Abstract
Background & objectives:
Prevalence of pulmonary tuberculosis (PTB) is known to be high in the indigenous tribal community Saharia in Madhya Pradesh, India. The risk factors for PTB are not well known among them. This study was done to determine various risk factors associated with PTB in the indigenous community Saharia.
Methods:
A prevalence survey was conducted among Saharias of Gwalior district of Madhya Pradesh. The population surveyed was 12,123 which was the source of cases and controls for the present study. All the bacillary-positive cases and controls in the ratio of 1:5 were included in the survey. Data were collected by the trained health workers from the patients and controls using a semi-structured pre-coded and pre-tested questionnaire which included data on risk factors including demographic factors, host-related factors and household factors. The individuals were also screened for diabetes mellitus and HIV.
Results:
Malnutrition and history of asthma were associated with an increased risk of PTB. More than 56 per cent cases were attributed to malnutrition and 12 per cent attributed to asthma. Low family income, alcohol consumption and smoking were the other contributors. The risk was higher in males as compared to females.
Interpretation & conclusions:
The study emphasized that the main contributors were social factors. Nutrition supplementation, especially in tuberculosis (TB) patients and integrated approach to improve their living conditions are needed to control TB in this community.
Keywords: Malnutrition, pulmonary tuberculosis, risk factors, Saharia
Tuberculosis (TB) remains a major global health problem. The major challenges to TB control today include multidrug-resistant-TB and HIV-TB co-infection. Another important challenge is the growing body of evidence, suggesting coexisting diabetes as a risk factor for new as well as reactivation of old cases of TB1. Apart from diabetes and HIV co-infection, there are many risk factors associated with TB infection and disease such as age, sex, malnutrition (biological factors), tobacco smoking, alcoholism (behavioural factors), poverty, overcrowding and poor housing (socio-economic factors)2,3,4.
Many studies have demonstrated a direct relationship between active smoking and TB5. India has a high burden of TB with an estimated prevalence of 230/100,000 population. In 2015, there were an estimated 10.4 million new (incident) TB cases worldwide, of which 2.8 million were estimated to have occurred in India6. However, there are only a few studies on risk factors for TB from India7,8.
Tribal population constitutes around 8.2 per cent of the total population of the country and one quarter of the total population in the State of Madhya Pradesh9. There are 46 ethnic groups in the State, and three among them have been categorized as Particularly Vulnerable Tribal Groups (PVTGs), earlier called as primitive tribal groups. The Saharia is one of these three PVTGs in the State. They are mainly located in Gwalior and Chambal divisions of Madhya Pradesh9. A high prevalence of pulmonary TB (PTB) (3294/100,000) has been reported among them in our earlier study10. Smoking and alcohol consumption have been shown to be associated with PTB disease in this population11. There is, however, no information on the role of other risk factors for PTB. Therefore, the present study was undertaken to find an association of various risk factors with PTB in this indigenous community.
Material & Methods
This study was conducted by the ICMR- National Institute for Research in Tribal Health, Jabalpur, India, after taking approval from the institutional ethics committee. A survey of PTB was carried out in four blocks of Gwalior district of Madhya Pradesh during March to November, 2013.
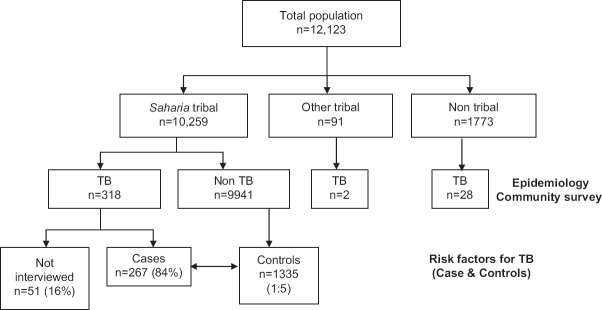
All individuals aged 15 yr and above were screened for PTB by chest symptoms such as persistent cough for two weeks or more, chest pain for one month or more, fever for one month or more and haemoptysis anytime in the last six months. All symptomatics were investigated by sputum smear and culture examinations. The population surveyed was 12,123 and was the source of cases and controls for the present study as described elsewhere10. The cases were defined as those who were sputum smear and/or culture positive for PTB and who were screened and declared not to have TB formed the controls (Fig. 1). A list of all the detected cases and controls was prepared, and those willing to participate and gave consent were included in the risk factors study.
