Abstract
Background & objectives:
Next generation transplantation medicine aims to develop stimulating cocktail for increased ex vivo expansion of primitive hematopoietic stem and progenitor cells (HSPC). The present study was done to evaluate the cocktail GF (Thrombopoietin + Stem Cell factor + Flt3-ligand) and homing-defining molecule Stromal cell-derived factor 1 (SDF1) for HSPC ex vivo expansion.
Methods:
Peripheral blood stem cell (n=74) harvests were analysed for CD34hi CD45lo HSPC. Immunomagnetically enriched HSPC were cultured for eight days and assessed for increase in HSPC, colony forming potential in vitro and in vivo engrafting potential by analyzing human CD45+ cells. Expression profile of genes for homing and stemness were studied using microarray analysis. Expression of adhesion/homing markers were validated by flow cytometry/ confocal microscopy.
Results:
CD34hi CD45lo HSPC expansion cultures with GF+SDF1 demonstrated increased nucleated cells (n=28, P< 0.001), absolute CD34+ cells (n=8, P=0.021) and increased colony forming units (cfu) compared to unstimulated and GF-stimulated HSPC. NOD-SCID mice transplanted with GF+SDF1-HSPC exhibited successful homing/engraftment (n=24, P< 0.001). Microarray analysis of expanded HSPC demonstrated increased telomerase activity and many homing-associated genes (35/49) and transcription factors for stemness/self-renewal (49/56) were significantly upregulated in GF+SDF1 stimulated HSPC when compared to GF-stimulated HSPC. Expression of CD44, CXCR4, CD26, CD14, CD45 and soluble IL-6 in expanded cultures were validated by flow cytometry and confocal microscopy.
Interpretation & conclusions:
Cocktail of cytokines and SDF1 showed good potential to successfully expand HSPC which exhibited enhanced ability to generate multilineage cells in short-term and long-term repopulation assay. This cocktail-mediated stem cell expansion has potential to obviate the need for longer and large volume apheresis procedure making it convenient for donors.
Keywords: Ex vivo expansion, flow cytometry, gene expression profiling, hematopoietic stem cells, homing and engraftment, stem cell self-renewal transcription factors, stromal cell, derived factor-1
Hematopoietic stem cell transplantation is the gold standard for cell-based therapy and has been preferred treatment for a number of benign and malignant hematologic diseases. Transplantation of stem cells helps to restore the patient's immune system. Hematopoietic engraftment rate post-transplantation of bone marrow (BM) harvest or peripheral blood stem cell (PBSC) harvest or cord blood is governed primarily by number of stem and progenitor cells in the infused product1,2. Early Engraftment is associated with fewer complications, lower overall treatment costs, and a higher potential for a successful transplant.
Many times stem cell yield is not sufficient for autologous and allogeneic transplants. In autologous transplant setting, insufficient stem cell yield occurs in situations such as involvement of marrow by disease and in patients receiving multiple lines of chemotherapy. Similarly in allogeneic transplant setting, occasionally due to recipient and donor disparity in body weight, enough stem cells may not be collected from PBSC or marrow. In patients being explored for cord transplant, the cord stem cell dose may be limiting for adult patients. Therefore in these situations, ability to expand stem cells in vitro to increase the fraction of primitive stem cells may allow more patients to undergo transplants.
Ex vivo expansion of primitive hematopoietic stem and progenitor cells (HSPC) is a key technology to the next generation transplantation medicine. Over the past 25 years, attempts have been made to determine the optimized condition to enable maximum stem cell expansion using different combination of cytokines3. Early acting cytokines such as stem cell factor (SCF), thrombopoietin (TPO), and Flt3-ligand (Flt3-L) [growth factor (GF)] in presence or absence of other cytokines/factors such as granulocyte macrophage colony-stimulating factor (GM-CSF), interleukin-6 (IL6), IL3, Notch-ligand, erythropoietin or angiopoietin have been used to expand HSPC4,5. van Hensbergen et al6 have used single GF for expansion of HSPC and evaluated their ability for better thrombopoiesis and megakaryopoiesis. Some of the studies have also used stromal cell support, copper chelators, aryl hydrocarbon receptor antagonist, three-dimensional matrix scaffold and overexpression of transcription factors5,7,8,9,10.
It is well documented that efficient BM homing is a prerequisite for successful engraftment of transplanted HSPC. The homing-defining molecule Stromal cell derived factor 1 (SDF1) is known to play an important role in B-cell differentiation and migration of HSPC and lymphocytes11. Biological SDF1 has not been found to be suitable for drug development due to its fast pharmacologic clearance and its potential for causing allergic reactions due to induction of antibodies and other complications12. Hence synthetic SDF1 agonists have been produced which possess superior stability and circumvent problems associated with biological entity.
In the current study, it was proposed to extensively investigate the ability of chemically synthesized chemokine SDF1 plus GF to expand HSPC in number and simultaneously manipulate their surface repertoire such that they improve HSPC homing and hematopoietic immune recovery. Besides short-term in vitro qualitative assessment of HSPC for transplantation using colony forming unit (cfu) assay, and long-term in vivo evaluation of engraftment potential in mice model, differential gene expression of ex vivo expanded human HSPC were also analyzed before and after culture with cytokines-chemokine mixture.
Material & Methods
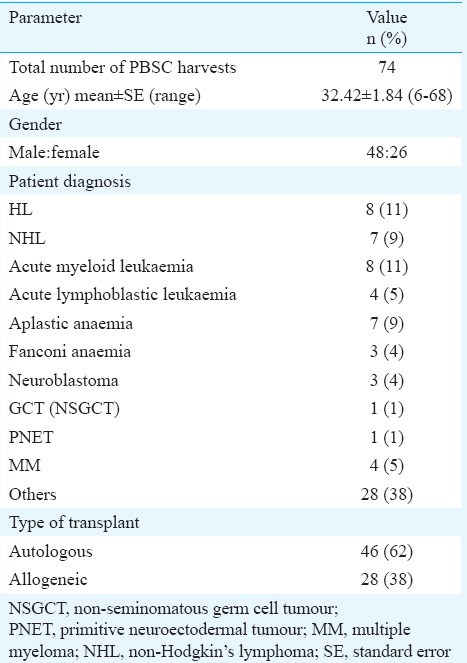
Human granulocyte colony-stimulating factor (G-CSF) mobilized leukapheresis samples were collected consecutively from December 2007 to May 2010, at Bone Marrow Transplant Unit, Advanced Centre for Treatment, Research & Education in Cancer (ACTREC), Tata Memorial Centre, Navi Mumbai, India. Patients (n=46) undergoing autologous transplants and HLA matched-related donors (n=28) of patients undergoing allogeneic transplants who consented to be part of the study were included. Stem cell harvests or leukapheresis samples were obtained after routine PBSC collection. The study protocol was approved by the Human Ethics Committee of Tata Memorial Centre, Mumbai. The characteristics, clinical history and treatment record of patients who underwent transplant are summarized in Table I.
Table I.
Details of peripheral blood stem cell (PBSC) harvest donors (n=74) for PBSC transplantation
Enrichment of human CD34+ cells and characterization by flow cytometry: PBSC harvests (n=74) were treated with ammonium chloride lysing solution for red blood cell lysis2. Nucleated cells obtained were analyzed by flow cytometry as described earlier13 in International Society for Hematotherapy and Graft Engineering (ISHAGE) protocol for CD34 analysis. Cells were stained with anti-human CD34 conjugated to Phycoerythrin (CD34-PE) and CD45 conjugated to Fluorescin-isothiocyanate (CD45-FITC, BD Pharmingen, San Jose, CA, USA) to analyze frequency of CD34hi CD45lo expression. Cells stained with Isotype-PE and CD45-FITC were used as control to set gates. Cells were acquired on a FACSCalibur flow cytometer (Becton Dickinson) and analyzed using CELLQuest Software (Becton Dickinson) or FlowJo Software (www.flowjo.com). CD34br CD45lo cells were gated on various gates in an organized sequential manner as per ISHAGE Guidelines13. Fraction of nucleated cells from a few harvests (n=35) were processed to enrich CD34+ cells using the Human Progenitor Cell Enrichment kit and EasySep Magnet (www.stemcell.com) according to the manufacturer's instruction. For evaluation of efficiency of enrichment protocol, post-enriched cells were analyzed for increase in CD34+ cells by flow cytometry and were used in cfu assays for increased progenitor potential and used in ex vivo expansion assay.
