tissue and tongue 2 or 42 hours following a single SI with 3′3′-cGAMP or CT revealed no morphological change or influx of immune cells (Figure 4A–4D). Conversely, CpG promoted an obvious thickening of the sublingual epithelium (Figure 4A, 4B), which was no longer seen at the 42-hour time point (Figure 4C). Flow cytometry analysis of sublingual tissues and tongues 2 hours after a single SI with 3′3′-cGAMP showed a small but significant increase in the relative percentage of mast cells in sublingual tissues (Figures 4D). The sublingual mucosa contains a large population of dendritic cells [32]. In our experiment, the largest population of leukocytes in the sublingual tissue and tongue were mast cells (Figure 4D–4F). Unlike CT, which reduced the number of neutrophils, 3′3′-cGAMP did not affect other myeoloid cell subsets (Figure 4E), but increased the number of T cells in both the tongue and sublingual tissue Figure 4F)
FIGURE 4.
Histological structures and immune cell subsets in tongue and sublingual tissues after sublingual immunization with 3′3′-cGAMP as adjuvant. Tongue and sublingual tissues were collected 2 hours (A, B) or 42 hours (C) after a single sublingual application of PA alone, PA plus cholera toxin (CT), PA plus CpG ODN 1826 (CpG), or PA plus the STING ligand 3′3′-cGAMP (3′3′-cGAMP). Thin sections (10 μM) of paraffin-embedded tissues were stained with hematoxylin & eosin. The sublingual mucosa was divided into thirds by length and the ratio of epithelium to the lamina propria was calculated every 100 μm and averaged for each third of the sublingual tissue. Potential changes in the structure of sublingual tissues were quantified by measuring the ratio of epithelial to lamina propria width in the caudal (Ca), middle (M) and rostral (Ro) portion. (D–F) Flow cytometry analysis of immune cell subsets 2 hours after sublingual application of the vaccine formulations. (D) c-Kit+ and CD11b+ cells; (E) myeloid cell subsets; (F) B and T cells. Results are expressed a mean ± SD (n=3–6 per group). *p ≤ 0.05 compared to the PA only (no adjuvant) group (Dunnet’s test).
Flow cytometry analysis of CLNs collected 4 hours following a single SI (Figure 5) showed that unlike CT, 3′3′-cGAMP did not increase the total number of B cells or the overall populations of IgG+ or IgA+ B cells in the CLNs (Figure 5A). Furthermore, unlike CT, 3′3′-cGAMP and CpG reduced the number of α4β7+ B cells (Figure 5B) and increased the number of GL7− IgG+ B cells (Figure 5C).
FIGURE 5.

B cell subsets in cervical lymph nodes after sublingual immunization with 3′3′-cGAMP as adjuvant. Cervical lymph nodes were collected 4 hours after a single sublingual application of PA alone, PA plus cholera toxin (CT), PA plus CpG ODN 1826 (CpG), or PA plus the STING ligand 3′3′-cGAMP (3′3′-cGAMP) and single cell suspensions were analyzed by flow cytometry. (A) B220+, IgG+, and IgA+ B cells; (B) Expression of gut homing receptors by B cells; (C) Expression of the germinal center marker GL7. Data are expressed as mean ± SD (n=3 per group). *p ≤ 0.05 compared to the PA only (no adjuvant) group (Dunnett’s post-test).
Innate signals induced by sublingual 3′3′-cGAMP for induction of anti-PA antibodies
Finally, to gain insight into potential mechanisms underlying the induction of IgA responses by 3′3′-cGAMP, we analyzed cytokine mRNA responses in CLNs and sublingual tissues cultured in vitro without or in the presence of 3′3′-cGAMP, or Poly I:C, an adjuvant known to induce IFN-β production [25]. Overnight culture with 3′3-cGAMP increased IFN-β and IL-10 mRNA responses in sublingual tissues (Figure 6A). The same 3′3-cGAMP treatment increased expression of IFN-β, IL-10 and TGF-β, but not CD40 in CLNs (Figure 6B). Token together, these data suggest that when used as adjuvant for a sublingual vaccine, the STING ligand 3′3′-cGAMP promotes IgA Ab responses via induction of cytokines that support immunoglobulin class switching to IgA.
