Abstract
Objectives
This meta-analysis aims to systematically measure the potential diagnostic value of anti-Helicobacter pylori IgG in urine for infection diagnosis, using all eligible studies published in English and Chinese languages.
Design
The random effect model was used to analyse the pooled sensitivity, specificity, positive likelihood ratio (PLR), negative LR (NLR), diagnostic OR (DOR), together with the summary receiver operator characteristic curve.
Setting
Literature searches of databases including PubMed, EMBASE, MEDLINE, Web of Science, Chinese National Knowledge Infrastructure and Wanfang databases were performed to retrieve studies evaluating the diagnostic value of urine IgG antibody for H.pylori infection.
Primary outcome measure
Twenty-three studies with 4963 subjects were included in the current meta-analysis.
Results
The pooled sensitivity, specificity, PLR, NLR, DOR and area under the curve (AUC) were 0.83 (95% CI 0.82 to 0.85), 0.89 (95% CI 0.88 to 0.90), 8.81 (95% CI 6.37 to 12.2), 0.13 (95% CI 0.09 to 0.2), 73 (95% CI 46.45 to 114.74) and 0.9551, respectively. Subgroup analyses showed that diagnostic accuracy of the urine IgG assay was no different in age, region, study population and assay method.
Conclusions
Anti-H. pylori antibody in urine might serve as a good marker in diagnosing H. pylori infection. However, further validation based on a larger sample is still required.
Keywords: H.pylori, urine IgG antibody, diagnosis, Meta analysis
Strengths and limitations of this study.
A comprehensive search of literature databases was performed to identify all eligible studies that reported the diagnostic performance of an anti-Helicobacter pylori antibody in urine.
The systematic meta-analysis used a standard protocol, strict inclusion criteria, standardised data extraction and independent reviewers.
We first assessed the summary predictive value of anti-H. pylori IgG in urine for infection diagnosis, and additional subgroup analyses based on study population, region, age and assay method were used to explore heterogeneity.
Unpublished research such as conference papers and studies published in languages other than English or Chinese were not included in this meta-analysis, so some relevant research may have been missed.
We selected the cut-off value according to the manufacturer’s recommendations, but this may not have been the most appropriate for specific areas.
Introduction
Helicobacter pylori is a bacterium that chronically infects more than half of the world’s population and plays a causative role in the pathogenesis of chronic gastritis, peptic ulcer diseases, gastric cancer and mucosa-associated lymphoid tissue lymphoma.1–4 The considerable burden of these H. pylori-related outcomes means that there is an acute demand for accurate diagnosis of this infection. Several detection methods have already been developed, such as culture, histological staining, the urea breath test (UBT) and the H. pylori stool antigen test (HpSA), but a simple, non-invasive, inexpensive and accurate diagnostic test remains the goal.
A number of methods have been developed for non-invasive H. pylori infection diagnosis using body fluids. Tests for the detection of serum anti-H. pylori antibodies are widely used because they are relatively straightforward, convenient and economical. Several studies have also reported the presence of specific anti-H. pylori antibodies in body fluids other than serum.5 6 For example, anti-H. pylori IgG is detectable in urine and has been used for the diagnosis of H. pylori infection. If urine samples could be used for the sensitive screening of H. pylori infection, this would be more convenient both for clinical practice and mass screening.
In 1993, Alemohammd et al reported that ELISA was both highly sensitive and specific for the detection of anti-H. pylori antibodies in urine. This was confirmed by another study from Japan.7 These studies laid the groundwork for the development of a urine-based ELISA kit and a rapid immunochromatography (IM) assay for H. pylori diagnosis.8 Evaluation of the IM assay in Japanese asymptomatic adults and patients with gastric disorders showed promising results compared with UBT (sensitivity: 86.3%–99%; specificity: 91.5%–100%).8 9 The use of ELISA to detect H. pylori in Japanese children also revealed high levels of sensitivity and specificity. When compared with 13C-UBT and/or HpSA, the ELISA sensitivity ranged from 92.3% to 94.4%, and specificity from 76.4% to 96.9%.10 11 Different findings were recorded, however, for the same kit when compared with gastrointestinal endoscopic testing for H. pylori, in line with European multicentre studies. Sensitivity and specificity in adults were 89.4% and 68%, respectively,12 and the corresponding figures in children were 63.2% and 97.3%, respectively.13 Subsequently, the accuracy and usefulness of the IM assay have been supported by several trials in different geographical areas, including Japan 14, Turkey,15 Hong Kong and Taiwan,16 the USA,17 and Europe.18
These variations in the sensitivity and specificity of anti-H. pylori IgG urine testing indicate the need for a comprehensive evaluation of the test performance before wider application. Therefore, this systematic review and meta-analysis was conducted to identify whether anti-H. pylori IgG in urine can serve as a valuable test for H. pylori diagnosis.
