Abstract
Introduction
Acne, a very common skin disease, can result in psychological distress and sustain impairment in quality of life. Data on the prevalence of acne and the differences in gender, region and age are limited. The aim of this review is to estimate the prevalence of acne in Mainland China comprehensively and to quantify its association with gender, region and age.
Methods
We searched electronic databases with predetermined search terms to identify relevant studies published between 1 January 1996 and 30 September 2016. We pointed out repeated results using Note Express software and evaluated the studies for inclusion. Two independent reviewers extracted the data, followed with statistical analyses using Comprehensive Meta-Analysis software version 2.0. A random effects model was adopted to calculate the overall pooled prevalence and to merge categories, including gender (males and females), region (Northern China and Southern China) and age (primary and secondary students: 7–17 years old; undergraduates: 18–23 years old; overall: no limits of age) for subgroup analyses. Logistic meta-regression analysis was used to clarify the associations between acne and the predictors age, gender and region using OR and their associated 95% CI.
Results
25 relevant studies were included in this meta-analysis. The overall pooled prevalence rates of acne were 39.2% (95% CI 0.310 to 0.479). The prevalence rates in different age groups were 10.2% overall (95% CI 0.059 to 0.171), 50.2% for primary and secondary students (95% CI 0.451 to 0.554), and 44.5% for undergraduates (95% CI 0.358 to 0.534); by gender, the prevalence rates were 35.7% for females (95% CI 0.275 to 0.448) and 39.7% for males (95% CI 0.317 to 0.482); and by region, the prevalence rates were 34.2% for Northern China (95% CI 0.242 to 0.458) and 46.3% for Southern China (95% CI 0.374 to 0.555). The associations between acne and the predictors age, gender and region were statistically significant.
Conclusions
In Mainland China, primary and secondary students exhibited higher prevalence rates than undergraduate students; males had higher prevalence rates of acne than females; and the prevalence rates of acne in Southern China was higher than Northern China.
Keywords: Acne, Dermatological epidemiology
Strengths and limitations of this study.
To our knowledge, this was the first systemic review evaluating the overall acne prevalence in Mainland China and analysing its associations with age, gender and region.
This systematic review, covering 12 different provinces, was composed of 25 studies, with 80 000 people over a 41-year period.
Some limitations should be considered: the diagnostic criteria for acne differed among the included studies; the age of the survey population could not be subdivided. Moreover due to heterogeneity among papers, the samples changed from variable to variable.
Introduction
Acne, a very common skin disease among adolescents, is the fourth most common reason for patients aged 11–21 years to visit a doctor in the USA.1 Acne is estimated to affect 9.4% of the world's population with the highest prevalence in adolescents.2 Acne vulgaris-associated disease burden exhibits global distribution and has continued to grow in prevalence over time within this population.3 In addition, a group of increasing epidemiological data suggests that acne also affects a considerable number of adults,4 and women are more frequently affected by adult acne than men.5 The incidence of acne is different from various countries and ethnic groups. The prevalence of acne is reported to be low in developing countries of Africa.6 7 In Northern Tanzania, the prevalence of acne is reported to be 0.1%,8 whereas the prevalence of acne is 3.9% in the German population aged between 16 and 70 years.9 However, a comprehensive systemic review of the prevalence of acne in the Chinese population is still lacking. Several studies have shown that the estimated prevalence rate of acne varies from 8.1% to 85.1% in China10–12 depending on the region, subjects’ ages and nationality studied.
Acne is a chronic inflammatory disease of the pilosebaceous unit resulting from androgen-induced increased sebum production, altered keratinisation, inflammation and bacterial colonisation of hair follicles by Propionibacterium acnes.13 Acne vulgaris alters the normal skin physiology, impairing stratum corneum and causing transepidermal water loss.14 The clinical features of acne include papules, pustules, cysts, comedos and nodules. Acne occurs primarily on the face, neck and upper trunk and can lead to scar formation if treated improperly. Acne scarring is a frequent complication of acne, and patients often lack effective and safe methods for managing this condition.15 The resulting scars may negatively impact on an affected person's psychosocial and physical well-being. It has been reported that acne can result in psychological distress and have profound effects on the patients’ self-esteem, which may lead to anxiety, depression, diminished self-confidence and communication difficulties.16 17
Many large population-based studies have been conducted to estimate the prevalence rates of acne in regional populations, but a comprehensive statistical analysis of the prevalence of Chinese acne has not emerged so far. China, being a vast region, comprises of 34 province-level administrative regions, with 56 ethnic groups and a population of 1.3 billion people. Therefore, a country-wide, population-based study of the prevalence rates of acne in China was needed. In this review, we examined the prevalence of acne in Mainland China systematically and analysed the effects of gender, region and age on acne.
Methods
Search strategy
Studies were aggregated from six databases between 1 January 1996 and 30 September 2016, including PubMed, EMBASE, Web of Science, the China National Knowledge Infrastructure, the VIP Database for Chinese Technical Periodicals and the Wan Fang Database for Chinese Periodicals. The following search terms were used to retrieve relevant studies: (acne), (prevalence or incidence) AND (China or Chinese). The combination of acne, prevalence, incidence, China and Chinese were used in varying combinations to identify relevant literature. Search strategies were customised to suit each database. The search strategy is presented in online supplementary table 1. In addition, a manual search was performed by checking the reference lists of eligible articles and relevant reviews.
bmjopen-2016-015354supp001.pdf (99.6KB, pdf)
Inclusion criteria
Studies that met all of the following criteria were included in the meta-analysis:
The study was a population-based survey.
The study evaluated the prevalence/incidence of acne.
The investigation involved random sampling or cluster sampling.
The sample size was >300.
A flow chart illustrating the article search process is presented in figure 1.
Figure 1.
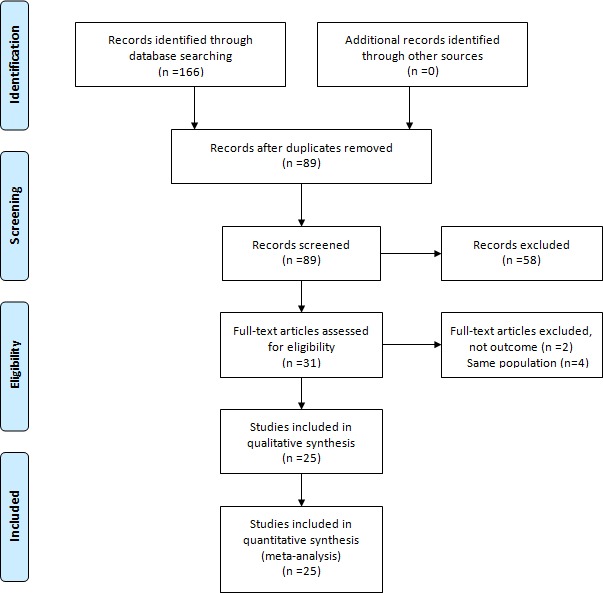
Flow-chart illustrating the article search process. First, we obtained 166 records identified through database searching. No additional records identified through other sources. Second, 89 records remained after duplicates were removed. Third, 58 studies were excluded after records screening. Then the remainder 31 studies were full-text articles assessed for eligibility and 6 studies were excluded. Finally 25 studies were included in the quantitative synthesis (meta-analysis).