Ex vivo expansion of CD34+ cells with stromal cell-derived factor 1 (SDF1) and a mixture of growth factors (GFs): Enriched CD34+ cells at 2 × 104/ml were cultured in Iscove's Modified Dulbecco's Medium (Life Technologies, USA) containing 10 per cent fetal calf serum (FCS, Life Technologies) in a 96-well flat bottom culture plate. The cultures were treated with recombinant TPO (50 ng/ml, Stemcell Technologies, Vancouver, Canada), SCF (50 ng/ml) [GF: SCF+TPO+Flt3-ligand (Flt3L)], Flt3L (50 ng/ml) and SDF1 peptide (10 ng/ml; A kind gift from Prof. Nobutaka Fujii and Dr Shinya Oishi, Kyoto University, Japan14) for eight days with appropriate controls3,12. Cells were incubated at 37°C in a humidified incubator with 5 per cent CO2. Medium along with GFs and/or SDF1 was replenished after four days. After eight days of stimulation, cells were counted for total nucleated cell count. Cells were also analyzed for CD34+HSPC in ex vivo expanded cultures.
Colony forming assay: The number of functional primitive and progenitor haematopoietic stem cells (HSCs) in samples of pre-enrichment PBSC cells, post-enriched cells and ex vivo expanded cultures were assessed by 14-day short-term cfu assay in methylcellulose cultures in the presence of erythropoietin, GM-CSF, IL3 and SCF3,12. Pre-enriched cells at 2×104/ml and enriched or expanded CD34+ cells at 1×102/ml were seeded and incubated for 14 days in humidified atmosphere at 37°C. Colonies of colony forming unit-erythrocyte (cfu-E), blast-forming unit-erythrocyte (bfu-E), colony-forming unit granulocyte macrophage (cfu-GM) and cfu-granulocyte erythrocyte monocyte, megakaryocyte (cfu-GEMM) were scored in a blinded manner using Laser Confocal Microscope LSM 510META (Carl Zeiss, Germany) as per the protocol described by the manufacturers of reagents (Stemcell Technologies). Area occupied by individual colony was marked and relative area was calculated using ImageJ software (NIH, USA).
Engraftment of expanded human haematopoietic stem and progenitor cell (HSPC) in NOD/SCID mice: Six to eight week long-term repopulating assay for evaluating in vivo engraftment potential of ex vivo expanded HSPC was performed by transplanting these cells in NOD/LtSz-SCID/SCID mice models to simulate process followed in human stem cell transplantation as per the methods reported previously4,12,15,16,17. All procedures were approved by the Animal Research Ethics Committee of ACTREC, Tata Memorial Centre, Navi Mumbai. NOD/LtSz-SCID/SCID mice were purchased from Jackson Laboratory, Bar Harbor, ME, USA. Mice were bred and maintained under defined flora conditions in individually ventilated (high-efficiency particle-arresting filtered air) sterile microisolator cages. Mice at 8-10 wk of age were irradiated (myeloablated) with sub-lethal dose of 375 cGy of total body irradiation from a 137Cs source (Bhabhatron, ACTREC). Initially, pilot studies (n=4), involving only a single animal, were used to test the logistics of a proposed experiment as exploratory study. The objective was to minimize loss and prevent unnecessary wastage of animals during standardization. Ex vivo expanded cells were injected and analyzed in mice (n=16) for HSPC from patients (n=4). Later, confirmatory study involving six animals per group (4 groups, n=24) was conducted using experiment method of design as ‘completely randomized design’. All animals were put together in a cage during sub-lethal irradiation treatment using irradiator. After the randomized allocation of animals to the treatment groups, animals were coded until the data were analyzed; 24-h later, ex vivo expanded HSPC from PBSC harvests were transplanted by tail vein injection into sex- and age-matched mice (0.5-2×104 CD34+ at day 0 per mouse). Two hours before and post-injection mice were kept on fasting to avoid regurgitation process. Second important reason as documented by Cheng et al18 is that fasting offers protection to long-term haematopoietic stem cell (LT-HSC) population within transplanted HSC (ex vivo expanded cultures) and promotes stress (myeloablation post-irradiation) - resistance, self-renewal and lineage-based immune reconstitution.
Four groups were studied; i.e. Group A: non-transplanted mice; Group B: mice transplanted with cultured unstimulated ex vivo expanded HSPC; Group C: mice transplanted with ex vivo expanded HSPC using GF (SCF+TPO+Flt3L); and Group D: mice transplanted with ex vivo expanded HSPC using GF+SDF1.
Blood from tail vein was collected after 48 h to check for homing of transplanted HSPC. Weekly, body weight was taken from each group animal and blood smear was done on the day of mice sacrificed for pathological and histopathological analysis. Experimental animals were euthanized by cervical dislocation 6-8 wk after transplantation. Blood by cardiac puncture and BM from femurs were collected to analyze human WBC, lymphocytes, monocytes and neutrophils by differential count and CD45+ cells by flow cytometry and laser confocal microscopy to study homing of transplanted cells. Blood smears were stained using May-Grunwald-Giemsa stain. Stained cells were observed under microscope and ImageJ software (NIH, USA) was used to measure the area occupied by platelets.
Gene expression profiling: To get deeper insight into effect of SDF1 on HSPC at molecular level, cultured and expanded HSPCs were processed for gene profiling study by microarray experiment as described by Oswald et al8. RNA was extracted from eight-day culture expanded HSPC (n=9) and microarray experiments were performed through customized service using Agilent Platform (Agilent, Palo Alto, CA, USA).
Microarray data analysis: Data extraction from images was done using Feature Extraction software v 9.5 of Agilent. Feature extracted data were analyzed using GeneSpring GX version 10 software from Agilent. Normalization of the data was done in GeneSpring GX using the 75th percentile shift and normalization to specific samples. Significant genes up- and downregulated showing one-fold and above among the samples were identified. Differentially regulated genes were classified into different functions and pathways. Heat maps were generated for differentially regulated genes using GeneSpring software.
Validation of markers by flow cytometry and confocal microscopy: Homing-related genes CD44, CXCR4, CD26, CD14 and IL-6 were validated in expanded cultures by flow cytometry and confocal microscopy. Ex vivo expanded HSPC were labelled with tracking dye PKH-26 (Sigma, USA) as described by Wysoczynski et al19. Cells were also stained with FITC- or PE-conjugated antibodies against human CD34, CD45, CXCR4, CD26, CD71, CD18 or CD44 and analyzed by laser confocal microscopy or flow cytometry2. Soluble IL-6 was measured by Cytokine Bead Array kit (BD Biosciences, CA, USA).
Statistical analysis: Data were presented as mean±standard error of the mean. The normality assumption was tested using Shapiro-Wilk test at five per cent level of significance. Continuous variables were compared by Student's t test, paired t test or Wilcoxon signed-rank test, as appropriate. Some of the figures were drawn by GraphPad Prism 5 for Windows (https://www.graphpad.com). For comparing more than two groups in in vivo study, the parametric analysis of variance (ANOVA) was applied. Further, the post-hoc tests were also applied to find the groups with significant difference. The entire statistical analyses were performed using SPSS 21.0 software (SPSS Inc., Chicago, IL, USA) and GPower 3.0.10 software (GPower, Düsseldorf, Germany).