Methods
Literature search strategy
Literatures of electronic databases including PubMed, EMBASE, MEDLINE, Web of Science, Chinese National Knowledge Infrastructure and Wanfang databases were searched by two independent researchers to identify relevant studies that evaluate the diagnostic value of urine IgG antibody for H.pylori infection. The last search date was 7 January 7 2016. The following search terms (in Title, Abstract or Keywords fields) were combined using Boolean rules: ‘H.pylori’, ‘Helicobacter pylori’, ‘urine IgG antibody’, ‘urine antibody’ (see online supplemental figure 1), with a filter for human studies published in English or Chinese. Two researchers (YG and QL) screened all the titles and abstracts; studies including data on H. pylori and urine IgG levels were read in full text. The reference lists of the selected papers were hand-searched to identify additional available papers. When multiple publications presented results using the same patient cohort, the most recent or the most complete publication was selected for inclusion. Review articles and references of the accepted articles were searched for additional papers.
bmjopen-2016-013248supp001.jpg (104.2KB, jpg)
Literature selection criteria
We included studies that met the following criteria: (1) Anti-H.pylori IgG antibody in urine was detected; (2) Investigation of the diagnostic accuracy of urine IgG of H.pylori compared with culture or histopathology or UBT or HpSA (based on only one or at least two reference methods); (3) Sensitivity, specificity and cut-off values can be found in identified studies or calculated from the provided data; (4) Publication with full text in a peer-reviewed scientific journal. While the exclusion criteria were listed as follows: (1) Studies with insufficient data to construct the 2×2 table; (2) Reviews, letters and conference abstracts; and (3) Publications identified as duplicates. If a study fulfilled the eligibility criteria, it was included in the systematic review. Any discrepancies were resolved with discussion.
Data extraction and quality Assessment of diagnostic accuracy studies (QUADAS)−2 assessment
The following variables were extracted from the original studies in a predefined data extraction form (see table 1): author, ethnicity, year of publication, number of cases, age (adults or children), study population (patients or healthy), reference standard and assay method (ELISA or IM technique). True positives (TPs), false positives (FPs), false negatives (FNs) and true negatives (TNs) for urine IgG antibody diagnoses were included. Extraction of studies was performed independently by two reviewers (YG and QL). Discrepancies were discussed with the third researcher (YY) and agreement was eventually reached. If a study was selected for the systematic review but did not provide data that could be included in the meta-analysis, the authors were contacted via email. If the authors did not reply or did not provide the requested information, then this article would be excluded. QUADAS-2 summary plots are outlined in table 2 and online supplemental figure 2.19
Table 1.
Characteristics of the studies included in the meta-analysis
| Author | Ethnicity | Year | Region | No. of cases | Age | Diseases | Reference standard | Blind design | Assay method | TP(a) | FP(b) | FN(c) | TN(d) |
| Mohammad M | American | 1993 | USA | 306 | Mix | Patient | C,H&E,R | NA. | ELISA | 237 | 6 | 10 | 53 |
| Kiyonori Katsuragi | Japanese | 1998 | Asia | 119 | NA | Mix | U | NA. | ELISA | 69 | 0 | 1 | 49 |
| Hiroto Miwa | Japanese | 1999 | Asia | 132 | Adult | Patient | U | Yes | ELISA | 63 | 5 | 10 | 54 |
| Mototsugu Kato | Japanese | 2000 | Asia | 189 | NA | Patient | C,H,R | NA. | ELISA | 127 | 12 | 5 | 45 |
| Soichiro Yamamoto | Japanese | 2000 | Asia | 117 | NA | Mix | H,S | NA. | IM | 81 | 2 | 7 | 27 |
| D Y Graham | American | 2001 | USA | 104 | Adult | Healthy | U | Yes | IM | 41 | 2 | 2 | 59 |
| Toru Fujisawa | Japanese | 2001 | Asia | 21 | Adult | Healthy | C,H,R | NA. | IM | 18 | 1 | 0 | 2 |
| Hiroto Miwa | Japanese | 2001 | Asia | 155 | Adult | Patient | U | NA. | IM | 93 | 7 | 4 | 51 |
| Kyoichi Adachi | Japanese | 2002 | Asia | 100 | Mix | Healthy | U | Yes | ELISA | 32 | 2 | 3 | 37 |
| IM | 30 | 1 | 5 | 38 | |||||||||
| WM Wong | Chinese | 2002 | Asia | 123 | Adult | Patient | R,H | Yes | IM | 58 | 3 | 2 | 60 |
| Youke Lu | Chinese | 2002 | Asia | 102 | Mix | Patient | C,R,H | NA. | ELISA | 60 | 4 | 2 | 27 |
| A Leodolter, D Vaira | European | 2003 | Europe | 449 | NA | Patient | C,H,R | NA. | IM | 178 | 34 | 38 | 170 |
| ELISA | 193 | 66 | 23 | 140 | |||||||||
| T Shimizu | Japanese | 2003 | Asia | 68 | Children | Patient | U, SA | NA. | ELISA | 12 | 13 | 1 | 42 |
| Antone R Opekun | American | 2004 | USA | 188 | Adult | Patient | U,S | Yes | IM | 72 | 0 | 8 | 87 |
| Fu-Chen Kuo | Chinese | 2005 | Asia | 317 | Mix | Patient | C,R,H,U | NA. | ELISA | 211 | 8 | 19 | 79 |
| Francis Megraud | European | 2005 | Europe | 316 | Children | Patient | C,H,R | Yes | ELISA | 86 | 4 | 50 | 176 |
| IM | 36 | 2 | 83 | 151 | |||||||||
| Yanfang Gong | Chinese | 2005 | Asia | 215 | Mix | Patient | U | Yes | ELISA | 80 | 19 | 16 | 100 |
| Chien-Yu Lu | Chinese | 2006 | Asia | 120 | NA | Patient | C,H&E,R,U | Yes | IM | 54 | 6 | 8 | 52 |
| Khitam Muhsen | Israeli Arab | 2006 | Asia | 159 | Children | Healthy | SA | NA. | ELISA | 27 | 3 | 52 | 77 |
| Lam Tung Nguyen | Vietnamese | 2010 | Asia | 148 | Mix | Patient | C,IM,S | Yes | IM | 66 | 6 | 17 | 59 |
| Demıray Gürbüz E | Turk | 2012 | Asia | 124 | Adult | Patient | C,H,R | Yes | IM | 61 | 8 | 21 | 34 |
| ELISA | 61 | 8 | 21 | 34 | |||||||||
| Masumi Okuda | Japanese | 2013 | Asia | 101 | Children | Healthy | U, SA | Yes | ELISA | 34 | 2 | 3 | 62 |
| IM | 29 | 0 | 7 | 64 | |||||||||
| Duc T Quach | Vietnamese | 2014 | Asia | 200 | Adult | Patient | R,H | NA. | IM | 94 | 9 | 17 | 80 |
C, culture; FN, false negative; FP, false positive; H, histology; IM: immunochromatography; R, rapid urease test; U, urea breath test; SA, stool; S, serology; TN, true negative; TP, true positive.