Data management
Any duplicate studies was removed by Note Express. Two reviewers (QC and DHL) independently evaluated the title and abstract of all studies identified through the search against the inclusion and exclusion criteria. The full text of all eligible studies were then retrieved. Any disagreement were resolved by a third reviewer. Excluded studies and the reasons for exclusion were recorded.
Data extraction
Customised data information was extracted independently by two investigators. The following information was collected for each study: (1) the name of the first author; (2) the location of the investigation area; (3) the year in which the investigation occurred; (4) the age range of the subjects; (5) the sample size (male, female and overall); (6) the prevalence rates obtained for males, females and overall; (7) the method used to sample subjects; (8) the criteria used to diagnose acne; and (9) the response rate.
Risk of bias (quality) assessment
Four key criteria18 19 were used by two independent investigators (YL and DHL) to estimate study quality: (1) the sampling scheme (random or consecutive); (2) whether the study included an adequate description of the characteristics of the study population; (3) whether a clear definition of the prevalence rate was provided; and (4) whether the response rate exceeded 75%. The scoring rule used was the following, for each quality item, ‘clear or adequate’ was scored as 1 point, whereas ‘no’ was scored as 0 point. The study was considered to be of adequate quality if the quality score was greater than or equal to 3. The two reviewers carefully assessed the included studies independently and agreed on the final grading. An additional reviewer was consulted should there be any uncertainty or disagreement.
Ethics and dissemination
Since primary data were not collected, formal ethical approval was not required. The results will be disseminated through peer-reviewed publications, conference presentations and the media.
Statistical analysis
The statistical analyses were performed using Comprehensive Meta-Analysis software V.2.0 (Biostat, Englewood Cliffs, New Jersey, USA; https://www.meta-analysis.com). We calculated the pooled prevalence estimates as proportions and 95% CIs for the subgroup categories weighted by the sample sizes of the individual studies and then pooled these data to derive an overall proportion and its associated 95% CI. The heterogeneity of the pooled prevalence was estimated using the χ2-based Q statistic. Meanwhile, I2 metrics were calculated to quantify heterogeneity. A random effects model was used if heterogeneity was observed (p<0.05); otherwise, a fixed effects model was applied. All studies were classified into one of two groups (North versus South) according to the geographical regions in which the studies were conducted. Similarly, the studies were classified into three groups based on the age of the participants in the samples, overall (no limits of age), undergraduate (18–23 years old) and primary and secondary students (‘p and s’, 7–17 years old). Subjects were also classified as male and female. The associations of age, gender and region with the prevalence of acne were evaluated using a logistic meta-regression model. The associations between age, gender, region and risk of acne were expressed as ORs and 95% CIs. A value of p<0.05 was considered statistically significant.
Analysis of the publication bias
To examine the authenticity of data, the Egger test and Funnel plots were produced using the Comprehensive Meta-Analysis software V.2.0. No publication bias exists if the studies arranged symmetrically around the central line with a p value of >0.05.
Results
Characteristics of the studies
Using the initial search strategy, 166 studies were identified. One hundred and forty-one studies were subsequently excluded (figure 1). A total of 25 studies10–12 20–41 involving 83 008 Chinese individuals were included in this meta-analysis. Detailed characteristics of each included study are presented in table 1. The overall quality of all included studies was found to be adequate (table 2).
Table 1.
Characteristics of the populations examined in studies reporting the prevalence of acne in Mainland China
| Name | Region | Year | Age range | Sample size | Prevalence (%) | ||
| Male % (n) | Female % (n) | Total (%) | |||||
| Zhang Feng27 | Dong guan | 2013 | Undergraduate | 669 | 40.37 (379) | 34.48 (290) | 37.82(253) |
| Liu Lijuan35 | Shi jiazhuang | 2014 | Undergraduate | 742 | 42.11 (418) | 36.11 (324) | 39.49(293) |
| Liu Qing36 | Zi bo | 2013 | 12–49 | 1455 | 9.4 (504) | 11.5 (951) | 8.32(121) |
| Li Shengjie34 | Tai an | 2010 | 17–24 | 1416 | 39.85 (532) | 46.61 (884) | 44.07(624) |
| Zhang Zhiyong40 | Han dan | 2010 | Undergraduate | 1582 | 59.71 (834) | 50.00 (748) | 55.12(872) |
| Cai Luanduan25 | Guang dong | 2010 | 9–18 | 2015 | 49.90 (986) | 59.86 (1029) | 54.99(1108) |
| Zhang Jian20 | Yi chun | 2011 | 18–26 | 448 | 45.16 (217) | 32.03 (231) | 38.39(172) |
| Wang Renli41 | Si chuan | 2008–2009 | All | 10,503 | 19.3 (4319) | 16.9 (6184) | 18.1(1901) |
| Pei Guangde37 | He nan | 2008 | All | 1547 | 8.80 (742) | 7.86 (805) | 8.39(129) |
| Chen Ying21 | Guang zhou | 2008 | Undergraduate | 2252 | 34.7 (1253) | 38.4 (999) | 36.4(819) |
| Zhang Hong39 | Jiang men | 2006–2008 | 17–18 | 12 450 | 55.9 (7134) | 46.02 (5136) | 51.83(6453) |
| Deng Bin31 | Hai nan | 2005 | 16–23 | 3500 | 34.8 (1990) | 32.4 (1510) | 33.8(1183) |
| Cui Jianping30 | Xing tai | 2007 | 10–18 | 2891 | 43.14 (1370) | 35.44 (1521) | 39.1(1130) |
| Li Min 22 | Fu jian | 2007 | 15–23 | 1484 | 69.5 (836) | 63.6 (648) | 66.9(992) |
| Feng Jieying24 | Guang zhou | 2004–2005 | 11–19 | 1561 | 51.95 (743) | 60.76 (818) | 56.57(883) |
| Gao Aili32 | Guang zhou | 2004 | 12–20 | 2552 | 34.2 (1151) | 31.6 (1305) | 32.8(837) |
| Wu Tieqiang26 | Zhu hai | 2006 | 10–18 | 3200 | 54.9 (1790) | 51.6 (1410) | 53.5(17182) |
| Sun Zhenxiu23 | Nan jing | 2005 | 16–23 | 2100 | 37.91 (1000) | 22 (1100) | 30.33(637) |
| Liu Zhaorui28 | Bei jing | 2003 | 16–17 | 4933 | 67.1 (2364) | 57.3 (2569) | 62.0(3058) |
| Zhang Aihua38 | Tai an, He ze | 1996 | 14–23 | 1510 | 38.24 (829) | 23.06 (581) | 20.99(317) |
| Fu Rui29 | Ji ning | 2012 | 11–17 | 2560 | 45.40 (1343) | 47.80 (1177) | 46.51(1190) |
| Li Bing33 | Shan xi | 2012 | 12–17 | 741 | 55.8 (335) | 49.2 (406) | 52.2(387) |
| Shen Yiwei 10 | China | 2012 | All | 17 345 | 10.4 (7858) | 6.1 (9487) | 8.1(1405) |
| Law MP11 | Hong Kong | 2010 | Undergraduate | 389 | N/A | N/A | 85.1(331) |
| Wu TQ12 | Guang dong | 2007 | 10–18 | 3163 | 51.3 (1785) | 58.6 (1378) | 53.5(1692) |
(n), the number of participants, N/A, not available.