Results
Per cent CD34+CD45lo HSPC in PBSC harvests: The volume of PBSC harvest ranged from 0.5 to 2.0 ml. Total nucleated cells in human G-CSF mobilized PBSC harvests ranged from 50 to 200×106. Flow cytometry analysis was performed on PBSC harvest (autologous n=46, allogeneic n=28) and enriched HSPC (n=35) as per standards of ISHAGE Gating Strategy. CD34hiCD45lo HSPC in PBSC harvest ranged from 0.004 to 1.7 per cent in cancer patients and 0.5 to 3.6 per cent in healthy allograft donors. As evident in Fig. 1A, per cent CD34+ cells in allogeneic cases (n=28, mean 0.69±0.09%) was comparable to that seen in autologous cases (n=46, mean 0.72±0.11%). Yield of enriched CD34+ cells ranged from 0.8 to 1.5×106. The comparison of per cent CD34hiCD45lo HSPC cells between pre-enriched (0.69±0.11, median=0.6) and post-enriched (13.33±2.34, median=9.38) PBSC harvests (autograft and allograft) was found to be of significance (n=35, P<0.001). A sample size of 35 achieves 99 per cent power to detect the effect size 0.92 and with a significance level (alpha) of 0.05 using post hoc power analysis for two dependent groups. The fold increase of enriched CD34+ cells as evaluated by ISHAGE guidelines ranged from 0.25 to 3050 per cent post-enrichment (Fig. 1B; mean fold increase: 122.14±88). Fold increase of CD34+HSPC post-enrichment was found to be more in autologous cases (189.1±159.1, median 21) than in allogeneic cases (42.6±22.4, median 15.9).
Fig. 1.
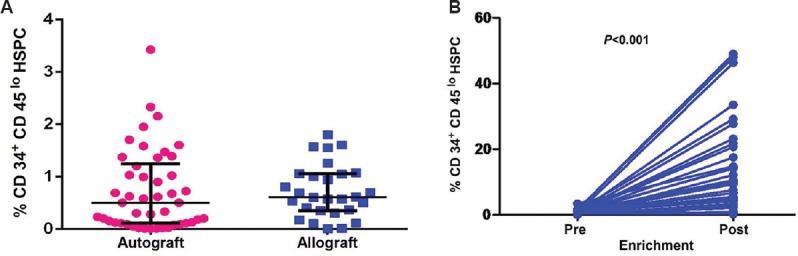
(A) Percentage of CD34+CD45lo haematopoietic stem and progenitor cells in PBSC harvests from cancer patients and healthy donors. The dot plot depicts percentage of CD34+CD45lo haematopoietic stem and progenitor cells in PBSC harvests of cancer patients (n=46; left side) and healthy donors (n=28; right side). Horizontal/vertical bars on each dot plot indicate mean±standard error. (B) Percentage of CD34+CD45lo haematopoietic stem and progenitor cells in PBSC harvests from cancer patients and healthy donors. Increase in per cent CD34+CD45lo haematopoietic stem and progenitor cells within pre- and post-enriched PBSC harvests (autograft and allograft; n=35). HSPC: Haematopoietic stem and progenitor cells; PBSC: Peripheral blood stem cell.
Ex vivo expansion of enriched HSPC: Enriched HSPC from PBSC harvests (cancer patients n=14, healthy allograft donors n=14) were expanded ex vivo without or with GF or GF+SDF1 for eight days. GF+SDF1-treated expansion cultures demonstrated increased number of nucleated cells compared to both the groups, i.e. untreated and GF-treated HSPC (Table II). The comparison of total cells in ex vivo expanded HSPC between all groups unstimulated, GF and GF+SDF1 was analyzed using Friedman test (P=0.001) and pairwise comparison between the three groups by Wilcoxon signed-rank test was significant (Table II). It was observed that HSPC from healthy allograft donors exhibited higher fold increase compared to those from patients (Table II).
Table II.
Comparison of total cells in ex vivo expanded haematopoietic stem and progenitor cells
The ex vivo expanded cultures (n=8) were analyzed for maintenance of stemness i.e. CD34+ cells during expansion. The comparison of per cent CD34+ cells in ex vivo expanded HSPC between all groups unstimulated, GF and GF+SDF1 was analyzed using Friedman test. There was increase in per cent CD34+ cells HSPC in GF+SDF1 group, but difference was not significant. However, as the cells expanded upon culture, the comparison of absolute CD34+ cells in ex vivo expanded HSPC between all groups unstimulated, GF and GF+SDF1 was found to be significant (P=0.021; Table III).
Table III.
Comparison of CD34+ cells in ex vivo expanded haematopoietic stem and progenitor cells
Assessment of human colony forming unit in PBSC harvests: Pre/post-enrichment cells from PBSC harvests and culture expanded HSPC were analyzed for long-term repopulating efficiency of enrichment protocol and ex vivo expansion by the assessment of increase in functional progenitor cells by 14-day long cfu assay. Fig. 2A depicts standard cfu colonies (cfu-E, bfu-E, cfu-GM eosinophils, cfu-GM neutrophils, cfu-M and cfu-GEMM) observed in our study. Number of cfu increased significantly post-enrichment (colony area, 24.9±5.1×105 μm2) compared to that before enrichment (colony area, 0.16±0.06×105 μm2), indicating efficiency of enrichment protocol (n=8). Power achieved for this test was 99 per cent with effect size 1.73. It was observed that colony unit area produced by HSPC from allograft healthy donors was significantly higher compared to that from patients (data not shown). Though the numbers of colonies increase to a marginal extent, the area occupied by these colonies was significantly enlarged in GF+SDF1-stimulated HSPC cultures over that of GF-stimulated cultures (data not shown). It was interesting to note that colonies given by GF+SDF1-stimulated HSPC cultures demonstrated increase in depth area compared to GF-stimulated cultures. Therefore, it was considered appropriate to report area instead of only numbers. During assessment of quality of ex vivo expanded cells by colony formation assay (CFA) (n=5), it was observed that number of functional progenitors were increased significantly in GF+SDF1-treated cells compared to GF-treated or untreated cells (Fig. 2B).
Fig. 2.
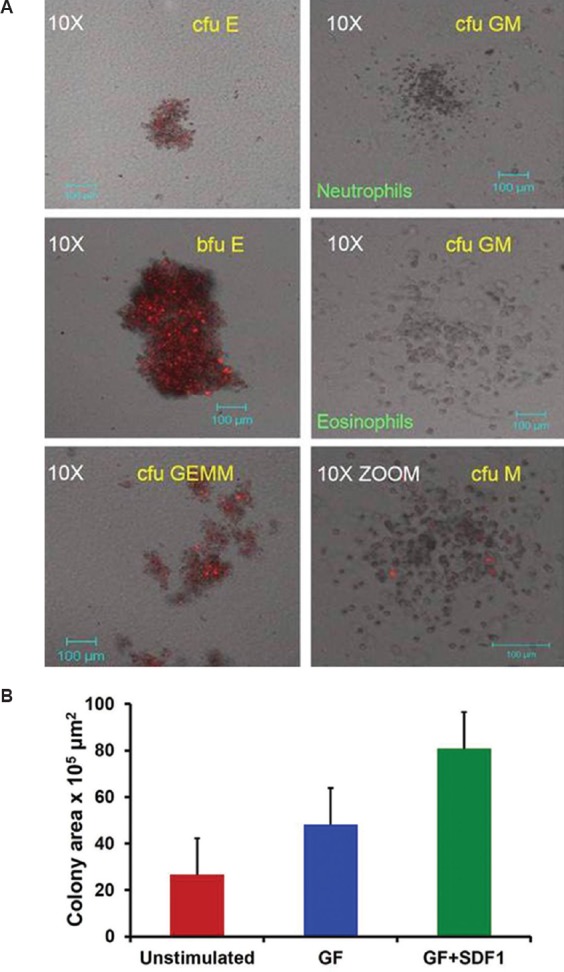
Stem cell progenitor assessment by colony formation assay (CFA). (A) Representative images of colony forming unit-erythrocyte (cfu-E), blast-forming unit-erythrocyte (bfu-E), colony-forming unit granulocyte macrophage (cfu-GM) (neutrophils), colony-forming unit granulocyte macrophage (cfu-GM) (eosinophils), cfu-granulocyte erythrocyte monocyte, megakaryocyte (cfu-GEMM), cfu-M colonies observed in CFA of enriched haematopoietic stem and progenitor cells under confocal microscope. Colonies were photographed after 14 days differentiation on methocult medium. (B) Stem cell progenitor assessment by colony formation assay. Total colony unit area (mean values) occupied by colonies generated by primitive and progenitor haematopoietic stem cells in ex vivo expanded haematopoietic stem and progenitor cells cultures (n=5).