Table 2.
Summary of data extraction and quality assessment of diagnostic accuracy studies (QUADAS)-2 assessments of included studies
| Author | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Quality |
| Mohammad M | N | Y | Y | Y | U | Y | N | Y | U | N | U | Y | Y | Y | 8 |
| Kiyonri Katsuragi | N | Y | Y | Y | U | Y | N | Y | U | N | U | Y | Y | Y | 8 |
| Hiroto Miwa | N | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | Y | Y | 11 |
| Mototsugu Kato | U | Y | Y | Y | U | Y | N | Y | U | N | U | Y | Y | Y | 8 |
| Soichiro Yamamoto | U | Y | Y | Y | U | Y | N | Y | U | N | Y | Y | Y | Y | 8 |
| DY Graham | N | Y | Y | Y | Y | Y | N | Y | Y | N | U | Y | Y | Y | 10 |
| Toru Fujisawa | U | Y | Y | Y | U | Y | N | Y | U | N | U | Y | Y | Y | 8 |
| Hiroto Miwa | Y | Y | Y | Y | U | Y | N | Y | U | N | Y | Y | Y | Y | 10 |
| Kyoichi Adachi | N | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | Y | Y | 11 |
| WM WONG | U | Y | Y | Y | Y | Y | N | Y | Y | N | U | Y | Y | Y | 10 |
| Youke Lu | Y | Y | Y | Y | U | Y | N | Y | U | N | Y | Y | Y | Y | 10 |
| A LEODOLTER, D. VAIRA | N | Y | Y | Y | U | Y | N | Y | U | N | U | Y | Y | Y | 8 |
| T Shimizu | N | Y | Y | Y | U | Y | N | Y | U | N | Y | Y | Y | Y | 9 |
| Antone R Opekun | Y | Y | Y | Y | U | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13 |
| Fu-Chen Kuo | N | Y | Y | Y | U | Y | N | Y | U | N | Y | Y | Y | Y | 9 |
| Francis Megraud | U | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | Y | Y | 11 |
| Chien-Yu Lu | N | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | Y | Y | 11 |
| Yanfang Gong | U | Y | N | Y | Y | Y | N | Y | Y | N | U | Y | Y | Y | 9 |
| Khitam Muhsen | N | Y | N | Y | U | Y | N | Y | U | N | U | Y | Y | Y | 7 |
| Lam Tung Nguyen | N | Y | Y | Y | Y | Y | N | Y | Y | N | U | Y | Y | Y | 8 |
| Demıray Gürbüz E | N | Y | Y | Y | U | Y | N | Y | Y | N | U | Y | Y | Y | 8 |
| Masumi Okuda | N | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | Y | Y | 9 |
| Duc T Quach | U | Y | Y | Y | U | Y | N | Y | U | N | U | Y | Y | Y | 8 |
1. Was a consecutive or random sample of patients enrolled?
2. Was a case-control design avoided?
3. Did the study avoid inappropriate exclusions?
4. Are there concerns that the included patients and setting do not match the review question?
5. Were the index test results interpreted without knowledge of the results of the reference standard?
6. If a threshold was used, was it prespecified?
7. Are there concerns that the index test, its conduct or its interpretation differ from the review question?
8. Is the reference standard likely to correctly classify the target condition?
9. Were the reference standard results interpreted without knowledge of the results of the index test?
10. Are there concerns that the target condition as defined by the reference standard does not match the question?
11. Was there an appropriate interval between the index test and reference standard?
12. Did all patients receive the same reference standard?
13. Were all patients included in the analysis?
14. Could the patient flow have introduced bias?
Y, yes; N, no; U, unclear.
bmjopen-2016-013248supp002.jpg (271KB, jpg)
Statistical analysis
The following parameters representing test accuracy were calculated based on the data (TP, FP, FN and TN) we extracted from each included study: the pooled sensitivity, specificity, PLR, NLR, DOR and corresponding 95% CIs. Simultaneously, the summary receiver operator characteristic (SROC) was also assessed. The heterogeneity was measured by Q test and the inconsistency index (I2), and p <0.05 and I2 >50% indicated significant heterogeneity among studies. The random-effect model (DerSimonian-Laird method) was conducted for the meta-analysis to calculate the pooled sensitivity, specificity and other related indexes of the studies, and meta-regression was performed to detect the source of the heterogeneity; otherwise, the fixed-effect model (Mantel-Haenszel method) was chosen.
In addition, the Spearman’s correlation coefficient was used to verify if the heterogeneity in meta-analysis could be explained by a threshold effect, which was defined as a positive correlation (p <0.05). Subgroup analyses were performed for region, age, study population and assay method. Deeks’ funnel plot asymmetry test was applied to determine the presence of publication bias using STATA V.12.1 software (Stata Corp, College Station, Texas, USA.).20 MetaDisc (V.1.4) software21 was also used to calculate other parameters of diagnostic performance. All p values were two-sided, and p <0.05 was considered statistically significant.