Fig. 1.
Flow chart showing intake of cases and controls.
The sample size for unmatched case–control study was calculated assuming the prevalence of an exposure of 25 per cent in controls, odds ratio (OR)=1.5, 95 per cent confidence interval (95% CI), 80 per cent power and 1:5 ratio of cases and controls. A minimum sample of 264 cases was calculated with its five time controls. The sample size was estimated using Kelsey's Method with the help of online OpenEpi calculator12. The controls were selected randomly from the same village from where cases were found13. For each TB case, five controls from the same village were randomly selected. The randomization was done by the survey team in the field in such a manner that five controls per case should be interview successfully.
Data collection: Using a semi-structured pre-coded and pre-tested questionnaire, the data on risk factors including demographic (age, sex) data were collected by the trained health workers from all the patients and controls. The interviews were conducted at home in their local language (Hindi). The data on host-related factors viz, education, occupation, bacillus Calmette–Guérin (BCG) vaccination, height and weight for computing body mass index (BMI), previous history of TB, smoking and alcohol intake were collected from all individuals. BMI <18.5 kg/m2 was considered as undernutrition14. A history of asthma was also noted. In addition, they were screened for blood glucose levels (to rule out diabetes mellitus) and HIV status. Information on household factors viz. household size, type of house, income, overcrowding, biomass fuel use and household member/relative with TB was also collected.
Quality check for data collection was inbuilt in the methodology. A 10 per cent random sample of individuals interviewed by the health worker was selected independently by a supervisor and the extent of agreement was ascertained for any corrective measures.
Blood sugar estimation: Capillary blood glucose using a glucometer (AccuChek Active, Australia) was done after overnight fasting to estimate blood sugar. Samples were collected by finger prick method, and the test was done as per the manufacturer's protocol. In case of abnormal blood sugar values (fasting glucose level >126 mg/dl)15, the patients were referred to primary health centres/community health centres/district TB centres for further evaluation to rule out diabetes mellitus.
HIV testing: After obtaining informed consent, HIV test was done on whole-blood samples collected by finger prick method. The test was done by rapid test (SD Bioline, Standard Diagnostics Inc., Germany). Reactive individuals were referred to nearest integrated counselling and testing centre for confirmation of HIV status. Pre- and post-test counselling was performed as per the standard guidelines16.
Data management and analysis: Data entry was done using the Census and Survey Processing System software package version 5.0. Data were analyzed using Statistical Package for the Social Sciences (SPSS/PC version 20.0; SPSS Inc., Chicago, IL, USA) package. The attributable proportion of all modifiable risk factors was calculated using the standard population attributable fraction formula -
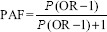
where P is prevalence of exposure in population and OR is the adjusted OR17.
In univariate analysis, the Chi-square test was used to compare cases and controls to assess the effect of each risk factors of interest. Multivariate model was constructed, including variables that showed a significant statistical effect in the prediction of TB in univariate analysis.
Results
During the survey, 318 TB cases were identified among 10,259 population from Saharia community15. Fifty one (16%) individuals could not be interviewed, 21 moved temporarily, seven were not available during our three visits, six refused to participate and 17 expired. The remaining 267 (84%) cases were interviewed at their residence (Fig. 1).
Profile of study population: The demographic and socio-economic profile of study population is shown in Table I. Age, education and housing (in terms of type, number of rooms and cooking place) were almost similar in both case and controls. Among 267 cases, 211 (79%) were males; whereas among 1335 controls, 660 (49%) were males. More cases were seen from the stone crushing workers and in low family annual income of  ≤10,000 (85 vs. 78%). The mean age of cases and controls was 39.42±13.5 and 39.46±13.4 yr, respectively.
≤10,000 (85 vs. 78%). The mean age of cases and controls was 39.42±13.5 and 39.46±13.4 yr, respectively.
Table I.