In vivo assessment of engraftment potential of ex vivo expanded HSPC: Immune reconstitution and repopulation ability of HSPC, previously ex vivo expanded using GF or GF+SDF1, were analyzed in sub-lethally irradiated (375 cGy) NOD-SCID mice. Expanded HSPC cells were injected into tail vein of 8-10 wk old mice. In each experiment, ex vivo expanded CD34+ cells from PBSC were transplanted into sex- and age-matched mice (0.5-2×104 CD34+ at day 0 per mouse).
For evaluation of homing of transplanted HSPC, CD45+ human cells which had migrated through endothelial vasculature were identified in circulating blood in mice 48-h post-transplantation. Mice transplanted with GF+SDF1-manipulated cells demonstrated increased human CD45+ cells in circulating blood compared to untreated HSPC or GF-treated HSPC group or non-transplanted (irradiated control) group which suggested faster homing of transplanted cells (data not shown).
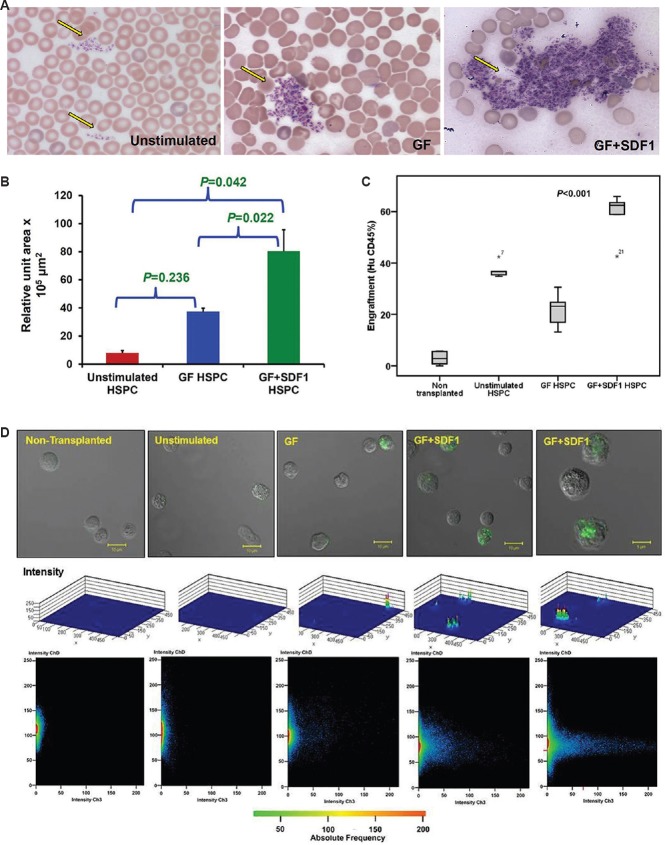
For engraftment of these mice at six weeks, platelets were analyzed in tail vein blood smear and were found to be increased in GF+SDF1-treated group compared to other groups (Fig. 3A and B). It was observed that mice transplanted with GF+SDF1-stimulated HSPC exhibited significant increased platelet formation over that seen with unstimulated HSPC (P=0.042, power=63% with effect size 1.7) and GF-stimulated HSPC (P=0.022, power=89% with effect size 3.8, Fig. 3B). BM cells of the mice were harvested and analyzed for expression of CD45+ human cells (n=6 per group; mean, non-transplanted Group A: 2.95±0.97%, unstimulated HSPC Group B: 36.94±1.4%, GF-treated HSPC Group C: 21.93±2.3%, GF+SDF1-treated HSPC Group D: 58.68±4.1%). The differences between per cent human CD45+ cells in all groups were significant (P<0.001; Fig. 3C and D). Power achieved for this test was 92 per cent with the effect size 0.93. Total BM cells were counted and used to calculate absolute number of CD45+ human cells in mouse BM (n=6 per group; mean, non-transplanted Group A: 0.11±0.13×105, unstimulated HSPC Group B: 4.44±0.93×105, GF-treated HSPC Group C: 2.41±0.42×105, GF+SDF1-treated HSPC Group D: 7.06±0.56×105). The comparative analysis of absolute number of CD45+ cells after engraftment of human transplanted HSPC revealed that increase in engraftment in mice transplanted with GF+SDF1-treated HSPC over other groups was of significance (P<0.001). Power achieved for this test was 85 per cent with the effect size 0.83.
Fig. 3.
Assessment of in vivo engraftment potential of ex vivo expanded haematopoietic stem and progenitor cells in mice. (A) Microscopic images (×10) of platelets stained at six-week engraftment. Drop of blood from tail vein was smeared and slides were stained with May-Grunwald-Giemsa stain. Left panel depicts platelets in mouse transplanted with unstimulated haematopoietic stem and progenitor cells while right panel exhibits platelets in mouse transplanted with growth factor+stromal cell-derived factor 1 (GF+SDF1) stimulated haematopoietic stem and progenitor cells. Yellow arrow in both panels denotes location of platelets. (B) Assessment of in vivo engraftment potential of ex vivo expanded haematopoietic stem and progenitor cells in mice. Mean values (4 experiments) of relative unit area ×103 μm2 occupied by platelets in peripheral blood of mice. P values obtained by paired t test. (C) Assessment of in vivo engraftment potential of ex vivo expanded haematopoietic stem and progenitor cells in mice. Per cent human CD45+ cells in bone marrow of mice transplanted with no haematopoietic stem and progenitor cells, unstimulated haematopoietic stem and progenitor cells (HSPC), growth factor-stimulated haematopoietic stem (GF HSPC) and progenitor cells and growth factor + stromal cell-derived factor 1 stimulated haematopoietic stem and progenitor cells (GF+ SDF1 HSPC). This represents box plot of engraftment data (n=6 in each group). (D) Assessment of in vivo engraftment potential of ex vivo expanded haematopoietic stem and progenitor cells in mice. Top subpanel, confocal microscopic view, middle subpanel, 3D plot, and bottom subpanel, intensity plot of human CD45+ cells stained with fluorescein-isothiocyanate (FITC) in bone marrow of transplanted mice.
The in vitro and in vivo study data implicated that CD34+ HSPC could be efficiently ex vivo expanded and chemokine-activated so as to bring about improved engraftment in patients with malignancies who are undergoing HSC transplantation.