Table 2.
Methodological quality of the studies reporting the prevalence of acne in Mainland China
| Name | Sampling scheme | Population characteristics | Prevalence definition | Diagnostic criteria | Response rate % | Score |
| Zhang Feng27 | Random stratified sample | Adequate | Clear | Clear | 95 | 4 |
| Liu Lijuan35 | Random stratified sample | Adequate | Clear | Clear | N/A | 3 |
| Liu Qing36 | Cluster sampling | Adequate | Clear | Clear | 48.5 | 3 |
| Li Shengjie34 | Random stratified sample | Adequate | Clear | Clear | 100 | 4 |
| Zhang Zhiyong40 | Cluster sampling | Adequate | Clear | Clear | 97.75 | 4 |
| Cai Luanduan25 | Random stratified sample | Adequate | Clear | Clear | 98.05 | 4 |
| Zhang Jian20 | Random stratified sample | Adequate | Clear | Clear | N/A | 3 |
| Wang Renli41 | Random stratified sample | Adequate | Clear | Clear | N/A | 3 |
| Pei Guangde37 | Random stratified sample | Adequate | Clear | Clear | 53.4 | 3 |
| Chen Ying21 | Random stratified sample | Adequate | Clear | Clear | 90.8 | 4 |
| Zhang Hong39 | Cross-sectional | Adequate | Clear | Clear | N/A | 3 |
| Deng Bin31 | Random stratified sample | Adequate | Clear | Clear | N/A | 3 |
| Cui Jianping30 | Random stratified sample | Adequate | Clear | Clear | 98.1 | 4 |
| Li Min22 | Random stratified sample | Adequate | Clear | Clear | N/A | 3 |
| Feng Jieying24 | Random stratified sample | aAequate | Clear | Clear | 98.24 | 4 |
| Gao Aili32 | Cross-sectional | Adequate | Clear | Clear | 96.2 | 4 |
| Wu Tieqiang26 | Cross-sectional | Adequate | Clear | Clear | 98.84 | 4 |
| Sun Zhenxiu23 | Random stratified sample | Adequate | Clear | Clear | N/A | 3 |
| Liu Zhaorui28 | Random stratified sample | Adequate | Clear | Clear | N/A | 3 |
| Zhang Aihua38 | Random stratified sample | Adequate | Clear | Clear | N/A | 3 |
| Fu Rui29 | Cross-sectional | Adequate | Clear | Clear | 99.06 | 4 |
| Li Bing33 | Random stratified sample | Adequate | Clear | Clear | 95.2 | 4 |
| Shen Yiwei 10 | Community-based study | Adequate | Clear | Clear | 86.84 | 4 |
| Law MP11 | Cross-sectional | Adequate | No | Clear | 99.3 | 4 |
| Wu TQ12 | Cross-sectional | Adequate | Clear | Clear | 98.8 | 4 |
N/A, not available.
The overall prevalence rates of acne in Mainland China
A total of 25 studies involving 83 008 Chinese people were included in this meta-analysis. However, there was significant heterogeneity in this meta-analysis (I2=99.797%, Q=11 823.369, p<0.001). Thus, a random effects model was selected for the analysis. The overall pooled prevalence of acne was 39.2% (95% CI 0.310 to 0.479; figure 2).
Figure 2.
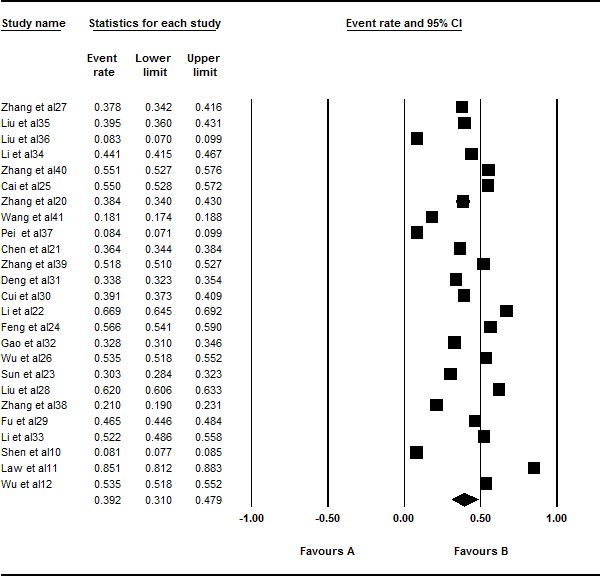
Forest plot of the overall prevalence rates of acne. A total of 25 studies were included in this meta-analysis. Through analysing in a random effects model, the overall pooled prevalence of acne was calculated as 39.2% (95% CI 0.310 to 0.479).
The prevalence rates of acne in Mainland China by gender
A total of 24 studies involving 40 712 males and 41 907 females were included in this subgroup meta-analysis. However, there was significant heterogeneity among both the males (I2=99.582%, Q=5508.959, p<0.001) and females (I2=99.614%, Q=5957.125, p<0.001) in this meta-analysis. Thus, a random effects model was selected for the analysis. Males (39.7%, 95% CI 0.317 to 0.482) exhibited a higher prevalence of acne than females (35.7%, 95% CI 0.275 to 0.448, Z=3.903, p<0.001) in the subgroup analysis (figure 3).
Figure 3.
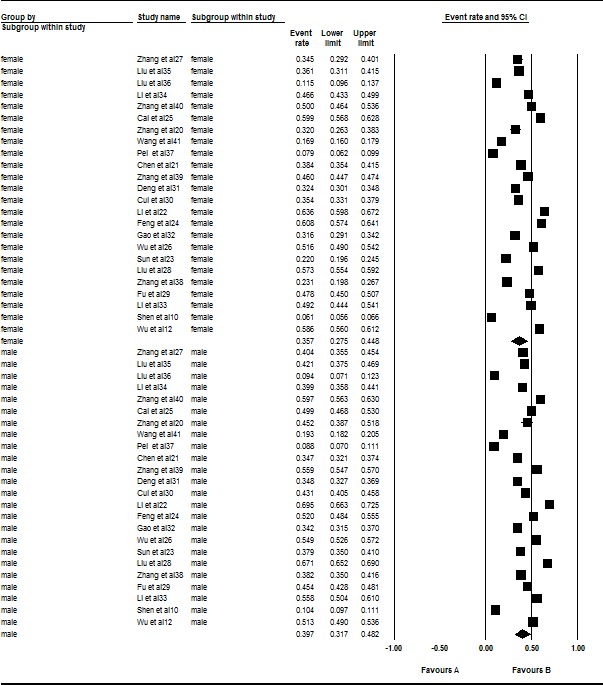
Forest plot of the prevalence rates of acne according to gender. A total of 24 studies were included in this subgroup meta-analysis. Through analysing in a random effects model, the prevalence rates of acne were 39.7% (95% CI 0.317 to 0.482) in males and 35.7% (95% CI 0.275 to 0.448) in females. Males (39.7%) exhibited a higher prevalence of acne than did females (35.7%) in the subgroup analysis (Z=3.903, p<0.001).