FIGURE 6.

The STING ligand 3′3′-cGAMP induces IFN-β and IgA-promoting cytokines in sublingual tissues and cervical lymph nodes. Tissues were collected from naïve mice and cultured overnight without addition of effector (no stimulation) or in the presence of 3′3′-cGAMP. Control cells were cultured in the presence of Polyinosinic:polycytidylic acid (Poly I:C). Cytokine (IFN-β, IL-10, TGF-β) and CD40 mRNA responses were measured by real-time quantitative PCR. (A) sublingual tissues; (B) cervical lymph nodes. Data are expressed as mean ± SD (n=3 per group). *p ≤ 0.05 compared to control non-stimulated cells (Dunnett’s post-test).
Discussion
Studies in experimental animal models have shown that needle-free vaccines delivered via mucosa represent the best strategy for promoting IgA responses in mucosal tissues. However, the development of mucosal vaccines has been hampered by the need to identify effective and safe vaccine adjuvants. Our results show the efficacy of the cyclic di-nucleotide 3′3′-cGAMP as an adjuvant for sublingual vaccination, which is capable of promoting broad immunity against anthrax toxins including serum anti-PA neutralizing and anti-PA SIgA responses in secretions of the airways. In addition, we show that this STING ligand promotes IgA responses via mechanisms distinct from the cAMP-promoting adjuvant CT.
Cyclic dinucleotides that bind STING (STING ligands) were recently shown to be potential alternatives to cAMP-inducing bacterial toxins and derivatives as vaccine adjuvant. For example, STING ligands of bacterial origin, including 3′3′-cGAMP, c-di-AMP, and c-di-GMP, effectively elicited mucosal and systemic immune responses following intranasal administration [33–35]. We now show that targeting STING with 3′3′-cGAMP is an effective strategy for enhancing the magnitude of immune responses induced by sublingual vaccines. The STING pathway is highly conserved in eukaryotes [36], and thus, our data suggest that STING ligands may represent a new class of molecular adjuvant that can be incorporated in future sublingual vaccines. STING ligands represent a heterogeneous group of molecules that differ in their binding affinities to mammalian STINGs [36]. For example, c-di-GMP has lower binding affinity than cGAMP and exposure of mammalian cells to 3′3′-cGAMP more closely mimics the IFN-β responses seen after exposure to endogenous 2′3′-cGAMP [37]. Future evaluation of STING ligands as adjuvant for sublingual vaccines should consider the polymorphisms of STING expression in the human population [38–40]. In this regard, STING ligands of bacterial origin such as 3′3′-cGAMP used in this study may also be detected by additional pathways such as the NLRP3 inflammasome. Thus, these ligands could act as successful adjuvants even in people with limited reactivity to STING ligands due to STING polymorphism [41].
Earlier studies with enterotoxin adjuvants that induce cAMP showned that the adjuvant activity of these molecules for nasal or oral vaccines was associated with Th2 or mixed Th2/Th1 responses. More recently, these adjuvants and other STING ligands were fund to also induce IL-17A responses [10, 28, 42, 43]. We report that although 3′3′-cGAMP and the cAMP-inducing bacterial toxin CT promoted high levels of antigen-specific IgA responses in the saliva, 3′3′-cGAMP as sublingual adjuvant promoted T helper responses that were clearly different than the Th17 and strong Th2 responses induced by CT. We also report that unlike CT, 3′3′-cGAMP resulted in higher frequency of antigen-specific IgA ASCs in the bone marrow. In this regard, we found that shortly after SI with 3′3′-cGAMP, the number of CD3+ T cells was increased in the tongue and sublingual tissues, and the frequency of GL7− B cells in CLNs was increased. On the other hand, SI with CT resulted in a higher frequency of GL7+ IgG+ B cells and GL7+ IgA+ B cells in the CLNs.