Differential gene expression of human CD34+ cells before and after culture with cytokines-chemokine mixture: Mobilized PBSC harvests, CD34-enriched PBSC and ex vivo expanded cells [unstimulated, SCF+TPO+Flt3 (GF)-treated and GF+SDF1-treated] were studied for gene expression profiles by microarray processing and data analysis using Agilent Platform. RNA was extracted from 21 samples, of which 10 could be processed further for hybridization based on RNA integrity and quantity. These included data of samples obtained from cancer patient (n=1; mobilized PBSC and CD34-enriched PBSC) and allogeneic healthy donors (n=4; mobilized PBSC and CD34-enriched PBSC, unstimulated HSPC, GF-HSPC, GF+SDF1 HSPC) Custom-designed human 8×60,000 array was used. Feature extracted data (n=9) were analyzed using GeneSpring GX version 10 software. Normalization of the data was done in GeneSpring GX using the 75th percentile shift and normalization to specific samples.
Gene profiles of whole peripheral blood (geodatabase n=2), mobilized PBSC samples (n=3), GF-stimulated HSPC (n=2) and GF+SDF1-stimulated HSPC (n=2) were compared. These have been submitted in NCBI-GEO. Many immune response genes, IL genes, DNA repair genes, chemokine genes, extracellular matrix genes and DNA damage repair genes were found to be upregulated (data available online in NCBI-GEO GSE37334). As observed in Fig. 4A, most of the homing genes were upregulated (71.4%; 35/49) in GF+SDF1-expanded HSPC over GF-expanded HSPC (mean data, n=2).
Fig. 4.
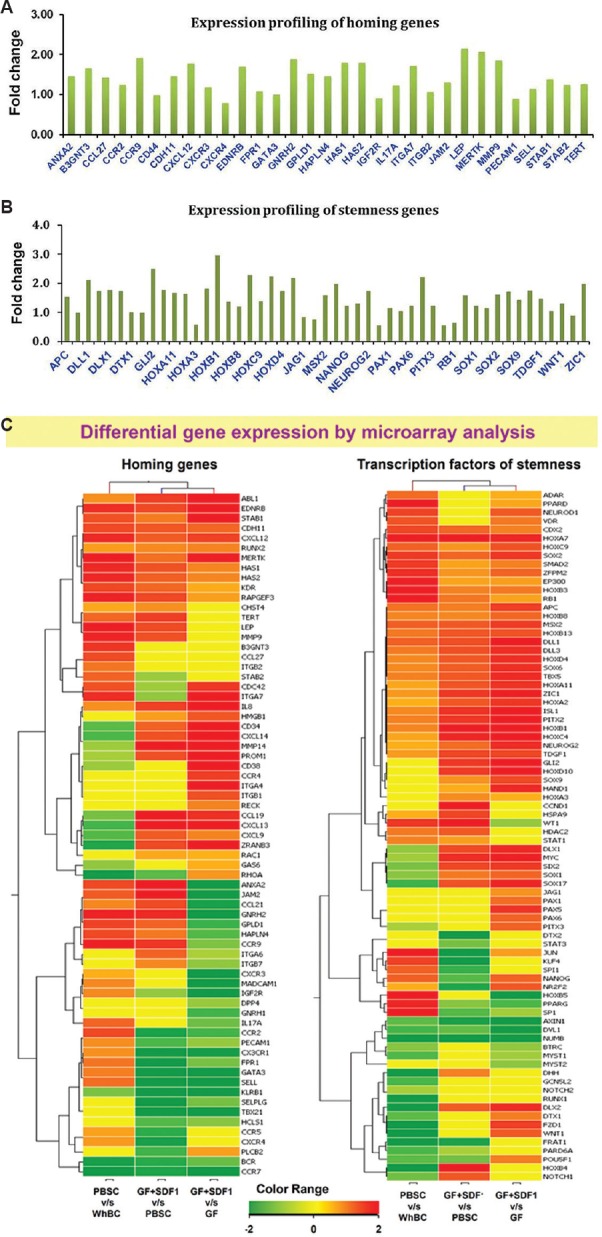
Gene expression profiling of growth factor-stimulated haematopoietic stem and progenitor cells cultures versus growth factor + stromal cell-derived factor 1 (GF+SDF1) stimulated haematopoietic stem and progenitor cells (HSPC) cultures. (A) Mean fold change for upregulated genes of homing from NCBI list in GF+ SDF1 stimulated HSPC over GF HSPC (n=2). Fold change depicted as 0.6 corresponds to 1.5 times increase of gene expression. (B) Mean fold change for genes for transcription factors involved in stem cell self-renewal, maintenance, organ morphogenesis and haematopoiesis (from NCBI list) in GF+SDF1 HSPC over GF HSPC cultures (n=2). Fold change depicted as 0.6 corresponds to 1.5 times increase of gene expression. (C) Heat maps of differential expression of genes involved in stem cell homing (left panel) and transcription factors for self renewal/ stemness (right panel) in PBSC harvest versus PBL from whole blood, GF+SDF1 stimulated HSPC versus cells from PBSC harvest and GF+SDF1 stimulated HSPC over GF-stimulated HSPC.
Moreover, GF+SDF1-expanded HSPC also exhibited increased expression (87.5%; 49/56) of genes involved in stem cell self-renewal markers, organ morphogenesis, stem cell maintenance, haematopoiesis and stem cell differentiation over GF-expanded HSPC (n=2; Fig. 4B). Functional pathway analysis of this set demonstrated that pathways of allograft rejection, graft-versus-host disease and cell cycle were significantly downregulated while pathways related to ABC transporters, calcium signalling pathway and cell adhesion molecules were significantly upregulated. These pathways play key role during maintenance of stemness post-expansion and manipulating cells such that they bring about better engraftment of transplanted HSPC.
Fig. 4C depicts comparative heat maps of fold increase/ decrease values of homing-associated genes and transcription factors involved in stem cell self renewal observed in gene profiles of mobilized PBSC versus whole blood lymphocytes, PBSC versus GF+SDF1 stimulated HSPC and GF stimulated HSPC versus GF+SDF1 stimulated HSPC samples.
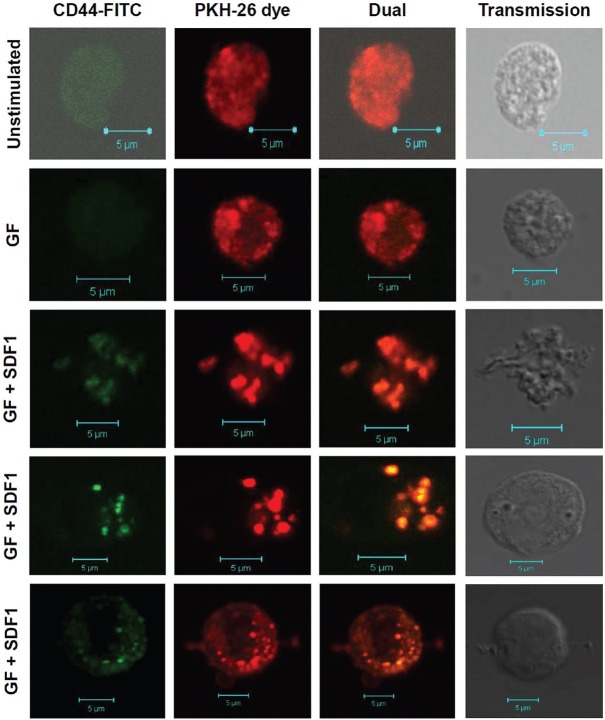
Validation of few markers by flow cytometry and confocal microscopy: Homing-related genes CD44, CXCR4, CD26, CD14 and IL-6 were validated by flow cytometry and confocal microscopy. Ex vivo expanded cells were intensely stained with lipid dye PKH-26. The lipid distribution was seen throughout cell membrane in unstimulated HSPC (Fig. 5). Cells responding to GF+SDF1 stimulation demonstrated distinct aggregation of lipid dye on membrane, indicating involvement of lipid rafts in SDF1-CXCR4 signalling. These cells demonstrated characteristic projections involved in migratory movement of cells, as observed in chemotactic cells. When cells were stained for CD44, it was observed that CD44 colocalized in lipid rafts at most events in GF+SDF1-stimulated cells indicating involvement of CD44 homing-related adhesion molecule in SDF1-CXCR4 signalling in these cells (Fig. 5). GF-stimulated cells showed CD44 aggregation in lipid rafts but to a lesser extent than that observed with GF+SDF1-stimulated cells. These observations corroborated well with microarray data (Fig. 5).