Results
Search results
This meta-analysis was organised according to the PRISMA statement (supplemental material). Figure 1 summarises the search process and numerical selection of the final papers that were included in the systematic review and meta-analysis. A systematic search of biomedical databases resulted in 423 hits, and after excluding duplicates, 246 citations were identified. No unpublished literature relevant to the topic was identified. Forty papers were selected based on their abstracts and titles and were read in full for eligibility. Two eligible studies referred to the same study group; hence, only one of these was included in the systematic review.10 18 Twenty-four individual studies fulfilled the eligibility criteria and were included in the systematic review.7–9 11–18 22–31 Of these, 23 studies had extractable data after contacting the authors and were included in the meta-analysis.7–9 11–13 15–18 22–24 26 28 30–33 A flow chart detailing the study selection process is shown in figure 1.
Figure 1.
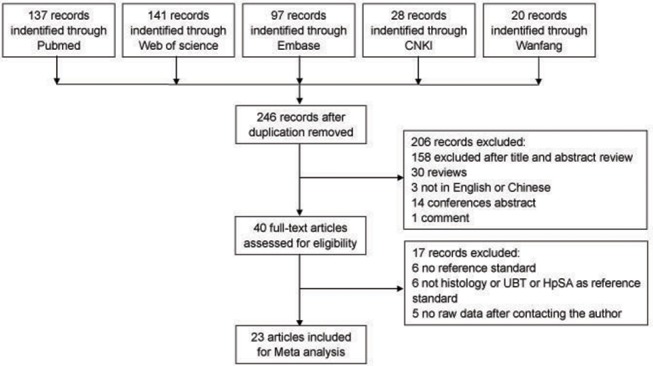
Flow diagram of the literature search. CNKI, Chinese National Knowledge Infrastructure; HpSA, Helicobacter pylori stool antigen test; UBT, urea breath test.
bmjopen-2016-013248supp005.pdf (216.5KB, pdf)
Study characteristics
Baseline characteristics of the eligible studies are summarised in table 1. A total of 23 studies with 4963 participants were included in the meta-analysis. Of these, three were conducted in the USA,7 17 23 two in Europe12 13 and the remaining 18 in Asia. All eligible studies were published between 2000 and 2014. Sample sizes ranged from 21 to 449. Urinary H. pylori IgG was detected using ELISA in nine studies, using IM in nine studies and using both assays in five studies. Key data were successfully extracted from all studies, including TPs, FPs, FNs and TNs. The number of TPs ranged from 12 to 237, FNs from 0 to 83, FPs from 0 to 66 and TNs from 2 to 176.
Diagnostic accuracy and threshold analysis
Spearman’s correlation coefficient was first used to determine the existence of the threshold effect because it is an important source of heterogeneity. The Spearman’s correlation coefficient for sensitivity and 1−specificity in the meta-analysis was 0.161, with a p value of 0.413, suggesting no heterogeneity from the threshold effect. Heterogeneity was measured using the Q test and the inconsistency index (I2) to choose the appropriate calculation model. Significant heterogeneity was detected in the pooled diagnostic OR (DOR) (DOR=73, I2=75%, p=0.0000) (figure 2). Therefore, the random effects model was used to calculate sensitivity, specificity, the positive likelihood ratio (PLR) and DOR.
Figure 2.
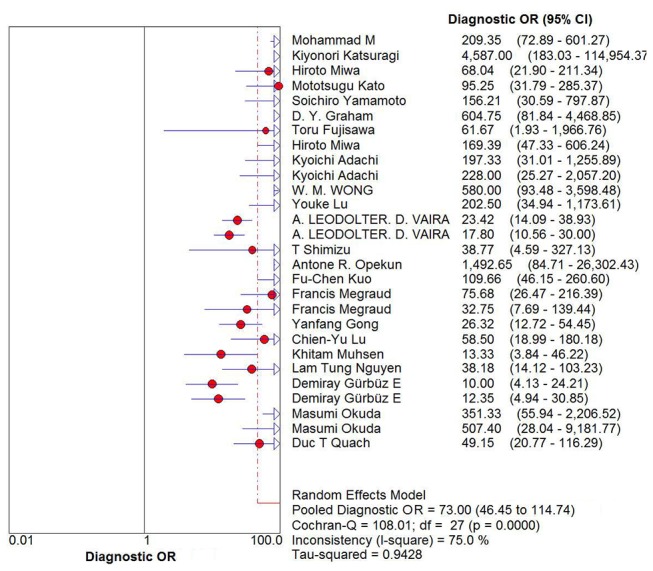
Forest plots of diagnostic OR (DOR) for Helicobacter pylori diagnosis by urine IgG antibody. The pooled DOR was 73 (95% CI 46.45 to 114.74).