The STING pathway has been shown to exert its effects via activation of IRF3 and induction of IFN-β (14–16, 18, 44, 23). In the present study, targeting STING in tissue culture led to higher IFN-β responses than the canonical IFN-β-inducer poly I:C [25, 44]. Because 3′3′-cGAMP also induced the IgA-promoting cytokines IL-10 and TGF-β in the CLNs, future studies will determine whether early recruitment of GL7− B cells in this environment contributed to the high levels of antigen-specific IgA ASCs in the bone marrow.
The sublingual mucosa and the CLNs are believed to be inductive sites for adaptive immune responses after SI. Thus, cell activation and/or recruitment in these sites could provide some clues about innate mechanisms that promote and/or shape immune responses after immunization, as well as information on potential adverse effects. The sublingual mucosa is made up of a pseudostratified epithelium overlying a thin lamina propria in which capillaries, mononuclear cells, and fibroblasts are present [32, 45]. No organized lymphoid tissue is present within the sublingual lamina propria in mice, but dendritic cells, macrophages, and langerins+ cells are the primary APCs and antigen presentation occurs in the CLNs [6, 32, 45]. We now report that c-kit+ cells (presumably mast cells) represent a large proportion of cells in both the tongue and sublingual tissues. To our knowledge, this is the first report to show that c-kit+ cells outnumber CD11b+ cells in these tissues. Since mast cell activation was shown to promote mucosal immunity and mucosal IgA responses [46, 47], future studies will address whether c-kit+ cells respond to direct stimulation by 3′3′-cGAMP, whether this could contribute to its ability to enhance IgA responses after SI.
Sublingual drug delivery and sublingual allergen-specific immunotherapy are currently in use in a variety of human and animal populations [26, 27]. Sublingual drug delivery is simple, involving application of liquids or rapidly dissolving tablets to the floor of the oral cavity [26]. It is also potentially safer than nasal vaccination: our result show that sublingual delivery of the STING ligand 3′3′-cGAMP does not result in an inflammatory responses at the site of delivery. Furthermore, SI with this STING ligand did not result in IgE stimulation, which supports the safety of this adjuvant. STING ligands have been used in the successful development of anti-tumor vaccines in mouse models, where they have been used to overcome the immune suppression promoted by tumors such as breast cancer [48–50]. Herein we show that SI with 3′3′-cGAMP induces mucosal IgA and high levels of serum neutralizing Abs against anthrax toxin, which indicate that targeting the STING pathway with 3′3′-cGAMP is a promising strategy for development of sublingual vaccines against infectious agents.
Acknowledgments
This work was supported by National Institute of Health grants AI18958 and DK101323
Abbreviations
- 3′-3′
cGAMP (3′-3′ cyclic GMP-AMP)
- cGAS
(cyclic GMP-AMP synthase)
- CLN
(cervical lymph node)
- CT
(cholera toxin)
- SIgA (secretory IgA)
STING (Stimulator of Interferon Gamma Genes)
- PA
(protective antigen of Bacillus anthracis).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boyaka PN, Tafaro A, Fischer R, Leppla SH, Fujihashi K, McGhee JR. Effective mucosal immunity to anthrax: neutralizing antibodies and Th cell responses following nasal immunization with protective antigen. Journal of immunology. 2003;170:5636–43. doi: 10.4049/jimmunol.170.11.5636. [DOI] [PubMed] [Google Scholar]