Fig. 5.
Colocalization of lipid grafts and CD44 in stromal cell-derived factor 1-CXCR4 signalling in haematopoietic stem and progenitor cells (HSPC). Ex vivo expanded cells were labelled with PKH-26 lipid dye. Later, these were stained with fluorescein-isothiocyanate-conjugated antibody against CD44 and analyzed by laser confocal microscopy. CD44 is colocalized in lipid rafts and is evenly distributed in unstimulated HSPC and GF HSPC. CD44 gets aggregated in lipid rafts upon stromal cell-derived factor 1 (SDF1) stimulation. Upon SDF1, stimulation cells become chemotactic and acquire migratory phenotype.
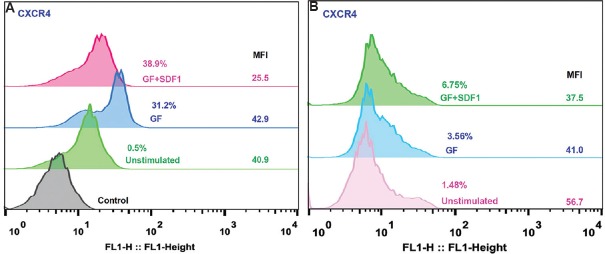
It was interesting to note that CXCR4-FITC-, CD34-PE-, CD45-FITC- and CD26-FITC-stained HSPC demonstrated colocalization and aggregation of markers CD34, CD26 and CD45 in lipid rafts (data not shown). Flow cytometry analysis exhibited that CD45, CXCR4 and CD14 expression was increased in GF+SDF1-stimulated cells as compared to GF-stimulated cells and unstimulated HSPC. However, expression of CD26 and CD71 was comparable in GF-stimulated and GF+SDF1-stimulated cells (data not shown). Though expression of CXCR4 was found to be increased in GF+SDF1-stimulated cells over GF-stimulated HSPC in ex vivo expanded cultures, the median fluorescence intensity (MFI) decreased in these cells (Fig. 6A). Similarly, human CXCR4 expressing cell per cent and absolute numbers were found to be increased in BM of mice transplanted with GF+SDF1-stimulated HSPC compared to that in mice transplanted with GF-stimulated HSPC or unstimulated HSPC. A similar phenomenon was observed in in vivo models also; CXCR4 receptor MFI decreased upon exposure to SDF1 ligand (Fig. 6B).
Fig. 6.
(A) CXCR4 expression and receptor downregulation in stromal cell-derived factor 1(SDF1) -CXCR4 signalling in haematopoietic stem and progenitor cells (HSPC). Ex vivo expanded cells stained with CXCR4 antibody - FITC conjugate. Cells were analyzed by flow cytometry. Histogram overlay of cells from unstimulated HSPC, GF HSPC and GF+SDF1 HSPC (representative of 5 experiments each). For each test, isotype antibody served as control; % expression and median fluorescence intensity (MFI) for each set are shown at right side of histogram. (B) CXCR4 expression and receptor downregulation in SDF1-CXCR4 signalling in HSPC. Flow cytometric analysis of bone marrow cells derived from mice transplanted with expanded HSPC were stained with CXCR4 antibody - FITC conjugate. Cells were analyzed by flow cytometry. Histogram overlay of cells from unstimulated HSPC, GF HSPC and GF+SDF1 HSPC (n=5). For each test, isotype antibody served as control; % expression and median fluorescence intensity for each set are shown at the right side of histogram.
Soluble IL6 was also found to be increased in supernatants of GF+SDF1-treated HSPC cultures compared to unstimulated HSPC cultures but showed similar increase as compared to GF-stimulated HSPC cultures (data not shown). These data corroborated well with microarray data.
Discussion
Many studies have attempted to enhance stem cell mobilization using G-CSF20, heparan sulphate mimetics21, therapeutic agents such as chemotherapy drugs (cyclophosphamide and 5-fluorouracil), cytokines
(such as IL-112 or IL-813), chemokines (such as MIP-1α15 or SDF122) or synthetic small molecules SDF1 agonist or CXCR4 antagonist such as AMD3100 or plerixafor11,22. Shaikh et al23 and Bhartiya24 have reported potential use of very small embryonal-like stem cells - a distinct subset of pluripotent stem cells in transplantation as well as haematopoietic regeneration; however, this concept is yet to be proved in clinical trials. On the other hand, ex vivo expansion requires proliferation of HSCs with maintenance of self-renewal and simultaneous inhibition of differentiation. Limited availability of ex vivo models that replicate the HSPC microenvironment interactions, limited understanding of mechanisms that control self-renewal and commitment of HSPC and delay in establishing safety of expanded cells have hindered their successful application in clinical therapy. Thus, approaches to circumvent low stem cell numbers and delayed engraftments have been continuously gathering attention. Simultaneously, efforts to get better understanding of mechanism underlying HSPC homing and engraftment have been initiated19.
Most of the efforts have been focussed on expansion of umbilical cord blood stem cells4,25. In the present study, we did not use cord blood as source of HSPC. We used HSPC from PBSC and investigated comparative potential of enriched HSPC for ex vivo expansion and retention of stemness, at in vitro as well as in vivo level.
It was observed that CD34+CD45lo HSPC numbers were comparable in autologous as well as allogeneic transplant donors. This indicated that PBSC mobilization in cancer patients was comparable to that seen in healthy donors. Cancer patients usually have received previous chemotherapeutic drugs which are also responsible for increased mobilization of CD34+ cells seen in autologous transplant PBSC harvests. Our data were in concordance with that reported in earlier studies1,25 that some cancer patients mobilized well while some healthy donors mobilized poorly. However, as exhibited by cfu assay, stem cell progenitors were found to be increased in allogeneic PBSC harvests compared to that from autologous donors. This could be attributed to prior myelotoxic chemotherapy in autologous transplant cases which may be possible reason for reduced expansion of HSPC from patients as compared to allogeneic donor cells that are healthy and have never received chemotherapy. Torretta et al25 have also observed similar results; however, they only analyzed cfu-GM progenitors. Cocktail of GF+SDF1 demonstrated significant ex vivo expansion of these HSPC over unstimulated or only GF-stimulated HSPC. We used enriched CD34+ HSPC i.e. the cell population also contained a few other cells from PBSC harvest. Others have used either purified CD34+ cells from cord blood6,22,26 or whole cord blood mononuclear cells5. In our study, enhanced repopulation ability of expanded HSPC cells or their ability to differentiate into multiple lineages could be attributed to the presence of mix population along with enriched CD34+ cells. Our observation was in concordance with data from a clinical trial by Delaney et al4. They observed successful engraftment when two cord blood units, i.e. one ex vivo expanded CD34+ cells and the other unmanipulated cord blood unit containing mixture of CD34+ and CD34− cells, were used. Immunodeficient mouse strains NOD-SCID or NOG models have been widely used to study engraftment and homing of HSPC cells4,15,27. We transplanted ex vivo expanded cells in NOD SCID mice and evaluated human CD45+ cells in mice BM which is widely accepted method. Platelet engraftment was analysed in these mice and significant results were observed in GF+SDF1-HSPC transplanted mice compared to other groups. Regarding platelet origin, it was not possible to distinguish human and mouse platelets as we had not performed flow cytometry for CD41/CD61 markers. However, during colony formation potential assay, ex vivo expanded HSPC demonstrated significant increase in cfu-GEMM which constitute progenitor of megakaryocytes (Mks). Thus, after transplantation of these expanded HSPC, Mk progenitors present in culture would have given rise to human platelets.