The prevalence rates of acne in Mainland China by region
A total of 24 studies involving 65 663 Chinese were included in this subgroup meta-analysis. Ten of these studies were conducted in Northern China (19 377 Chinese), and 14 studies were conducted in Southern China (46 286 Chinese). However, significant heterogeneity was found in both the North (I2=99.573%, Q=2109.204, p<0.001) and the South (I2=99.689%, Q=4176.791, p<0.001) subgroups in this meta-analysis. Thus, a random effects model was selected for the analysis. The South (46.3%, 95% CI 0.374 to 0.555) had a higher prevalence of acne than the North (34.2%, 95% CI 0.242 to 0.458, Z=2.498, p=0.012) in the subgroup analysis (figure 4).
Figure 4.
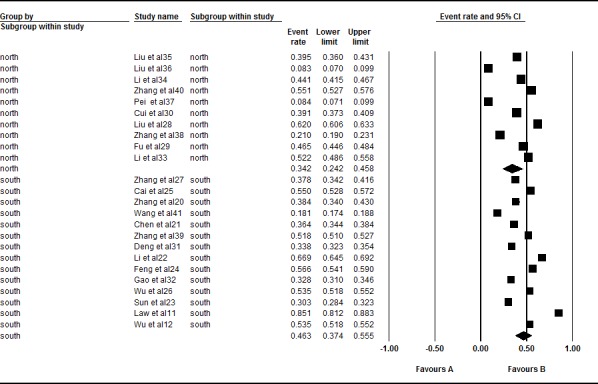
Forest plot of the prevalence rates of acne according to region. A total of 24 studies were included in this subgroup meta-analysis. Ten of these studies were conducted in Northern China and 14 studies were conducted in Southern China. Through analysing in a random effects model, the prevalence rates of acne in South was 46.3% (95% CI 0.374 to 0.555) and 34.2% in North (95% CI 0.242 to 0.458). The South (46.3%) had a higher prevalence of acne than the North (34.2%) in the subgroup analysis (Z=2.498, p=0.012).
The prevalence rates of acne in Mainland China by age
A total of 25 studies, consisting of four studies that examined the overall population, 10 p and s studies, and 11 undergraduate studies, were included in this subgroup meta-analysis. However, significant heterogeneity was found overall (I2=99.534%, Q=643.149, p<0.001), for the p and s subgroup (I2=98.860%, Q=789.719, p<0.001) and for the undergraduates (I2=99.130%, Q=1149.658, p <0.001) in this meta-analysis. Thus, a random effects model was selected for the analysis. The prevalence of acne overall ages was 10.2% (95% CI 0.059 to 0.171). The p and s students (50.2%, 95% CI 0.451 to 0.554) had a higher prevalence of acne than did the undergraduates (44.5%, 95% CI 0.358 to 0.534, Z=2.411, p=0.016) in the subgroup analysis (figure 5).
Figure 5.
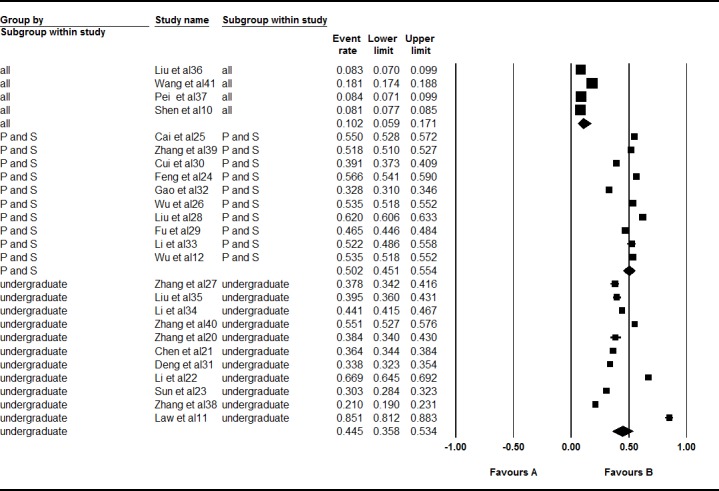
Forest plot of the prevalence rates of acne according to age. All=overall age; p and s=primary and secondary students. A total of 25 studies, consisting of four studies that examined the overall population, 10 p and s studies and 11 undergraduate studies were included in this subgroup meta-analysis. Through analysing in a random effects model, the prevalence of acne over all ages was 10.2% (95% CI 0.059 to 0.171). The prevalence of acne in the primary and secondary students was 50.2% (95% CI 0.451 to 0.554) and 44.5% (95% CI 0.358 to 0.534) in the undergraduates. The primary and secondary students (50.2%) had a higher prevalence of acne than the undergraduates (44.5%) in the subgroup analysis (Z=2.411, p=0.016).
Logistic meta-regression analysis of the associations between age, gender and region and the prevalence of acne
The associations between acne and the predictors age, gender and region were statistically significant (table 3) as measured by the corresponding ORs and their associated 95% CIs. The OR of acne prevalence was 1.217 (95% CI 0.109 to 14.681, p=0.024) between male and female subgroups. Suggesting that Chinese males might be more susceptible to acne than females. The OR between Southern China and Northern China was 1.184 (95% CI 0.002 to 3.833, p=0.028) for acne. The geographical factors of Southern China might be a risk factor for acne. The OR between p and s students and undergraduate was 3.127 (95% CI 0.001 to 3.838, p=0.012) for acne; therefore, age might have a vital role in the development of acne.
Table 3.
Adjusted OR for acne obtained by logistic meta-regression analysis
| Factor | Number of studies | OR (95% CI) | p Value |
| Age of range | |||
| Undergraduate | 11 | 1.00 | |
| Primary and secondary school students | 10 | 3.127 (0.001 to 3.838) | 0.012 |
| Gender | |||
| Female | 24 | 1.00 | |
| Male | 24 | 1.217 (0.109 to 14.681) | 0.024 |
| Location | |||
| North | 10 | 1.00 | |
| South | 14 | 1.184 (0.002 to 3.833) | 0.028 |
Analysis of the publication bias
There were no obvious asymmetries in the Funnel plots and the p-value exceeded 0.05 for the following groups: the overall pooled studies (t=0.030, p=0.976, 95% CI −0.215 to 0.222) (see online supplementary figure 1), male subgroup (t=0.346, p=0.733, 95% CI −0.185 to 0.132) (see online supplementary figure 2), female subgroup (t=0.143, p=0.887, 95% CI −0.164 to 0.188) (see online supplementary figure 3), subgroup of all age layers (t=0.501, p=0.666, 95% CI −0.831 to 0.658) (see online supplementary figure 4), subgroup of p and s students (t=0.580, p=0.578, 95% CI −0.246 to 0.147) (see online supplementary figure 5), subgroup of undergraduates (t=1.061, p=0.316, 95% CI −0.121 to 0.335) (see online supplementary figure 6) and Southern China subgroup (t=0.441, p=0.667, 95% CI −0.194 to 0.293) (see online supplementary figure 7). Only the studies of the Northern China subgroup had publication bias (t=3.369, p=0.01, 95% CI −0.520 to −0.974) (see online supplementary figure 8).