Distribution of demographic and socio-economic, clinical and lifestyle risk factors for pulmonary tuberculosis
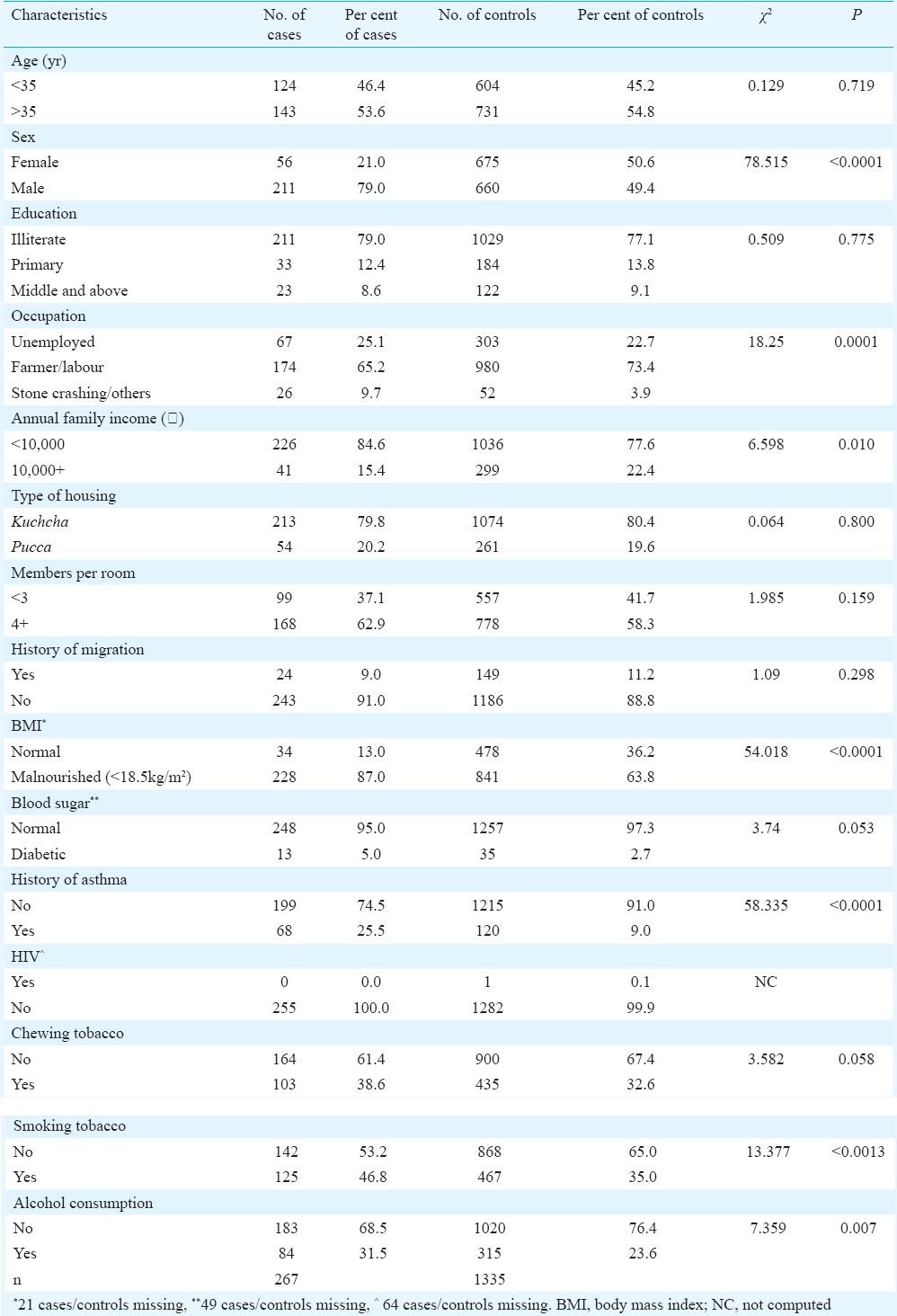
Investigation of risk factors for pulmonary tuberculosis (PTB): Results from univariate analysis carried out on 267 cases and 1335 controls to investigate the demographic and socio-economic risk factors are displayed in Table II. Male gender (OR=3.85; 95% CI 2.82-5.27; P<0.001); stone crushing workers (OR=2.26; 95% CI 1.32-3.88; P<0.01) and low income (OR=1.59; 95% CI 1.11-2.27; P<0.001) were found to increase the risk of PTB. It was observed that malnutrition (OR=3.81; 95% CI 2.61-5.56; P<0.001) and history of asthma (OR=3.46; 95% CI 2.48-4.83; P<0.001) were associated with increased risk of PTB. Prevalence of HIV was low among controls with one HIV-positive individual (Table II). Abnormal blood sugar was associated with high risk, but the difference was not found to be significant. The risk of TB was higher in tobacco smokers (OR=1.64; 95% CI 1.25-2.13; P<0.001) and alcohol consumers (OR=1.49; 95% CI 1.11-1.98; P<0.01) (Table II). Alcohol consumption and smoking were meagre in women.