- 2.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11:S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 3.Kweon MN. Sublingual mucosa: A new vaccination route for systemic and mucosal immunity. Cytokine. 2011;54:1–5. doi: 10.1016/j.cyto.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12:592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 5.Cuburu N, Kweon MN, Hervouet C, Cha HR, Pang YY, Holmgren J, et al. Sublingual immunization with nonreplicating antigens induces antibody-forming cells and cytotoxic T cells in the female genital tract mucosa and protects against genital papillomavirus infection. Journal of immunology. 2009;183:7851–9. doi: 10.4049/jimmunol.0803740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGhee JR, Fujihashi K. Inside the mucosal immune system. PLoS Biol. 2012;10:e1001397. doi: 10.1371/journal.pbio.1001397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Negri DR, Riccomi A, Pinto D, Vendetti S, Rossi A, Cicconi R, et al. Persistence of mucosal and systemic immune responses following sublingual immunization. Vaccine. 2010;28:4175–80. doi: 10.1016/j.vaccine.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Raghavan S, Ostberg AK, Flach CF, Ekman A, Blomquist M, Czerkinsky C, et al. Sublingual immunization protects against Helicobacter pylori infection and induces T and B cell responses in the stomach. Infect Immun. 2010;78:4251–60. doi: 10.1128/IAI.00536-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng E, Cardenas-Freytag L, Clements JD. The role of cAMP in mucosal adjuvanticity of Escherichia coli heat-labile enterotoxin (LT) Vaccine. 1999;18:38–49. doi: 10.1016/s0264-410x(99)00168-1. [DOI] [PubMed] [Google Scholar]
- 10.Duverger A, Carre JM, Jee J, Leppla SH, Cormet-Boyaka E, Tang WJ, et al. Contributions of edema factor and protective antigen to the induction of protective immunity by Bacillus anthracis edema toxin as an intranasal adjuvant. Journal of immunology. 2010;185:5943–52. doi: 10.4049/jimmunol.0902795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eriksson AM, Schon KM, Lycke NY. The cholera toxin-derived CTA1-DD vaccine adjuvant administered intranasally does not cause inflammation or accumulate in the nervous tissues. Journal of immunology. 2004;173:3310–9. doi: 10.4049/jimmunol.173.5.3310. [DOI] [PubMed] [Google Scholar]
- 12.Gagliardi MC, De Magistris MT. Maturation of human dendritic cells induced by the adjuvant cholera toxin: role of cAMP on chemokine receptor expression. Vaccine. 2003;21:856–61. doi: 10.1016/s0264-410x(02)00532-7. [DOI] [PubMed] [Google Scholar]
- 13.Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N Engl J Med. 2004;350:896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 14.van Ginkel FW, Jackson RJ, Yuki Y, McGhee JR. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. Journal of immunology. 2000;165:4778–82. doi: 10.4049/jimmunol.165.9.4778. [DOI] [PubMed] [Google Scholar]
- 15.Dubensky TW, Jr, Kanne DB, Leong ML. Rationale, progress and development of vaccines utilizing STING-activating cyclic dinucleotide adjuvants. Ther Adv Vaccines. 2013;1:131–43. doi: 10.1177/2051013613501988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Yeruva L, Marinov A, Prantner D, Wyrick PB, Lupashin V, et al. The DNA sensor, cyclic GMP-AMP synthase, is essential for induction of IFN-beta during Chlamydia trachomatis infection. Journal of immunology. 2014;193:2394–404. doi: 10.4049/jimmunol.1302718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhat N, Fitzgerald KA. Recognition of cytosolic DNA by cGAS and other STING-dependent sensors. Eur J Immunol. 2014;44:634–40. doi: 10.1002/eji.201344127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–8. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carroll EC, Jin L, Mori A, Munoz-Wolf N, Oleszycka E, Moran HB, et al. The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons. Immunity. 2016;44:597–608. doi: 10.1016/j.immuni.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Shu C, Yi G, Chaton CT, Shelton CL, Diao J, et al. Cyclic GMP-AMP synthase is activated by double-stranded DNA-induced oligomerization. Immunity. 2013;39:1019–31. doi: 10.1016/j.immuni.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science. 