bmjopen-2016-015354supp002.jpg (15.8KB, jpg)
bmjopen-2016-015354supp003.jpg (15.8KB, jpg)
bmjopen-2016-015354supp004.jpg (15.8KB, jpg)
bmjopen-2016-015354supp005.jpg (15.8KB, jpg)
bmjopen-2016-015354supp006.jpg (15.8KB, jpg)
bmjopen-2016-015354supp007.jpg (15.8KB, jpg)
bmjopen-2016-015354supp008.jpg (15.8KB, jpg)
bmjopen-2016-015354supp009.jpg (15.8KB, jpg)
Discussion
This meta-analysis, based on 83 008 subjects, derived from 25 studies, covering 12 provinces and municipalities in China, enables us to assess reliable prevalence rates of acne at the national level. Our results showed that the overall pooled prevalence of acne was 39.2%. Other studies have reported a range of acne prevalence from different countries, such as 3.9% in the German population aged between 16 and 70 years9 and 61.5% in the Portugal population between the ages of 20 and 60 years old.42 The differences in age range, ethnic background, regions and pollution might explain the difference in the incidence of acne. Skin is the outermost barrier and various air pollutants such as oxides, particulate matter, ultraviolet (UV) radiation, polycyclic aromatic hydrocarbons and the ozone can all affect the skin. Puri43 reported that air pollutants can cause damage to the skin by inducing oxidative stress, and prolonged or repetitive exposure to high levels of these pollutants may have profound negative effects on the skin. The various degrees of air pollution in different countries over the years have had different effects on the human skin. Males (39.7%) exhibited a higher prevalence of acne than females (35.7%, Z=3.903, p<0.001) in the subgroup analysis of gender. Meanwhile, the South (46.3%) had a higher prevalence of acne than the North (34.2%, Z=2.498, p=0.012) in the regional subgroup analysis. Moreover, the primary and secondary students (50.2%) had a higher prevalence of acne than undergraduates (44.5%, Z=2.411, p=0.016) in the subgroup analysis of age. There was heterogeneity among the 25 included studies in this meta-analysis (I2=99.797%, Q=11 823.369, p<0.001), and the following reasons might explain the phenomenon: (1) Differences existed in the design of studies, such as non-unified age range, collection of data and research objects, explaining the deviation of the prevalence of acne. (2) Evidence for seasonality was observed, with lower lipid production and reduced barrier function during the winter.44 A correlation between the incidence of acne and skin surface lipid34 45 has been observed before, thus the included articles may have been conducted in different seasons,26 29 35 40 which resulted in different incidences of acne.
The age-related subgroup prevalence rates were also calculated in this study. The prevalence of primary and secondary students was 50.2%, which was consistent with the results of Wei et al 46 (51.3%). This result was similar to those reported by Karciauskiene et al’s47 results among schoolchildren aged 7–19 years, which was 55.4% in Lithuania. However, these results are lower than a study conducted in Brazil, where a prevalence rate of 96% was found in adolescents aged 10–1748. This difference may also be attributed to the different age range, regions and ethnic backgrounds of the study subjects. Our results suggest that primary and secondary students (50.2%) had a higher prevalence of acne than undergraduates (44.5%, Z=2.411, p=0.016), which is in agreement with the findings of Shen et al.10 Moreover, using Logistic meta-regression analysis, we found that the OR between primary and secondary school students and undergraduate was 3.127 (95% CI 0.001 to 3.838, p=0.012) for acne. Vilar et al 49 and Yentzer et al 50 also reported that primary and secondary students (89.3%) had a higher prevalence of acne than the undergraduate subjects (61.9%) in the US population. Choi et al 51 reported that in patients with late onset acne, the number of comedones and total number of acne lesions and the proportions of comedones were significantly less than in patients with early onset acne. The age of onset had a negative correlation with the number of comedones and the proportion of comedones in the T-zone and the entire face, namely, acne. The clinical differences in acne based on age appeared to be mainly a result of age of onset and not from the progression of the acne.52 53
In the present study, males (39.7%) had a 1.112 times higher prevalence rate of acne than did females (35.7%, Z=3.903, p<0.001). This result is in agreement with the findings of Schafer et al 54 that acne was more in men (29.9%) than women (23.7%) in the city of Hamburg (Germany). Adityan and Thappa55 showed that the male to female ratio was 1.25:1 in South India. In Auckland, Lello et al 56 reported that males (91%) were more susceptible to acne compared with females (79%) and severe and moderately severe acne were significantly more common in males (OR=2.6, 95% Cl 1.73 < OR <3.9) as well. Furthermore, moderate and severe acne (27.1% and 0.4 l% of patients, respectively) were significantly (p<0.01) common in males (36.2% and 0.9%, respectively) than females (21.2% and 0.2%, respectively) in Egypt.57 This indicated that males had a higher risk of occurrence and development of acne than females. (1) This difference might be related to lifestyle. It is well known to us that males accounted for a larger proportion than females in drinking and smoking.58–60 (2) Males also tend to pay less attention to skin health than females in China. Inappropriate personal hygiene (use of abrasive soaps, harsh detergents and excessive scrubbing) can contribute to the pathological process of acne.61 62 (3) Androgen levels elevation is greater in males than females during adolescence, and increased androgen levels is a risk factor of acne.63 The interplay of growth hormone, insulin and insulin-like growth factor-1 signalling during puberty may also have a causal role in the pathogenesis of acne by influencing adrenal and gonadal androgen metabolism.64 Adult women in different age categories have a lower prevalence of acne than adolescence. But recent research has shown that acne is affecting an increasing number of adults, particularly females. Adult female acne should be considered as a specific acne subtype distinct from adolescent acne, and a review on the topical and oral treatment options suitable for treating acne in adult females5 is expected in the future.