Table II.
Results of univariate and multivariate analysis of all risk factors for pulmonary tuberculosis (n=1602)
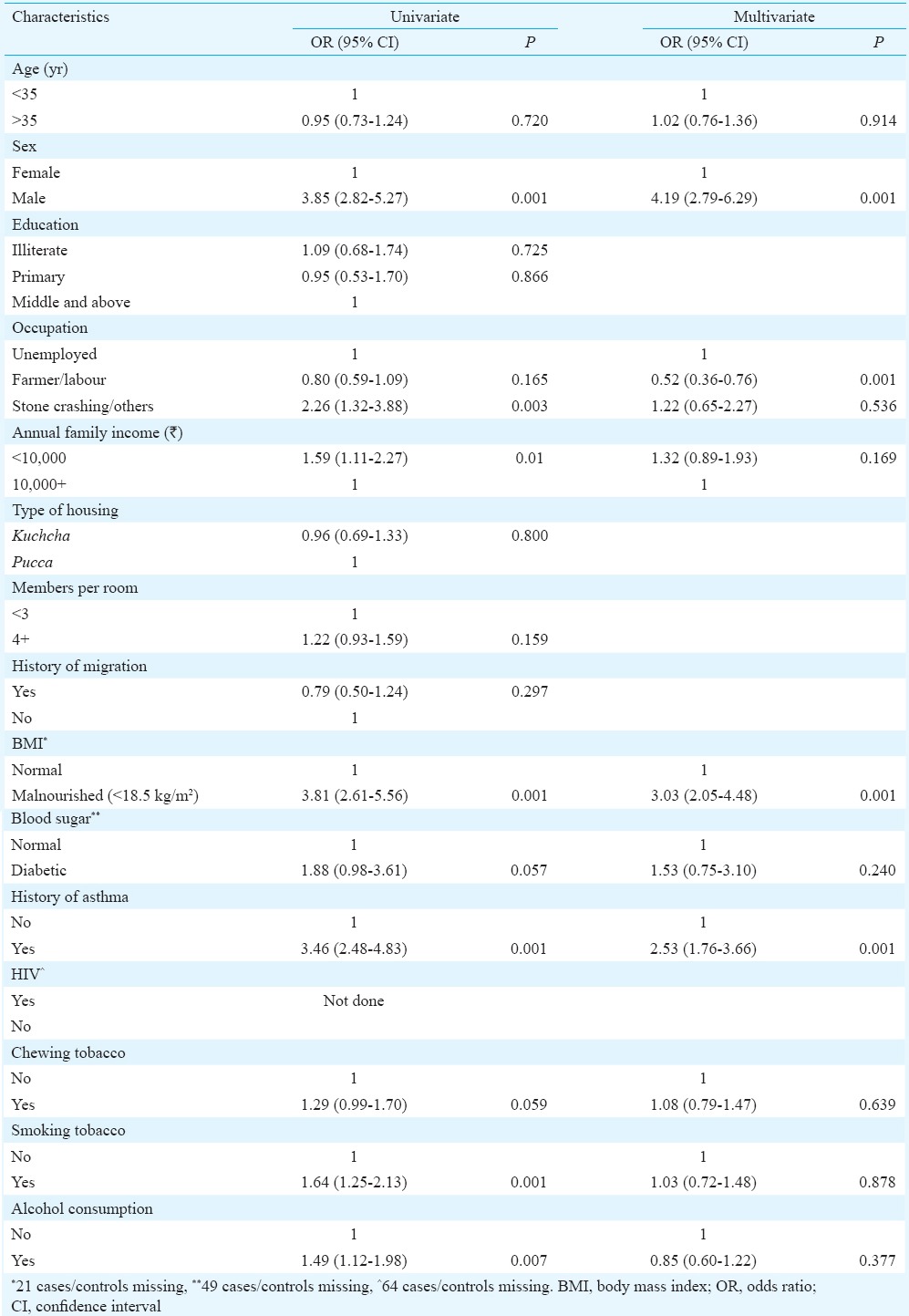
A multivariate model (binary logistic regression) was constructed, including variables of interest that univariate analysis showed a significant association with TB. The risk of TB was found to be associated with male sex, malnutrition and asthma. In multivariate analysis, the risk factors that stood out were malnutrition and history of asthma (Table II). Stone crushing workers were not a risk factor in multivariate analysis as the number was small.