Importance of SDF1-CXCR4 pathway contribution to BM homing has been shown earlier by Kollet et al17. They demonstrated that incubation of cord blood CD34+ cells with anti-CXCR4 antibodies virtually abrogated their BM homing in NOD-SCID mice. In our study, CXCL12/CXCR4 pathway was seen to be activated in GF+SDF1-stimulated HSPC as CXCR4, CXCR7 and CXCL12 genes were significantly augmented in these cultures over GF-stimulated cultures. Our data were in concordance with that reported by Yellowley28. In our study, gene profiling analysis exhibited that SDF1 upregulated other homing-related molecules which included adhesion molecules as well. Larochelle et al29 have demonstrated role of CXCR4, tetraspanins, VLA-4, L-selectin, CD44 and E-selectin in homing and engraftment. They also showed importance of polarized membrane domain in engraftment of long-term repopulating cells in vivo. In our study, GF+SDF1-stimulated HSPC exhibited upregulation of CXCR4, tetraspanins, CD44, L-selectin and P-selectin while VLA-4 and prominin1 were found to be unaltered. CXCR4 protein expression was validated by flow cytometry of ex vivo expanded cultures and BM of HSPC-transplanted mice after 6-8 wk of engraftment. We also observed increased polarized membrane domain upon GF+SDF1 stimulation of HSPC compared to GF-stimulated or non-stimulated cultured HSPC. CD45 and CD26 were also found to be involved in lipid rafts upon activation in HSPC2. Polarized membrane domain of HSPC was attributed to stem cell maintenance in hypoxic conditions29. As reported by Wysoczynski et al19 increased homing demonstrated in our study could be due to polarization of CXCR4, CD26, CD45 noted in cell membranes in GF+SDF1-stimulated cultures over GF-activated HSPC cultures (data not shown). Soluble IL6 was also found to be increased in supernatants of GF+SDF1-treated HSPC cultures (data not shown). Improved HSPC engraftment could be due to IL6 observed in ex vivo expanded cultures as reported by other groups15.
CD34, CD45, CD26, CXCR4 and CD71 markers were analysed on cells isolated from BM of mice transplanted with expanded primitive and progenitor HSC. These receptors constitute markers of primitive HSC and show engraftment after 6-8 wk post-transplantation indicating their long-term repopulation ability17,23. Shaikh et al23 have demonstrated that Lin-CD34+CD45-CD133+SSEA+primitive stem cells express CXCR4 marker. Our data were in concordance with this finding and HSPC enriched from PBSC harvest in our study also demonstrated these markers in in vitro cultures. In mice model also, BM of transplanted cells at 6-8 wk post-transplantation exhibited CD34, CD45 and CXCR4 markers, indicating the presence of long-term-repopulation ability of HSC.
Studies have also demonstrated importance of bioactive phospholipids (sphingosine-1-phosphate) and complement cascade factors (C3a and cathelicidin) in HSPC mobilization and engraftment30,31. In agreement with these data, an increase in sphingolipid machinery and complement components C3-cathelicidin in GF+SDF1-stimulated HSPC cultures (data not shown) was seen compared to GF-stimulated HSPC cultures.
Others have reported importance of absolute numbers of CD34+ cells for successful engraftment in human transplant cases4,32. A significant increase has been observed in absolute numbers of CD34 in ex vivo expanded cultures and human CD45 in in vivo model in our study. All these data support the fact that the method of expansion described in this study allows expansion of primitive HSCs along with progenitor cells.
Second, we employed CFA to demonstrate the presence of primitive HSCs as per methods reported by others12,16. Primitive HSCs relate to cells capable of giving rise to lineages of lymphoid and myeloid progenitors viz. cfu-GEMM. In our study, HSPC population exhibited colonies belonging to cfu-E, bfu-E, cfu-GM as well as cfu-GEMM. In animal model of six-week engraftment experiment, expression of human lymphocytes (CD45), monocytes (CD14) and megakaryocyte progenitors (CD71) as well as Mks in terms of platelets was observed.
Within the expanded cultures, the maximum expansion potential will be possessed by the most primitive stem cells. We have hypothesized that SDF1 stimulation manoeuvres modulation of stem cell repertoire and genetic makeup such that genes involved in proliferative capacity as well as maintenance of self-renewal/stemness get upregulated. Significant fold increase of transcription factors HOXC4, HOXB4, SOX17, PU.1, SET, nanog, oct3/4, c-myc, TERT and sox2 in GF+SDF1-activated HSPC over only GF-stimulated HSPC was observed. These transcription factors were shown to be involved in expansion and self-renewal of primitive HSCs27,33. Shaikh et al23 have demonstrated that Lin-CD133+SSEA4+CD34+CD45-primitive stem cells exhibit telomerase activity and express pluripotency markers nanog, sox2, etc. Hiyama and Hiyama34 have also reported that telomerase reverse transcriptase (TERT) and telomerase activity is upregulated in cytokine-induced proliferation and cell cycle activation in primitive HSC.
In conclusion, our study demonstrated that ex vivo expansion mediated by SDF1 along with early-acting cytokines increased primitive as well as progenitor HSCs that were functionally active and gave rise to multi-lineage cells in short-term and long-term assays. These data provided important leads that SCF+TPO+Flt3L+SDF1 cocktail could be further explored. Further studies to refine this method are required before ex vivo expanded cells can be used in the clinic. If stem cell expansion technique is developed successfully, then it will obviate the need for longer and large volume apheresis procedure making it convenient for donors. The same method can be used to expand cord blood stem cells which may prove beneficial for many patients for whom cord blood transplants may be currently not feasible due to inadequate doses.
Acknowledgment
Authors acknowledge the Indian Council of Medical Research (ICMR), New Delhi, for providing financial support for this project (Grant No. 80/20/2006/STM-BMS). Part of this work was also supported by Intramural grant by Tata Memorial Centre No.361. The fourth and sixth authors (VA and RK) were supported by ICMR Senior Research Fellowship. Authors thank Dr Shinya Oishi for providing SDF1 synthetic analogue for in vitro work, and acknowledge Genotypic Technology Pvt. Ltd., Bengaluru, for the microarray processing and data analysis.
Footnotes
Conflicts of Interest: None.