The prevalence rates of acne were also different in different regions. The prevalence of acne among individuals in Southern China (46.3%) was higher than those in Northern China (34.2%, Z=2.498, p=0.012). This is consistent with Subramaniyan,65 who considered that the prevalence of acne was different between the East of the Indian mainland and its southern part. This difference may be due to several factors. (1) UV radiation. Enhanced sebum excretion, colonisation of the pilosebaceous duct with P acnes and resultant inflammation were thought to play a critical role in the pathogenesis of acne.66 The function of the sebaceous gland as an endocrine skin organ, which is mainly composed of sebocytes, has an important role in the occurrence and development of acne.67 Excessive sebum production and its abnormal lipid ingredients from the sebaceous gland contributed to the formation of primary acne lesions.68 Sebocytes also produce inflammatory cytokines and they have a vital effect on the formation and aggravation of acne lesions.69 Southern China has longer hours of sunshine than Northern China. Solar radiation contains UV radiation, which is an external environmental factor causing many skin diseases. UV radiation can affect the glands through direct or indirect pathways.70 It is widely accepted that sebaceous gland hyperplasia and increased sebum secretion occur after irradiation of UV-B. The expression of inflammatory cytokines, especially interleukin (IL) 1b, IL-6, IL-8 and tumor necrosis factor (TNF)-α are significantly increased in cultured sebocytes after treatment with UV-B.71 Meanwhile, correlational research showed that time-distinct gene induction of TNF-a, IL-1b and matrilysin in cultured HaCaT cells may be involved in UV-induced cellular responses.72 Although, mRNA levels do not always correspond to protein level in vivo, mRNA levels of inflammatory cytokines such as IL-1, IL-6 and TNF-a are also identified to be increased through studies using whole mouse skin and human skin exposed to UV-B.73 74 Furthermore, it has been known that epithelial keratinocytes contain a functional peroxisome proliferator-activated receptor gamma (PPAR)-gamma system, and this system is a target for UV-B radiation.75 The synthesis of free fatty acids by PPAR-gamma stimulates the production of pro-inflammatory cytokines, such as IL-1b and TNF-a, in sebaceous glands.71 (2) Humidity. There is greater humidity in southern parts of China than Northern China. Subramaniyan65 reported that the prevalence of dermatoses, including acne, is much higher in humid regions than arid regions. (3) Diet. Chili is a popular food for southern residents compared with the northern residents and is consumed in greater quantities than northern residents. Spicy food has been identified as a risk factor for the development of acne.33 In simple terms, both environmental factors (climate and humidity) and diet may lead to the different prevalence rates of acne between Northern and Southern China.
This systematic review is likely limited by the different diagnostic criteria for acne among the included studies. Due to the enormous number of Chinese people, it was difficult to implement an unitive diagnostic criteria. In addition, the age of the survey population could not be subdivided, which made further detailed analysis of age groups impossible. More research are needed on the national prevalence of acne to provide better baseline data and to monitor the effect of acne over time in China.
Conclusion
The overall pooled prevalence rate of acne was 39.2% in Mainland China. Primary and secondary students exhibited higher prevalence rates than undergraduate students. Due to differences in lifestyle, skincare routines and androgen levels, males showed higher prevalence rates of acne than females. Possible etiological factors for the difference in prevalence rates of acne between Southern China and Northern China may be due to the varying UV radiation, humidity and dietary habits. The evidence generated from this paper may prove beneficial in terms of understanding the age and regional distribution and prevalence rates of acne among the Chinese population, which may help in identifying target prevention and treatment strategies for this cohort of patients.
bmjopen-2016-015354supp010.doc (64.5KB, doc)
Supplementary Material
Footnotes
Contributors: SJL and DHL conceived the study and participated in drafting the final manuscript. QC and YL analysed the data and completed the final draft of the manuscript. TTL and WHT prepared all the figures. All authors reviewed the manuscript.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Ziv A, Boulet JR, Slap GB. Utilization of physician offices by adolescents in the united states. Pediatrics 1999;104:35–42. 10.1542/peds.104.1.35 [DOI] [PubMed] [Google Scholar]
- 2. Tan JK, Bhate K. A global perspective on the epidemiology of acne. Br J Dermatol 2015;172 Suppl 1:3–12. 10.1111/bjd.13462 [DOI] [PubMed] [Google Scholar]
- 3. Lynn DD, Umari T, Dunnick CA, et al. . The epidemiology of acne vulgaris in late adolescence. Adolesc Health Med Ther 2016;7:13–25. 10.2147/AHMT.S55832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Su P, Chen Wee Aw D, Lee SH, et al. . Beliefs, perceptions and psychosocial impact of acne amongst singaporean students in tertiary institutions. J Dtsch Dermatol Ges 2015;13:227–33. 10.1111/ddg.12578 [DOI] [PubMed] [Google Scholar]
- 5. Dréno B. Treatment of adult female acne: a new challenge. J Eur Acad Dermatol Venereol 2015;29 Suppl 5:14–19. 10.1111/jdv.13188 [DOI] [PubMed] [Google Scholar]
- 6. Hogewoning AA, Koelemij I, Amoah AS, et al. . Prevalence and risk factors of inflammatory acne vulgaris in rural and urban ghanaian schoolchildren. Br J Dermatol 2009;161:475–7. 10.1111/j.1365-2133.2009.09259.x [DOI] [PubMed] [Google Scholar]
- 7. Cordain L, Lindeberg S, Hurtado M, et al. . Acne vulgaris: a disease of Western civilization. Arch Dermatol 2002;138:1584–90. [DOI] [PubMed] [Google Scholar]
- 8. Gibbs S. Skin disease and socioeconomic conditions in rural africa: tanzania. Int J Dermatol 1996;35:633–9. 10.1111/j.1365-4362.1996.tb03687.x [DOI] [PubMed] [Google Scholar]
- 9. Augustin M, Herberger K, Hintzen S, et al. . Prevalence of skin lesions and need for treatment in a cohort of 90 880 workers. Br J Dermatol 2011;165:865–73. 10.1111/j.1365-2133.2011.10436.x [DOI] [PubMed] [Google Scholar]