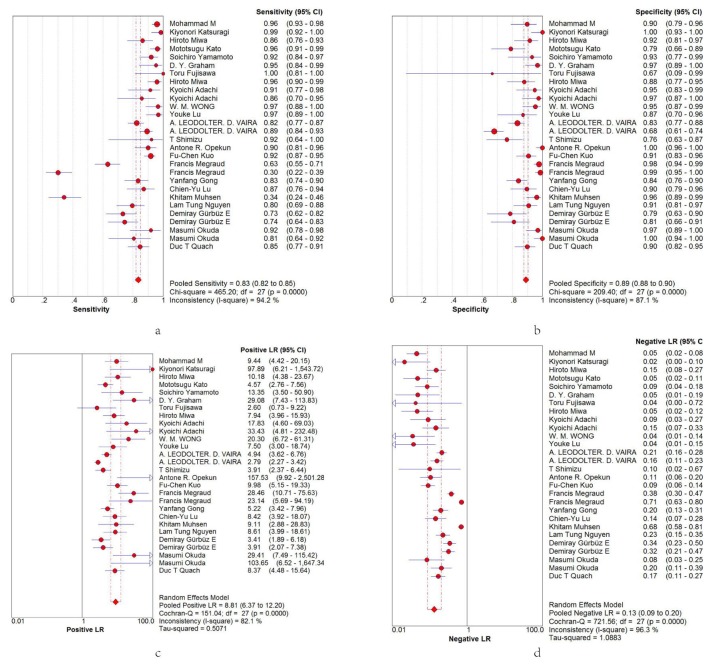
Based on TP, TN, FP and FN data extracted from the included studies, we evaluated the diagnostic accuracy of urinary IgG in H. pylori diagnosis from the following quantitative parameters: pooled sensitivity and specificity were 0.83 (95% CI 0.82 to 0.85; figure 3A) and 0.89 (95% CI 0.88 to 0.90; figure 3B), respectively; pooled PLR and negative likelihood ratio (NLR) were 8.81 (95% CI 6.37 to 12.2; figure 3C) and 0.13 (95% CI 0.09 to 0.2; figure 3D), respectively. The SROC curve for urinary IgG was positioned near the desirable upper left corner, and the area under the curve (AUC) was 0.9551, indicating that the level of overall accuracy was high (supplemental figure 3).
Figure 3.
Forest plots of sensitivity, specificity, PLR and NLR for H. pylori diagnosis by urine IgG antibody. (A) The summary sensitivity was 0.83 (95% CI 0.82 to 0.85; I2=94.4%). (B) The summary specificity was 0.89 (95% CI 0.87 to 0.90; I2=86.1%). (C) The summary PLR was 8.5 (95% CI 6.27 to 12.2; I2=81.0%). (D) The summary NLR of all articles was 0.13 (95% CI 0.09 to 0.20; I2=96.3%). NLR, negative likelihood ratio; PLR, positive likelihood ratio.
bmjopen-2016-013248supp003.jpg (119.4KB, jpg)
Subgroup analysis
Subgroup analysis was conducted based on age, region, study population and assay method. Pooled results are shown in table 3. A random effects model was used because significant heterogeneity was observed (all I2 >50%). The differences between subgroups were conclusions based on whether there was the overlap of the 95% CI for each AUC.
Table 3.
Group/subgroup analysis of pooled estimates with 95% CI for sensitivity, specificity, positive likelihood ratio (LR) and negative LR
| Group/subgroup | Spearman ’s p | Cochrane Q test | Pooled sensitivity (95% CI) |
Pooled specificity (95% CI) |
Pooled positive LR (95% CI) |
Pooled Negative NR (95% CI) |
Area under the curve (95% CI) |
|
| diagnostic OR (95% CI) | p | |||||||
| Overall | 0.413 | 73 (46.45 to 114.74) |
0.0000 | 0.83 (0.82 to 0.85) |
0.89 (0.88 to 0.90) |
8.81 (6.37 to 12.2) |
0.13 (0.09 to 0.2) |
0.96 (0.94 to 0.97) |
| Age | ||||||||
| Children | 0.397 | 61.62 (22.16 to 171.32) |
0.0335 | 0.53 (0.48 to 0.58) |
0.96 (0.94 to 0.97) |
17.93 (4.83 to 62.59) |
0.35 (0.22 to 0.58) |
0.96 (0.91 to 1.01) |
| Adult | 0.732 | 85.12 (29.81 to 243.06) |
0.0000 | 0.87 (0.84 to 0.89) |
0.91 (0.88 to 0.94) |
8.13 (4.61 to 14.33) |
0.13 (0.07 to 0.22) |
0.96 (0.92 to 1.0) |
| Region | ||||||||
| Asian | 0.724 | 73.75 (43.38 to 125.38) |
0.0000 | 0.86 (0.84 to 0.88) |
0.9 (0.88 to 0.92) |
7.74 (5.77 to 10.39) |
0.12 (0.07 to 0.20) |
0.96 (0.94 to 0.97) |
| Europe and USA | 0.645 | 73.75 (29.26 to 125.38) |
0.0000 | 0.80 (0.77 to 0.82) |
0.88 (0.86 to 0.90) |
12.05 (5.22 to 27.8) |
0.16 (0.07 to 0.38) |
0.96 (0.91 to 1.0) |
| Study population | ||||||||
| Patient | 0.616 | 54.29 (34.07 to 86.51) |
0.0000 | 0.84 (0.82 to 0.85) |
0.87 (0.85 to 0.89) |
7.17 (5.18 to 9.93) |
0.14 (0.09 to 0.23) |
0.94 (0.92 to 0.96) |
| Healthy | 0.294 | 156.11 (41.44 to 588.04) |
0.0073 | 0.75 (0.69 to 0.80) |
0.97 (0.94 to 0.98) |
16.25 (6.94 to 38.06) |
0.13 (0.03 to 0.53) |
0.98 (0.96 to 1.0) |
| Assay method | ||||||||
| Immunochromatography | 0.5940 | 82.94 (41.62 to 165.29) |
0.0000 | 0.81 (0.78 to 0.83) |
0.92 (0.90 to 0.94) |
9.81 (6.28 to 15.34) |
0.14 (0.07 to 0.28) |
0.96 (0.93 to 0.98) |
| ELISA | 0.7820 | 67.46 (35.58 to 127.9) |
0.0000 | 0.86 (0.84 to 0.87) |
0.87 (0.84 to 0.88) |
7.92 (5.02 to 12.5) |
0.12 (0.07 to 0.23) |
0.95 (0.93 to 0.98) |
Age analysis
Seven studies containing 1047 adults (>17 years of age) were evaluated. Pooled sensitivity, specificity, PLR and NLR were 0.87 (95% CI 0.84 to 0.89), 0.91 (95% CI 0.88 to 0.94), 8.13 (95% CI 4.61 to 14.33) and 0.13 (95% CI 0.07 to 0.22), respectively, with a DOR of 85.12 and an AUC value of 0.9593 (95% CI 0.92 to 1.0). The diagnostic performance of urinary IgG was evaluated for young people in the four other studies containing 644 children (≤17 years of age). Pooled sensitivity, specificity, PLR and NLR were 0.53 (95% CI 0.48 to 0.58), 0.96 (95% CI 0.94 to 0.97), 17.93 (95% CI 4.83 to 62.59) and 0.35 (95% CI 0.22 to 0.58), respectively, with a DOR of 61.62 and an AUC value of 0.9632 (95% CI 0.91 to 1.01). There was no significant difference in the AUC values between adults and children.