References
- 1.Lemoli RM, de Vivo A, Damiani D, Isidori A, Tani M, Bonini A, et al. Autologous transplantation of granulocyte colony-stimulating factor-primed bone marrow is effective in supporting myeloablative chemotherapy in patients with hematologic malignancies and poor peripheral blood stem cell mobilization. Blood. 2003;102:1595–600. doi: 10.1182/blood-2003-02-0440. [DOI] [PubMed] [Google Scholar]
- 2.Prabhash K, Khattry N, Bakshi A, Karandikar R, Joshi A, Kannan S, et al. CD26 expression in donor stem cell harvest and its correlation with engraftment in human haematopoietic stem cell transplantation: potential predictor of early engraftment. Ann Oncol. 2010;21:582–8. doi: 10.1093/annonc/mdp342. [DOI] [PubMed] [Google Scholar]
- 3.Tanavde VM, Malehorn MT, Lumkul R, Gao Z, Wingard J, Garrett ES, et al. Human stem-progenitor cells from neonatal cord blood have greater hematopoietic expansion capacity than those from mobilized adult blood. Exp Hematol. 2002;30:816–23. doi: 10.1016/s0301-472x(02)00818-4. [DOI] [PubMed] [Google Scholar]
- 4.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–6. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madkaikar M, Ghosh K, Gupta M, Swaminathan S, Mohanty D. Ex vivo expansion of umbilical cord blood stem cells using different combinations of cytokines and stromal cells. Acta Haematol. 2007;118:153–9. doi: 10.1159/000108630. [DOI] [PubMed] [Google Scholar]
- 6.van Hensbergen Y, Schipper LF, Brand A, Slot MC, Welling M, Nauta AJ, et al. Ex vivo culture of human CD34+ cord blood cells with thrombopoietin (TPO) accelerates platelet engraftment in a NOD/SCID mouse model. Exp Hematol. 2006;34:943–50. doi: 10.1016/j.exphem.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Schuster JA, Stupnikov MR, Ma G, Liao W, Lai R, Ma Y, et al. Expansion of hematopoietic stem cells for transplantation: current perspectives. Exp Hematol Oncol. 2012;1:12. doi: 10.1186/2162-3619-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oswald J, Steudel C, Salchert K, Joergensen B, Thiede C, Ehninger G, et al. Gene-expression profiling of CD34+ hematopoietic cells expanded in a collagen I matrix. Stem Cells. 2006;24:494–500. doi: 10.1634/stemcells.2005-0276. [DOI] [PubMed] [Google Scholar]
- 9.Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–8. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higuchi A, Yanga ST, Lia PT, Changc Y, Tsaic EM, Chenc YH, et al. Polymeric materials for ex vivo expansion of hematopoietic progenitor and stem cells. Polym Rev. 2009;49:181–200. [Google Scholar]
- 11.Suárez-Álvarez B, López-Vázquez A, López-Larrea C. Mobilization and homing of hematopoietic stem cells. Adv Exp Med Biol. 2012;741:152–70. doi: 10.1007/978-1-4614-2098-9_11. [DOI] [PubMed] [Google Scholar]
- 12.Li K, Chuen CK, Lee SM, Law P, Fok TF, Ng PC, et al. Small peptide analogue of SDF-1alpha supports survival of cord blood CD34+ cells in synergy with other cytokines and enhances their ex vivo expansion and engraftment into nonobese diabetic/severe combined immunodeficient mice. Stem Cells. 2006;24:55–64. doi: 10.1634/stemcells.2005-0082. [DOI] [PubMed] [Google Scholar]
- 13.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5:213–26. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 14.Oishi S, Fujii N. Peptide and peptidomimetic ligands for CXC chemokine receptor 4 (CXCR4) Org Biomol Chem. 2012;10:5720–31. doi: 10.1039/c2ob25107h. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed F, Ings SJ, Pizzey AR, Blundell MP, Thrasher AJ, Ye HT, et al. Impaired bone marrow homing of cytokine-activated CD34+ cells in the NOD/SCID model. Blood. 2004;103:2079–87. doi: 10.1182/blood-2003-06-1770. [DOI] [PubMed] [Google Scholar]
- 16.Pike BL, Robinson WA. Human bone marrow colony growth in agar-gel. J Cell Physiol. 1970;76:77–84. doi: 10.1002/jcp.1040760111. [DOI] [PubMed] [Google Scholar]
- 17.Kollet O, Spiegel A, Peled A, Petit I, Byk T, Hershkoviz R, et al. Rapid and efficient homing of human CD34(+)CD38(-/low)CXCR4(+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice. Blood. 2001;9:3283–91. doi: 10.1182/blood.v97.10.3283. [DOI] [PubMed] [Google Scholar]
- 18.Cheng CW, Adams GB, Perin L, Wei M, Zhou X, Lam BS, et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14:810–23. doi: 10.1016/j.stem.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wysoczynski M, Reca R, Ratajczak J, Kucia M, Shirvaikar N, Honczarenko M, et al. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005;105:40–8. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- 20.Hopman RK, DiPersio JF. Advances in stem cell mobilization. Blood Rev. 2014;28:31–40. doi: 10.1016/j.blre.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Giacomo F, Lewandowski D, Cabannes E, Nancy-Portebois V, Petitou M, Fichelson S, et al. Heparan sulfate mimetics can efficiently mobilize long-term hematopoietic stem cells. Haematologica. 2012;97:491–9. doi: 10.3324/haematol.2011.047662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broxmeyer HE, Vadhan-Raj S. Preclinical and clinical studies with the hematopoietic colony-stimulating factors and related interleukins. Immunologic research. 1989;8:185–201. doi: 10.1007/BF02918144. [DOI] [PubMed] [Google Scholar]
- 23.Shaikh A, Nagvenkar P, Pethe P, Hinduja I, Bhartiya D. Molecular and phenotypic characterization of CD133 and SSEA4 enriched very small embryonic-like stem cells in human cord blood. Leukemia. 2015;29:1909–17. doi: 10.1038/leu.2015.100. [DOI] [PubMed] [Google Scholar]
- 24.Bhartiya D. Stem cells, progenitors & regenerative medicine: A retrospection. Indian J Med Res. 2015;141:154–61. doi: 10.4103/0971-5916.155543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torretta L, Perotti C, Dornini G, Danova M, Locatelli F, Pedrazzoli P, et al. Circulating progenitor cell collection: experience from 275 leukaphereses in various malignancies and in healthy donors. Haematologica. 1996;81:208–15. [PubMed] [Google Scholar]
- 26.Broxmeyer HE, Kohli L, Kim CH, Lee Y, Mantel C, Cooper S, et al. Stromal cell-derived factor-1/CXCL12 directly enhances survival/antiapoptosis of myeloid progenitor cells through CXCR4 and G(alpha)i proteins and enhances engraftment of competitive, repopulating stem cells. J Leukoc Biol. 2003;73:630–8. doi: 10.1189/jlb.1002495. [DOI] [PubMed] [Google Scholar]
- 27.Nishino T, Osawa M, Iwama A. New approaches to expand hematopoietic stem and progenitor cells. Expert Opin Biol Ther. 2012;12:743–56. doi: 10.1517/14712598.2012.681372. [DOI] [PubMed] [Google Scholar]
- 28.Yellowley C. CXCL12/CXCR4 signaling and other recruitment and homing pathways in fracture repair. Bonekey Rep. 2013;2:300. doi: 10.1038/bonekey.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larochelle A, Gillette JM, Desmond R, Ichwan B, Cantilena A, Cerf A, et al. Bone marrow homing and engraftment of human hematopoietic stem and progenitor cells is mediated by a polarized membrane domain. Blood. 2012;119:1848–55. doi: 10.1182/blood-2011-08-371583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ratajczak MZ, Kim C. Bioactive sphingolipids and complement cascade as new emerging regulators of stem cell mobilization and homing. J Stem Cell Res Ther. 2011;1 doi: 10.4172/2157-7633.1000e102. pii: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adamiak M, Borkowska S, Wysoczynski M, Suszynska M, Kucia M, Rokosh G, et al. Evidence for the involvement of sphingosine-1-phosphate in the homing and engraftment of hematopoietic stem cells to bone marrow. Oncotarget. 2015;6:18819–28. doi: 10.18632/oncotarget.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu JT, Cheng SB, Yang Y, Chang KH, Hwang WL, Teng CL. Circulating hematopoietic progenitors and CD34(+) cells predicted successful hematopoietic stem cell harvest in myeloma and lymphoma patients: experiences from a single institution. J Blood Med. 2016;7:5–11. doi: 10.2147/JBM.S95679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chotinantakul K, Prasajak P, Leeanansaksiri W. Wnt1 accelerates an ex vivo expansion of human cord blood CD34(+)CD38(-) cells. Stem Cells Int. 2013;2013:909812. doi: 10.1155/2013/909812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiyama E, Hiyama K. Telomere and telomerase in stem cells. Br J Cancer. 2007;96:1020–4. doi: 10.1038/sj.bjc.6603671. [DOI] [PMC free article] [PubMed] [Google Scholar]