- 10. Shen Y, Wang T, Zhou C, et al. . Prevalence of acne vulgaris in Chinese adolescents and adults: a community-based study of 17,345 subjects in six cities. Acta Derm Venereol 2012;92:40–4. 10.2340/00015555-1164 [DOI] [PubMed] [Google Scholar]
- 11. Law MP, Chuh AA, Lee A, et al. . Acne prevalence and beyond: acne disability and its predictive factors among Chinese late adolescents in Hong Kong. Clin Exp Dermatol 2010;35:16–21. 10.1111/j.1365-2230.2009.03340.x [DOI] [PubMed] [Google Scholar]
- 12. Wu TQ, Mei SQ, Zhang JX, et al. . Prevalence and risk factors of facial acne vulgaris among chinese adolescents. Int J Adolesc Med Health 2007;19:407–12. [DOI] [PubMed] [Google Scholar]
- 13. Williams HC, Dellavalle RP, Garner S. Acne vulgaris. The Lancet 2012;379:361–72. 10.1016/S0140-6736(11)60321-8 [DOI] [PubMed] [Google Scholar]
- 14. McCarty M. Evaluation and management of refractory acne vulgaris in adolescent and adult men. Dermatol Clin 2016;34:203–6. 10.1016/j.det.2015.11.007 [DOI] [PubMed] [Google Scholar]
- 15. Abdel Hay R, Shalaby K, Zaher H, et al. . Interventions for acne scars. Cochrane Database Syst Rev 2016;4:CD011946 10.1002/14651858.CD011946.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lauermann FT, Almeida HL, Duquia RP, et al. . Acne scars in 18-year-old male adolescents: a population-based study of prevalence and associated factors. An Bras Dermatol 2016;91:291–5. 10.1590/abd1806-4841.20164405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mulder MM, Sigurdsson V, van Zuuren EJ, et al. . Psychosocial impact of acne vulgaris. evaluation of the relation between a change in clinical acne severity and psychosocial state. Dermatology 2001;203:124–30. [DOI] [PubMed] [Google Scholar]
- 18. Cheng JW, Cheng SW, Ma XY, et al. . The prevalence of primary Glaucoma in mainland China: a systematic review and meta-analysis. J Glaucoma 2013;22:301–6. 10.1097/IJG.0b013e31824083ca [DOI] [PubMed] [Google Scholar]
- 19. Say L, Donner A, Gülmezoglu AM, et al. . The prevalence of stillbirths: a systematic review. Reprod Health 2006;3:1 10.1186/1742-4755-3-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang J. Prevalence of acne in college students and the analysis of psychological condition of the patient. J Yichun Coll 2011;33:89–90. [Google Scholar]
- 21. Ying C, Wu J, Liu Y, et al. . The prevalence and related factors of acne in Guangzhou college students. South Chin J Dermato-Venereology 2009;16:131–2. [Google Scholar]
- 22. Li M, Candong L, Donghua C. Epidemiology investigation of acne in adolescents of different ages. J Gansu Coll Tradit Chin Med 2008;25:27–9. [Google Scholar]
- 23. Zhenxiu S. Investigation of the incidence of acne in college students. Chin J Leprosy Skin Dis 2005;21:994–5. [Google Scholar]
- 24. Jieying F, Ruiqiang F. Epidemiology of acne of 1561 middle school students in Guangzhou. South Chin J Dermato-Venereol 2008;15:49–51. [Google Scholar]
- 25. Luanduan C, Dinan Z, Yang L, et al. . Epidemiological investigations of 2015 secondary students of acne. Chin Mod Doctor 2011;49:6–7. [Google Scholar]
- 26. Wu T, Mei S, Zhang J, et al. . Prevalence and risk factors of acne vulgaris in adolescents 10-18 years old. Int J Dermatol Venereol 2006;32:201–4. [Google Scholar]
- 27. Zhang F, Liu J, Lin X, et al. . Analysis of prevalence and awareness of acne of college students in Dongguan City. Med Soc 2014;27:68–70. [Google Scholar]
- 28. Liu Z, Huang Y, Zhang H, et al. . Prevalence of the knowledge and attitude behavior on acne of students grade 2 in senior high school in Beijing. Chin J Dermatol 2003;36:519–20. [Google Scholar]
- 29. Rui F, Furen Z. Epidemiological investigation of acne vulgaris in Jining area junior high school students. Chin J Leprosy Skin Dis 2014;30:214–5. [Google Scholar]
- 30. Jianping C, Dapeng D, Yun S. Prevalence of acne among primary and middle school students in urban and rural area of Xingtai city. Chin J School Health 2008;29:995–6. [Google Scholar]
- 31. Bin D, Zeng H, Yongjiang D, et al. . Survey of acne vulgaris in college students in Hainan Province and logistic analysis of risk factors. Chin Trop Med 2008;8:1867–8. [Google Scholar]
- 32. Gao A, Zhang H, Zeng H, et al. . The prevalence and risk factors analysis of adolescent acne in Guangzhou City tianhe district. Chin J Leprosy Skin Dis 2007;23:1052–3. [Google Scholar]
- 33. Bing L, Cangzhen H, Yanjie W. Investigation and analysis of risk factors for the occurrence of acne among middle school students in Weinan City. Chin Health Care Nutri 2012;22:2444–5. [Google Scholar]
- 34. Shengjie L, Zhang Y, Zheng G, et al. . Investigation and analysis of sick status and influencing factors on the undergraduates facial acne vulgaris in Taian. Chin J Dermato-venereol 2012;26:625–7. [Google Scholar]
- 35. Liu L, Shiheng Song LC, et al. . Prevalence and awareness of risk factors of acne among college students in Shijiazhuang City. Chin J Dermato-venereol 2014;28:171–2. [Google Scholar]
- 36. Liu Q, Gao X, Liu X, et al. . An epidemiological survey on acne in zibo district, shandong province. J Prac Dermatol 2013;16:149–51. [Google Scholar]
- 37. Pei G, Du J, Huang Y, et al. . An epidemiological survey on acne in Jiaozuo district of henan province. Chin J Dermato-venereol 2010;24:1129–31. [Google Scholar]
- 38. Aihua Z, Qingjuan G, Wanfa R, et al. . Investigation on incidence rate of acne in youth. Hebei Medicine 1996;2:78–9. [Google Scholar]
- 39. Hong Z, Xiaobing H, Lichun F. The situation and strategies of prevention about acne of the Jiangmen middle-school students. J Prac Dermatol 2009;2:14–16. [Google Scholar]
- 40. Zhiyong Z, Ziyin L, Hui L, et al. . Investigation of acne about related factors of college students in Handan City. J Hainan Med Univ 2011;17:1718–20. [Google Scholar]
- 41. Wang R, Ma A, Zhang X, et al. . A survey about the prevalence of acne in Liangshan district of sichuan province. Chin J Dermatol 2010;43:875–7. [Google Scholar]
- 42. Semedo D, Ladeiro F, Ruivo M, et al. . Adult acne: prevalence and portrayal in primary healthcare patients, in the greater Porto Area, Portugal. Acta Med Port 2016;29:507–13. 10.20344/amp.6626 [DOI] [PubMed] [Google Scholar]
- 43. Puri P, Nandar SK, Kathuria S, et al. . Effects of air pollution on the skin: a review. Indian J Dermatol Venereol Leprol 2017;7:1–9. [DOI] [PubMed] [Google Scholar]
- 44. Meyer K, Pappas A, Dunn K, et al. . Evaluation of seasonal changes in facial skin with and without acne. J Drugs Dermatol 2015;14:593–601. [PubMed] [Google Scholar]
- 45. Ikaraoha CI, Ikaraocha CI, Taylor GO, et al. . Pattern of skin surface lipids in some south-western nigerians with acne vulgaris. West Afr J Med 2004;23:65–8. 10.4314/wajm.v23i1.28086 [DOI] [PubMed] [Google Scholar]