Regional analysis
Of the 23 included studies, five were from Europe or USA and the remaining 18 were from Asia. For studies from Europe and USA, the analysis showed a pooled sensitivity of 0.80 (95% CI 0.77 to 0.82) and a pooled specificity of 0.88 (95% CI 0.86 to 0.90). Combined PLR was 12.05 (95% CI 5.22 to 27.8), NLR was 0.16 (95% CI 0.07 to 0.38), and AUC and DOR were 0.9557 (95% CI 0.91 to 1.0) and 73.75, respectively. For studies from Asia, the pooled sensitivity was 0.86 (95% CI 0.84 to 0.88) and the pooled specificity was 0.9 (95% CI 0.88 to 0.92). Combined PLR was 7.74 (95% CI 5.77 to 10.39), NLR was 0.12 (95% CI 0.07 to 0.2), DOR was 73.75 and AUC was 0.9553 (95% CI 0.94 to 0.97). There was no significant difference in the AUC values between Europe or USA.
Study population analysis
Study population analysis, of both patients and healthy controls, was performed in the systematic review. A total of 16 patient studies and 5 studies of healthy controls or individuals with no upper abdominal symptoms were evaluated. In the patient population, pooled sensitivity, specificity, PLR and NLR were 0.84 (95% CI 0.82 to 0.85), 0.87 (95% CI 0.85 to 0.89), 7.17 (95% CI 5.18 to 9.93) and 0.14 (95% CI 0.09 to 0.23), respectively, with a DOR of 54.29 and AUC value of 0.9436 (95% CI 0.92 to 0.96). In the healthy population, pooled sensitivity, specificity, PLR and NLR were 0.75 (95% CI 0.69 to 0.80), 0.97 (95% CI 0.94 to 0.98), 16.25 (95% CI 6.94 to 38.06) and 0.13 (95% CI 0.03 to 0.53), respectively, with a DOR of 156.11 and AUC value of 0.98 (95% CI 0.96 to 1.0). Except for pooled sensitivity, the diagnostic performance of the urine IgG assay was better for the healthy population than the patient population. However, there was no significant difference in AUC values between patients and controls.
Assay method analysis
Of all studies included, urinary H. pylori IgG was detected using ELISA in nine, IM in nine and both assays in five. For studies that used ELISA, the pooled sensitivity was 0.86 (95% CI 0.84 to 0.87) and pooled specificity was 0.87 (95% CI 0.84 to 0.88). Combined PLR was 7.92 (95% CI 5.02 to 12.5), NLR was 0.12 (95% CI 0.07 to 0.23), and AUC and DOR were 0.9521 and 67.46, respectively. For studies that used IM, pooled sensitivity, specificity, PLR and NLR were 0.81 (95% CI 0.78 to 0.83), 0.92 (95% CI 0.89 to 0.93), 9.81 (95% CI 6.28 to 15.34) and 0.14 (95% CI 0.07 to 0.28), respectively, with a DOR of 82.94 and AUC value of 0.9584. No statistically significant difference was detected between ELISA and IM for the diagnostic accuracy of urine antibody detection.
Meta-regression analysis
Heterogeneity was found in summary estimates for sensitivity, specificity, PLR, NLR and DOR. Therefore, meta-regression was conducted to examine the source of heterogeneity based on region, sample size, age, study population, blind design, quality of study and assay method. The results indicated that study population and quality of study were the important factors contributing to heterogeneity (p=0.0189 and p=0.0295, respectively) (table 4).
Table 4.
Meta-regression of potential heterogeneity within the included studies
| Variables | Constant coefficient | SE | p Value | RDOR | (95% CI) |
| Constant coefficient | −0.98 | 3.4737 | 0.781 | ---- | ---- |
| S | 0.309 | 0.1614 | 0.0706 | ---- | ---- |
| Region | −0.459 | 0.8022 | 0.574 | 0.63 | (0.12 to 3.39) |
| Sample size | −0.001 | 0.0041 | 0.8856 | 1 | (0.99 to 1.01) |
| Age | −0.093 | 0.2489 | 0.7117 | 0.91 | (0.54 to 1.53) |
| Study population | 1.367 | 0.5326 | 0.0189 | 3.92 | (1.29 to 11.96) |
| blinded design | 0.144 | 0.6537 | 0.8282 | 1.15 | (0.29 to 4.54) |
| Assay method | 0.008 | 0.4155 | 0.9841 | 1.01 | (0.42 to 2.41) |
| Quantity | 0.518 | 0.22 | 0.0295 | 1.68 | (1.06 to 2.66) |
RDOR, relative diagnostic OR; S, statistic.