2013;341:1390–4. doi: 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blaauboer SM, Gabrielle VD, Jin L. MPYS/STING-mediated TNF-alpha, not type I IFN, is essential for the mucosal adjuvant activity of (3′–5′)-cyclic-di-guanosine-monophosphate in vivo. Journal of immunology. 2014;192:492–502. doi: 10.4049/jimmunol.1301812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao D, Wu J, Wu YT, Du F, Aroh C, Yan N, et al. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341:903–6. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrasek J, Iracheta-Vellve A, Csak T, Satishchandran A, Kodys K, Kurt-Jones EA, et al. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc Natl Acad Sci U S A. 2013;110:16544–9. doi: 10.1073/pnas.1308331110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swanson CL, Wilson TJ, Strauch P, Colonna M, Pelanda R, Torres RM. Type I IFN enhances follicular B cell contribution to the T cell-independent antibody response. J Exp Med. 2010;207:1485–500. doi: 10.1084/jem.20092695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeBoer DJ, Verbrugge M, Morris M. Clinical and immunological responses of dust mite sensitive, atopic dogs to treatment with sublingual immunotherapy (SLIT) Vet Dermatol. 2016;27:82–7e23. doi: 10.1111/vde.12284. [DOI] [PubMed] [Google Scholar]
- 27.Nolte H, Bernstein DI, Nelson HS, Kleine-Tebbe J, Sussman GL, Seitzberg D, et al. Efficacy of house dust mite sublingual immunotherapy tablet in North American adolescents and adults in a randomized, placebo-controlled trial. The Journal of allergy and clinical immunology. 2016;138:1631–8. doi: 10.1016/j.jaci.2016.06.044. [DOI] [PubMed] [Google Scholar]
- 28.Jee J, Bonnegarde-Bernard A, Duverger A, Iwakura Y, Cormet-Boyaka E, Martin TL, et al. Neutrophils negatively regulate induction of mucosal IgA responses after sublingual immunization. Mucosal immunology. 2015;8:735–45. doi: 10.1038/mi.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duverger A, Jackson RJ, van Ginkel FW, Fischer R, Tafaro A, Leppla SH, et al. Bacillus anthracis edema toxin acts as an adjuvant for mucosal immune responses to nasally administered vaccine antigens. Journal of immunology. 2006;176:1776–83. doi: 10.4049/jimmunol.176.3.1776. [DOI] [PubMed] [Google Scholar]
- 30.Boyaka PN, Ohmura M, Fujihashi K, Koga T, Yamamoto M, Kweon MN, et al. Chimeras of labile toxin one and cholera toxin retain mucosal adjuvanticity and direct Th cell subsets via their B subunit. Journal of immunology. 2003;170:454–62. doi: 10.4049/jimmunol.170.1.454. [DOI] [PubMed] [Google Scholar]
- 31.Bonnegarde-Bernard A, Jee J, Fial MJ, Aeffner F, Cormet-Boyaka E, Davis IC, et al. IKKbeta in intestinal epithelial cells regulates allergen-specific IgA and allergic inflammation at distant mucosal sites. Mucosal immunology. 2014;7:257–67. doi: 10.1038/mi.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuburu N, Kweon MN, Song JH, Hervouet C, Luci C, Sun JB, et al. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine. 2007;25:8598–610. doi: 10.1016/j.vaccine.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 33.Ebensen T, Libanova R, Schulze K, Yevsa T, Morr M, Guzman CA. Bis-(3′,5′)-cyclic dimeric adenosine monophosphate: strong Th1/Th2/Th17 promoting mucosal adjuvant. Vaccine. 2011;29:5210–20. doi: 10.1016/j.vaccine.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 34.Ebensen T, Schulze K, Riese P, Morr M, Guzman CA. The bacterial second messenger cdiGMP exhibits promising activity as a mucosal adjuvant. Clin Vaccine Immunol. 2007;14:952–8. doi: 10.1128/CVI.00119-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanchez MV, Ebensen T, Schulze K, Cargnelutti D, Blazejewska P, Scodeller EA, et al. Intranasal delivery of influenza rNP adjuvanted with c-di-AMP induces strong humoral and cellular immune responses and provides protection against virus challenge. PLoS One. 2014;9:e104824. doi: 10.1371/journal.pone.0104824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu X, Wu FH, Wang X, Wang L, Siedow JN, Zhang W, et al. Molecular evolutionary and structural analysis of the cytosolic DNA sensor cGAS and STING. Nucleic Acids Res. 2014;42:8243–57. doi: 10.1093/nar/gku569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Motani K, Ito S, Nagata S. DNA-Mediated Cyclic GMP-AMP Synthase-Dependent and -Independent Regulation of Innate Immune Responses. Journal of immunology. 2015;194:4914–23. doi: 10.4049/jimmunol.1402705. [DOI] [PubMed] [Google Scholar]