- 46. Wei B, Pang Y, Zhu H, et al. . The epidemiology of adolescent acne in north east China. J Eur Acad Dermatol Venereol 2010;24:953–7. 10.1111/j.1468-3083.2010.03590.x [DOI] [PubMed] [Google Scholar]
- 47. Karciauskiene J, Valiukeviciene S, Stang A, et al. . Beliefs, perceptions, and treatment modalities of acne among schoolchildren in Lithuania: a cross-sectional study. Int J Dermatol 2015;54:e70–e78. 10.1111/ijd.12753 [DOI] [PubMed] [Google Scholar]
- 48. Bagatin E, Timpano DL, Guadanhim LR, et al. . Acne vulgaris: prevalence and clinical forms in adolescents from são paulo, Brazil. An Bras Dermatol 2014;89:428–35. 10.1590/abd1806-4841.20142100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vilar GN, Santos LA, Sobral Filho JF. Quality of life, self-esteem and psychosocial factors in adolescents with acne vulgaris. An Bras Dermatol 2015;90:622–9. 10.1590/abd1806-4841.201533726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yentzer BA, Hick J, Reese EL, et al. . Acne vulgaris in the United States, a descriptive epidemiology. Cutis 2010;86:94–9. [PubMed] [Google Scholar]
- 51. Choi CW, Lee DH, Kim HS, et al. . The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol 2011;25:454–61. 10.1111/j.1468-3083.2010.03813.x [DOI] [PubMed] [Google Scholar]
- 52. Williams C, Layton AM. Persistent acne in women : implications for the patient and for therapy. Am J Clin Dermatol 2006;7:281–90. [DOI] [PubMed] [Google Scholar]
- 53. Marks R. Acne and its management beyond the age of 35 years. Am J Clin Dermatol 2004;5:459–62. 10.2165/00128071-200405060-00011 [DOI] [PubMed] [Google Scholar]
- 54. Schäfer T, Nienhaus A, Vieluf D, et al. . Epidemiology of acne in the general population: the risk of smoking. Br J Dermatol 2001;145:100–4. 10.1046/j.1365-2133.2001.04290.x [DOI] [PubMed] [Google Scholar]
- 55. Adityan B, Thappa DM. Profile of acne vulgaris--a hospital-based study from south India. Indian J Dermatol Venereol Leprol 2009;75:272–8. 10.4103/0378-6323.51244 [DOI] [PubMed] [Google Scholar]
- 56. Lello J, Pearl A, Arroll B, et al. . Prevalence of acne vulgaris in Auckland senior high school students. N Z Med J 1995;108:287–9. [PubMed] [Google Scholar]
- 57. El-Khateeb EA, Khafagy NH, Abd Elaziz KM, et al. . Acne vulgaris: prevalence, beliefs, patients' attitudes, severity and impact on quality of life in Egypt. Public Health 2014;128:576–8. 10.1016/j.puhe.2014.03.009 [DOI] [PubMed] [Google Scholar]
- 58. Kegler MC, Hua X, Solomon M, et al. . Factors associated with support for smoke-free policies among government workers in six chinese cities: a cross-sectional study. BMC Public Health 2014;14:1130 10.1186/1471-2458-14-1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang MJ. The chinese drinking problem: a review of the literature and its implication in a cross-cultural study. Kaohsiung J Med Sci 2002;18:543–50. [PubMed] [Google Scholar]
- 60. Yang X, Lu X, Wang L, et al. . Common variants at 12q24 are associated with drinking behavior in han chinese. Am J Clin Nutr 2013;97:545–51. 10.3945/ajcn.112.046482 [DOI] [PubMed] [Google Scholar]
- 61. Kilkenny M, Merlin K, Plunkett A, et al. . The prevalence of common skin conditions in australian school students: 3. acne vulgaris. Br J Dermatol 1998;139:840–5. 10.1046/j.1365-2133.1998.02510.x [DOI] [PubMed] [Google Scholar]
- 62. Alexis AF, Lamb A. Concomitant therapy for acne in patients with skin of color: a case-based approach. Dermatol Nurs 2009;21:1. [PubMed] [Google Scholar]
- 63. Henze C, Hinney B, Wuttke W. Incidence of increased androgen levels in patients suffering from acne. Dermatology 1998;196:53–4. 10.1159/000017867 [DOI] [PubMed] [Google Scholar]
- 64. Kumari R, Thappa DM. Role of insulin resistance and diet in acne. Indian J Dermatol Venereol Leprol 2013;79:291–9. 10.4103/0378-6323.110753 [DOI] [PubMed] [Google Scholar]
- 65. Subramaniyan R. Pattern of dermatoses among nicobarese in a community health camp at Nancowry, andaman and Nicobar islands. Indian J Dermatol 2016;61:187–9. 10.4103/0019-5154.177766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cunliffe WJ. The sebaceous gland and acne-40 years on. Dermatology 1998;196:9–15. 10.1159/000017859 [DOI] [PubMed] [Google Scholar]
- 67. Gollnick HP, Dreno B. Pathophysiology and management of acne. J Eur Acad Dermatol Venereol 2015;29 Suppl 4:1–2. 10.1111/jdv.13182 [DOI] [PubMed] [Google Scholar]
- 68. Zouboulis CC. Acne and sebaceous gland function. Clin Dermatol 2004;22:360–6. 10.1016/j.clindermatol.2004.03.004 [DOI] [PubMed] [Google Scholar]
- 69. Zouboulis CC, Adjaye J, Akamatsu H, et al. . Human skin stem cells and the ageing process. Exp Gerontol 2008;43:986–97. 10.1016/j.exger.2008.09.001 [DOI] [PubMed] [Google Scholar]
- 70. Roberts JE, Light RJE. Light and immunomodulation. Ann N Y Acad Sci 2000;917:435–45. [DOI] [PubMed] [Google Scholar]
- 71. Lee WJ, Park KH, Sohn MY, et al. . Ultraviolet B irradiation increases the expression of inflammatory cytokines in cultured sebocytes. J Dermatol 2013;40:993–7. 10.1111/1346-8138.12330 [DOI] [PubMed] [Google Scholar]
- 72. Skiba B, Neill B, Piva TJ. Gene expression profiles of TNF-alpha, TACE, Furin, IL-1beta and matrilysin in UVA- and UVB-irradiated HaCat cells. Photodermatol Photoimmunol Photomed 2005;21:173–82. 10.1111/j.1600-0781.2005.00162.x [DOI] [PubMed] [Google Scholar]
- 73. Murphy GM, Dowd PM, Hudspith BN, et al. . Local increase in interleukin-1-like activity following UVB irradiation of human skin in vivo. Photodermatol 1989;6:268–74. [PubMed] [Google Scholar]
- 74. Scordi IA, Vincek V. Timecourse study of UVB-induced cytokine induction in whole mouse skin. Photodermatol Photoimmunol Photomed 2000;16:67–73. 10.1034/j.1600-0781.2000.d01-6.x [DOI] [PubMed] [Google Scholar]
- 75. Zhang Q, Southall MD, Mezsick SM, et al. . Epidermal peroxisome proliferator-activated receptor gamma as a target for Ultraviolet B radiation. J Biol Chem 2005;280:73–9. 10.1074/jbc.M409795200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-015354supp001.pdf (99.6KB, pdf)
bmjopen-2016-015354supp002.jpg (15.8KB, jpg)
bmjopen-2016-015354supp003.jpg (15.8KB, jpg)
bmjopen-2016-015354supp004.jpg (15.8KB, jpg)
bmjopen-2016-015354supp005.jpg (15.8KB, jpg)
bmjopen-2016-015354supp006.jpg (15.8KB, jpg)
bmjopen-2016-015354supp007.jpg (15.8KB, jpg)
bmjopen-2016-015354supp008.jpg (15.8KB, jpg)
bmjopen-2016-015354supp009.jpg (15.8KB, jpg)
bmjopen-2016-015354supp010.doc (64.5KB, doc)