Fig. 2 shows the unadjusted (based on OR) and adjusted [based on adjusted OR (AOR)] attributable proportions for the modifiable risk factors for TB such as nutritional status, annual family income, occupation, smoking tobacco, history of asthma, alcohol consumption and blood sugar level. It showed that 64 per cent cases were attributed to malnutrition, 31 per cent attributed to low annual family income, 18 per cent attributed to tobacco smoking, 18 per cent attributed to asthma, 10 per cent attributed to alcohol consumption, about five per cent attributed to stone crushing work and two per cent attributed to abnormal blood sugar (diabetics). After adjusting (based on AOR), it was found that 56 per cent of the cases were attributed to malnutrition and 12 per cent attributed to asthma.
Fig. 2.
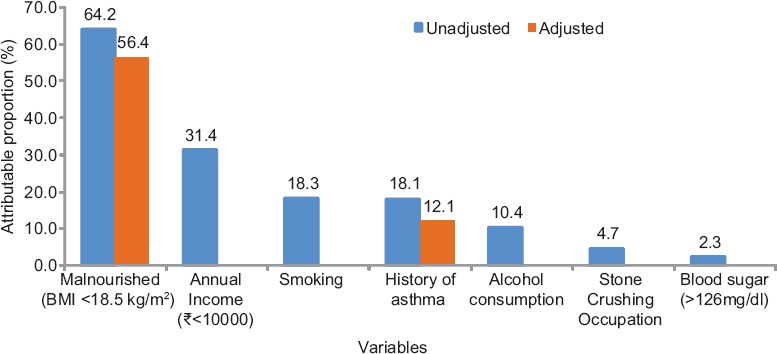
Attributable proportions for the modifiable risk factors of tuberculosis.
Discussion
In our study, males had significantly higher prevalence of TB than females. This difference was not attributed to gender bias because the coverage for screening examinations in this active case detection survey was uniformly high for both males and females. The immune response is known to be different in the two sexes, indicating sexual dimorphism. Evidence suggests that at physiological levels, oestrogen is beneficial to the immune system, whereas the male sex hormone, testosterone, is immunosuppressive18. Habits such as smoking and alcoholism might have also contributed to TB in men as these habits were found to be negligible among women.
TB has been found more in stone crushing workers in earlier study19. Indoor air pollution has been considered as an important risk factor for TB in India by other authors20. Although these factors were observed in many cases in our study, these were not found to be significant. Majority of people in these areas were exposed to these factors. Underlying silicosis in these stone crushing workers cannot be denied; however, we could not evaluate the same as this was a field-based study and X-ray facility was not available.
In a study by Lienhardt et al21, TB was shown to be associated with male sex, family history of TB, absence of a BCG scar, smoking, alcohol, anaemia, HIV infection and history and treatment of worm infection. In another study in a tertiary care hospital from south India, diabetes was found to be a predominant risk factor22. Kolappan et al2 found that risk factors such as age, male sex, smoking and alcoholism were independently associated with pulmonary TB. In our study, age did not have an effect on TB. In another study from south India, TB was associated with low education level, kitchen type and diabetes, reflecting the complex interaction between non-communicable disease, urbanization and a changing economic climate23. Smoking increases mortality from TB by increasing the incidence of the disease24. Both smoking and alcoholism were important risk factors for TB in our study; however, these did not turn out to be significant in multivariate analysis.
HIV co-infection is a known risk factor for progression of Mycobacterium tuberculosis infection to active disease, increasing the risk of latent TB reactivation 20-fold25. HIV was not found to be a risk factor in the present study as the prevalence of HIV was found to be very low among this community. Diabetes prevalence was also found to be low in the study population and it was not significant in TB cases.