- 38.Blaauboer SM, Mansouri S, Tucker HR, Wang HL, Gabrielle VD, Jin L. The mucosal adjuvant cyclic di-GMP enhances antigen uptake and selectively activates pinocytosis-efficient cells in vivo. Elife. 2015;4 doi: 10.7554/eLife.06670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skrnjug I, Guzman CA, Rueckert C. Cyclic GMP-AMP displays mucosal adjuvant activity in mice. PLoS One. 2014;9:e110150. doi: 10.1371/journal.pone.0110150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yi G, Brendel VP, Shu C, Li P, Palanathan S, Cheng Kao C. Single nucleotide polymorphisms of human STING can affect innate immune response to cyclic dinucleotides. PLoS One. 2013;8:e77846. doi: 10.1371/journal.pone.0077846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdul-Sater AA, Tattoli I, Jin L, Grajkowski A, Levi A, Koller BH, et al. Cyclic-di-GMP and cyclic-di-AMP activate the NLRP3 inflammasome. EMBO Rep. 2013;14:900–6. doi: 10.1038/embor.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brereton CF, Sutton CE, Ross PJ, Iwakura Y, Pizza M, Rappuoli R, et al. Escherichia coli heat-labile enterotoxin promotes protective Th17 responses against infection by driving innate IL-1 and IL-23 production. Journal of immunology. 2011;186:5896–906. doi: 10.4049/jimmunol.1003789. [DOI] [PubMed] [Google Scholar]
- 43.Mattsson J, Schon K, Ekman L, Fahlen-Yrlid L, Yrlid U, Lycke NY. Cholera toxin adjuvant promotes a balanced Th1/Th2/Th17 response independently of IL-12 and IL-17 by acting on Gsalpha in CD11b(+) DCs. Mucosal immunology. 2015;8:815–27. doi: 10.1038/mi.2014.111. [DOI] [PubMed] [Google Scholar]
- 44.Ireton RC, Gale M., Jr RIG-I like receptors in antiviral immunity and therapeutic applications. Viruses. 2011;3:906–19. doi: 10.3390/v3060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song JH, Kim JI, Kwon HJ, Shim DH, Parajuli N, Cuburu N, et al. CCR7-CCL19/CCL21-regulated dendritic cells are responsible for effectiveness of sublingual vaccination. Journal of immunology. 2009;182:6851–60. doi: 10.4049/jimmunol.0803568. [DOI] [PubMed] [Google Scholar]
- 46.Fang Y, Zhang T, Lidell L, Xu X, Lycke N, Xiang Z. The immune complex CTA1-DD/IgG adjuvant specifically targets connective tissue mast cells through FcgammaRIIIA and augments anti-HPV immunity after nasal immunization. Mucosal immunology. 2013;6:1168–78. doi: 10.1038/mi.2013.16. [DOI] [PubMed] [Google Scholar]
- 47.McGowen AL, Hale LP, Shelburne CP, Abraham SN, Staats HF. The mast cell activator compound 48/80 is safe and effective when used as an adjuvant for intradermal immunization with Bacillus anthracis protective antigen. Vaccine. 2009;27:3544–52. doi: 10.1016/j.vaccine.2009.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chandra D, Quispe-Tintaya W, Jahangir A, Asafu-Adjei D, Ramos I, Sintim HO, et al. STING ligand c-di-GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol Res. 2014;2:901–10. doi: 10.1158/2326-6066.CIR-13-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med. 2015;7:283ra52. doi: 10.1126/scitranslmed.aaa4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Temizoz B, Kuroda E, Ohata K, Jounai N, Ozasa K, Kobiyama K, et al. TLR9 and STING agonists synergistically induce innate and adaptive type-II IFN. Eur J Immunol. 2015;45:1159–69. doi: 10.1002/eji.201445132. [DOI] [PMC free article] [PubMed] [Google Scholar]