Publication bias
Because publication bias is recognised as an important factor that influences the results of meta-analyses,34 the Deeks’ funnel plot asymmetry test was performed to examine publication bias (supplemental Figure 4). The test returned a p value of 0.124, suggesting no statistically significant publication bias was found in the pooled analysis of the studies.
bmjopen-2016-013248supp004.jpg (41.9KB, jpg)
Discussion
Non-invasive tests for the assessment of H. pylori status have become part of patient management strategies.35–37 Preliminary studies have explored the diagnostic accuracy of testing for anti-H. pylori antibodies in urine, but the results are inconclusive. In the present study, we performed comprehensive database searches for all eligible studies reporting the diagnostic accuracy of testing for anti-H. pylori antibodies in urine. Our meta-analysis was strengthened by the use of a standard protocol, strict inclusion criteria, standardised data extraction and independent reviewers. To the best of our knowledge, this is the first study assessing the summary predictive value of anti-H. pylori IgG in urine for infection diagnosis.
Anti-H. pylori IgG in urine is detectable and has been used for the diagnosis of H. pylori infection, but a comprehensive evaluation of the test performance is needed before its wider application. After pooling data, we obtained a pooled sensitivity of 0.83 and a pooled specificity of 0.89, which represent good markers for H. pylori diagnosis. The SROC curve, which assesses overall test performance by showing the trade-off between sensitivity and specificity,38 39 had an AUC of 0.9551, suggesting a good level of accuracy. Another indicator of diagnostic accuracy is DOR, which combines sensitivity and specificity data into a single number ranging from 0 to infinity, with higher values indicating better discriminatory test performances.40 The mean DOR in the meta-analysis was 73, suggesting that testing for anti-H. pylori antibodies in urine should be helpful in the diagnosis of H. pylori infection. We further examined the diagnostic accuracy of an anti-H. pylori antibody in urine by calculating the PLR and NLR, which can be easier to relate to clinical practice than SROC and DOR. The pooled PLR was 8.81 and the pooled NLR was 0.13, indicating that the presence of anti-H. pylori antibodies in urine has an important function in diagnosing H. pylori infection. Substantial heterogeneity was found with meta-analysis, so the random effects model was used to synthesise the above data. Our results show that anti-H. pylori IgG represents a good marker for the diagnosis of H. pylori infection.
Heterogeneity is an important factor that can affect the results of meta-analysis. Therefore, we used the Spearman’s correlation coefficient to clarify whether the threshold effect contributed to the source of heterogeneity. The Spearman’s correlation coefficient was 0.193, with a p value of 0.334, suggesting that heterogeneity among the included studies could not have been induced by the threshold effect. We further used subgroup analysis based on study population, region, age and assay method to explore heterogeneity. No significant difference in age, region or assay method was detected, but subgroup analysis for the study population revealed a little difference in AUC values between patients and controls, suggesting a relatively high level of diagnostic accuracy in the healthy population, although there was the overlap of the 95% CI for each AUC of study population subgroup. In meta-analysis, the patient population included dyspeptic, chronic gastritis and patients with peptic ulcer among others. It is possible that the disease condition in the stomach may cause a change in H. pylori colonisation.41 On the other hand, H. pylori IgG is not synchronised with the H. pylori infection process, so the delayed generation or disappearance of H. pylori colonisation for several months may affect the level of anti-H. pylori IgG in the urine.42 Indeed, Graham et al 23 reported that urine tests may remain positive for an extended time after successful treatment of the infection. This may be an important factor affecting the accuracy of the antibody test in the diseased population. The meta-regression analysis also demonstrated that study population was an important factor contributing to heterogeneity, which is consistent with subgroup analysis. These findings indicate that H. pylori infection diagnosis by anti-H. pylori IgG in the urine requires extra caution in diseased populations.
The QUADAS tool was developed and evaluated by Whiting et al 43 and is recommended by the Cochrane diagnostic accuracy systematic reviews44 to provide a methodological assessment of the quality of diagnostic accuracy studies. Experience, reports from users and feedback from the Cochrane Collaboration suggested the potential for improvements; therefore, QUADAS-2 was developed19 and has been shown to be a considerable improvement over the original tool. The responses to QUADAS-2 signalling questions are assessed in terms of risk of bias or concerns regarding applicability. In the present meta-analysis, 23 of the included studies were qualified using QUADAS-2 assessment, which included a score of 7 for one study, a score of 8 for nine studies, a score of 9 for four studies, and a score of 10 or more for nine studies. Meta-regression analysis showed that the quality of included studies was another factor for heterogeneity. Therefore, a difference in diagnostic accuracy was present between low and high scoring studies according to regression analysis. This indicates that meta-analyses should include as many high quality articles as possible to improve their accuracy.
There are several limitations to our meta-analysis that should be borne in mind when interpreting the results. First, the studies included are not an exhaustive list because the search range was limited to published studies. Unpublished research, such as conference papers, cannot be obtained so it is possible that some relevant literature has been missed. Additionally, only studies published in English or Chinese were included. Second, for articles that contained different cut-off values within the same study, we selected cut-off values according to the manufacturers’ recommendations. However, these may not be the most appropriate values for specific areas.
In conclusion, testing for anti-H. pylori antibodies in urine appears to have an important function and represents a good marker for the diagnosis of H. pylori infection. Sources of heterogeneity were found to come from the quality of the studies included, and from the study population. Further large-scale, well designed studies examining different study populations are required to confirm the results of this meta-analysis.
Supplementary Material
Footnotes
Funding: This work was supported by a grant from Natural Science Foundation of Liaoning Province (201602822).
Competing interests: None declared.
Data sharing statement: No additional data are available.