In this study majority of cases of TB were attributed to malnutrition, followed by low annual family income, tobacco smoking, asthma and alcohol consumption. This is a marginalized community with no sustainable income and poor knowledge about the disease. Modelling study data based on National Family Health Survey (NFHS) showed that about 50 per cent of TB cases could be attributed to undernutrition, thereby implying that improving nutritional status could have a dramatic impact on TB incidence26. A study from Chhattisgarh, Central India, also found undernutrition in a large number of TB cases and that undernourished patients had more severe disease27. Padmapriyadarsini et al28 have recommended nutrition supplementation for TB patients. An integrated approach to improve their living conditions is also needed to control TB in this community. The Revised National Tuberculosis Control Programme has special provisions for tribal areas under its tribal plan such as additional facilities, incentives for workers and patients29 which needs to be effectively implemented.
The limitation of this study was that the individuals with abnormal blood sugar level were not confirmed as diabetics though they were referred to health centre for further testing. In addition, the exposure levels of indoor air pollution could not be measured.
In conclusion, the present survey showed that the Saharia, one of the PVTGs residing in Central India, had PTB due to the major risk factors of malnutrition, history of asthma and their living conditions. Appropriate interventions need to be developed for nutritional supplementation for effective management of TB in this vulnerable population.
Acknowledgment
The authors are grateful to late Dr Neeru Singh, Director, NIRTH, Jabalpur for her encouragement and support. The contributions of the District Tuberculosis Officer, the WHO/RNTCP consultant, District tribal welfare authorities, Block Medical Officers, Ashram School authorities and peripheral field staff in the district are acknowledged. Authors also acknowledge the help of laboratory and field staffs, and assistance provided by Ms Preeti Tiwari, Swati Chouhan, Sneha Patel, and Srimati Priyashri Tiwari Pandey. Financial support by Adim Jati Kalyan Vibhag, through the District Administration, Gwalior, India is also acknowledged.
Footnotes
Conflicts of Interest: None.
References
- 1.Sally M. Beyond DOTS - The new stop TB strategy and its implementation. Challenges of tuberculosis control. Can Med Assoc J. 2006;174:33–4. doi: 10.1503/cmaj.051504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolappan C, Gopi PG, Subramani R, Narayanan PR. Selected biological and behavioural risk factors associated with pulmonary tuberculosis. Int J Tuberc Lung Dis. 2007;11:999–1003. [PubMed] [Google Scholar]
- 3.Kim MJ, Kim HR, Hwang SS, Kim YW, Han SK, Shim YS, et al. Prevalence and its predictors of extrapulmonary involvement in patients with pulmonary tuberculosis. J Korean Med Sci. 2009;24:237–41. doi: 10.3346/jkms.2009.24.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lönnroth K, Williams BG, Stadlin S, Jaramillo E, Dye C. Alcohol use as a risk factor for tuberculosis - A systematic review. BMC Public Health. 2008;8:289. doi: 10.1186/1471-2458-8-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowe CR. An association between smoking and respiratory tuberculosis. Br Med J. 1956;2:1081–6. doi: 10.1136/bmj.2.5001.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Global Tuberculosis Report 2016. Geneva: WHO; 2016. [Google Scholar]
- 7.Chelleng PK, Devi KR, Borbora D, Chetia M, Saikia A, Mahanta J, et al. Risk factors of pulmonary tuberculosis in tea garden communities of Assam, India. Indian J Med Res. 2014;140:138–41. [PMC free article] [PubMed] [Google Scholar]
- 8.Gaude GS, Hattiholli J, Kumar P. Risk factors and drug-resistance patterns among pulmonary tuberculosis patients in northern Karnataka region, India. Niger Med J. 2014;55:327–32. doi: 10.4103/0300-1652.137194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Government of India. Statistical Profile of Scheduled Tribes in India. Ministry of Tribal Affairs. Statistics Division. Government of India. 2010. [accessed on April 28, 2014]. Available from: https://tribal.nic.in/ST/StatisticalProfileofSTs2013.pdf .
- 10.Rao VG, Bhat J, Yadav R, Muniyandi M, Sharma R, Bhondeley MK. Pulmonary tuberculosis - a health problem amongst Saharia tribe in Madhya Pradesh. Indian J Med Res. 2015;141:630–5. doi: 10.4103/0971-5916.159560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao VG, Gopi PG, Bhat J, Yadav R, Selvakumar N, Wares DF. Selected risk factors associated with pulmonary tuberculosis among Saharia tribe of Madhya Pradesh, central India. Eur J Public Health. 2012;22:271–3. doi: 10.1093/eurpub/ckr009. [DOI] [PubMed] [Google Scholar]
- 12.Kelsey JL, Whittemore AS, Evans AS, Thompson D, editors. Methods in observational epidemiology. 2nd ed. New York: Oxford Press; 1996. [Google Scholar]
- 13.Hennessy S, Bilker WB, Berlin JA, Strom BL. Factors influencing the optimal control-to-case ratio in matched case-control studies. Am J Epidemiol. 1999;149:195–7. doi: 10.1093/oxfordjournals.aje.a009786. [DOI] [PubMed] [Google Scholar]
- 14.A Manual: Measuring and Interpreting Malnutrition and Mortality. Ch. 1. Geneva: WHO; 2000. World Health Organization. Defining and measuring malnutrition. [Google Scholar]
- 15.New Delhi: Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India; 2009. Government of India-WHO. National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Disease and Stroke. A Manual for Medical Officer. [Google Scholar]
- 16.New Delhi: NACO. Ministry of Health & Family Welfare, Government of India; 2015. National AIDS Control Organisation. Guidelines for HIV Testing. [Google Scholar]
- 17.Kolappan C, Subramani R. Association between biomass fuel and pulmonary tuberculosis: A nested case-control study. Thorax. 2009;64:705–8. doi: 10.1136/thx.2008.109405. [DOI] [PubMed] [Google Scholar]
- 18.Bellamy R, Beyers N, McAdam KPWJ, Ruwende C, Gie R, Samaai P, et al. Genetic susceptibility to tuberculosis in Africans: A genome-wide scan. Proc Natl Acad Sci U S A. 2000;97:8005–9. doi: 10.1073/pnas.140201897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiwari RR, Sharma YK, Saiyed HN. Tuberculosis among workers exposed to free silica dust. Indian J Occup Environ Med. 2007;11:61–4. doi: 10.4103/0019-5278.34530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sehgal M, Rizwan SA, Krishnan A. Disease burden due to biomass cooking-fuel-related household air pollution among women in India. Glob Health Action. 2014;7:25326. doi: 10.3402/gha.v7.25326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lienhardt C, Fielding K, Sillah JS, Bah B, Gustafson P, Warndorff D, et al. Investigation of the risk factors for tuberculosis: A case-control study in three countries in West Africa. Int J Epidemiol. 2005;34:914–23. doi: 10.1093/ije/dyi100. [DOI] [PubMed] [Google Scholar]
- 22.Gupta S, Shenoy VP, Mukhopadhyay C, Bairy I, Muralidharan S. Role of risk factors and socio-economic status in pulmonary tuberculosis: A search for the root cause in patients in a tertiary care hospital, South India. Trop Med Int Health. 2011;16:74–8. doi: 10.1111/j.1365-3156.2010.02676.x. [DOI] [PubMed] [Google Scholar]
- 23.Shetty N, Shemko M, Vaz M, D’Souza G. An epidemiological evaluation of risk factors for tuberculosis in South India: A matched case control study. Int J Tuberc Lung Dis. 2006;10:80–6. [PubMed] [Google Scholar]
- 24.Gajalakshmi V, Peto R. Smoking, drinking and incident tuberculosis in rural India: Population-based case-control study. Int J Epidemiol. 2009;38:1018–25. doi: 10.1093/ije/dyp225. [DOI] [PubMed] [Google Scholar]
- 25.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: The epidemiology and the response. Clin Infect Dis. 2010;50(Suppl 3):S201–7. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- 26.Murray M, Oxlade O, Lin HH. Modeling social, environmental and biological determinants of tuberculosis. Int J Tuberc Lung Dis. 2011;15(Suppl 2):S64–70. doi: 10.5588/ijtld.10.0535. [DOI] [PubMed] [Google Scholar]
- 27.Bhargava A, Chatterjee M, Jain Y, Chatterjee B, Kataria A, Bhargava M, et al. Nutritional status of adult patients with pulmonary tuberculosis in rural central India and its association with mortality. PLoS One. 2013;8:e77979. doi: 10.1371/journal.pone.0077979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padmapriyadarsini C, Shobana M, Lakshmi M, Beena T, Swaminathan S. Undernutrition & tuberculosis in India: Situation analysis & the way forward. Indian J Med Res. 2016;144:11–20. doi: 10.4103/0971-5916.193278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.New Delhi: Directorate General of Health Services, Ministry of Health & Family Welfare; 2013. Central TB Division. Revised National Tuberculosis Control Programme Social Action Plan (Including the Tribal Action Plan) [Google Scholar]