References
- 1. Covacci A, Telford JL, Del Giudice G, et al. Helicobacter pylori virulence and genetic geography. Science 1999;284:1328–33. 10.1126/science.284.5418.1328 [DOI] [PubMed] [Google Scholar]
- 2. Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer 2002;2:28–37. 10.1038/nrc703 [DOI] [PubMed] [Google Scholar]
- 3. Suerbaum S, Michetti P. Helicobacter Pylori infection. N Engl J Med 2002;347:1175–86. 10.1056/NEJMra020542 [DOI] [PubMed] [Google Scholar]
- 4. Plebani A, Notarangelo LD, Monafo V, et al. A new immunoperoxidase assay for Lolium perenne-specific IgE in serum based on the biotin/avidin system (BAS). Clin Allergy 1984;14:373–8. 10.1111/j.1365-2222.1984.tb02218.x [DOI] [PubMed] [Google Scholar]
- 5. Yamamoto T, Tamura M, Ishii T, et al. Urinary antibody titers to Helicobacter pylori and an impact of clinical characteristics. J Clin Gastroenterol 2003;36:329–31. 10.1097/00004836-200304000-00010 [DOI] [PubMed] [Google Scholar]
- 6. Yamamoto T, Kojima K, Sanaka M, et al. Reliability of rapid urinary test for antibody to Helicobacter pylori in adult patients with proteinuria. Diagn Microbiol Infect Dis 2006;54:105–8. 10.1016/j.diagmicrobio.2005.09.009 [DOI] [PubMed] [Google Scholar]
- 7. Alemohammad MM, Foley TJ, Cohen H. Detection of immunoglobulin G antibodies to Helicobacter pylori in urine by an enzyme immunoassay method. J Clin Microbiol 1993;31:2174–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Katsuragi K, Noda A, Tachikawa T, et al. Highly sensitive urine-based enzyme-linked immunosorbent assay for detection of antibody to Helicobacter pylori. Helicobacter 1998;3:289–95. 10.1046/j.1523-5378.1998.08045.x [DOI] [PubMed] [Google Scholar]
- 9. Miwa H, Hirose M, Kikuchi S, et al. How useful is the detection kit for antibody to Helicobacter pylori in urine (URINELISA) in clinical practice? Am J Gastroenterol 1999;94:3460–3. 10.1111/j.1572-0241.1999.01608.x [DOI] [PubMed] [Google Scholar]
- 10. Okuda M, Nakazawa T, Booka M, et al. Evaluation of a urine antibody test for Helicobacter pylori in japanese children. J Pediatr 2004;144:196–9. 10.1016/j.jpeds.2003.10.057 [DOI] [PubMed] [Google Scholar]
- 11. Shimizu T, Yarita Y, Haruna H, et al. Urine-based enzyme-linked immunosorbent assay for the detection of Helicobacter pylori antibodies in children. J Paediatr Child Health 2003;39:606–10. 10.1046/j.1440-1754.2003.00213.x [DOI] [PubMed] [Google Scholar]
- 12. Leodolter A, Vaira D, Bazzoli F, et al. European multicentre validation trial of two new non-invasive tests for the detection of Helicobacter pylori antibodies: urine-based ELISA and rapid urine test. Aliment Pharmacol Ther 2003;18:927–31. 10.1046/j.1365-2036.2003.01761.x [DOI] [PubMed] [Google Scholar]
- 13. Mégraud F. Comparison of non-invasive tests to detect Helicobacter pylori infection in children and adolescents: results of a multicenter European study. J Pediatr 2005;146:198–203. 10.1016/j.jpeds.2004.10.044 [DOI] [PubMed] [Google Scholar]
- 14. Quach DT, Hiyama T, Shimamoto F, et al. Value of a new stick-type rapid urine test for the diagnosis of Helicobacter pylori infection in the vietnamese population. World J Gastroenterol 2014;20:5087–91. 10.3748/wjg.v20.i17.5087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Demıray Gürbüz E, Gönen C, Bekmen N, et al. The diagnostic accuracy of urine IgG antibody tests for the detection of Helicobacter pylori infection in Turkish dyspeptic patients. Turk J Gastroenterol 2012;23:753–8. 10.4318/tjg.2012.0497 [DOI] [PubMed] [Google Scholar]
- 16. Lu CY, Kuo FC, Wang SW, et al. The clinical applications and accuracy of 2 rapid near-patient tests in detecting Helicobacter pylori infection. Diagn Microbiol Infect Dis 2006;56:241–6. 10.1016/j.diagmicrobio.2006.04.008 [DOI] [PubMed] [Google Scholar]
- 17. Opekun AR, Luu P, Gotschall AB, et al. Point-of-care Helicobacter pylori urine antibody detection in a multi-ethnic adult population in the United States. Transl Res 2006;148:13–18. 10.1016/j.lab.2006.03.008 [DOI] [PubMed] [Google Scholar]
- 18. Okuda M, Kamiya S, Booka M, et al. Diagnostic accuracy of urine-based kits for detection of Helicobacter pylori antibody in children. Pediatr Int 2013;55:337–41. 10.1111/ped.12057 [DOI] [PubMed] [Google Scholar]
- 19. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 20. Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication Bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882–93. 10.1016/j.jclinepi.2005.01.016 [DOI] [PubMed] [Google Scholar]
- 21. Zamora J, Abraira V, Muriel A, et al. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006;6:31 10.1186/1471-2288-6-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamamoto S, Uemura N, Okamoto S, et al. A new rapid test for detecting anti-Helicobacter pylori antibody excreted into urine. Helicobacter 2000;5:160–4. 10.1046/j.1523-5378.2000.00025.x [DOI] [PubMed] [Google Scholar]
- 23. Graham DY, Reddy S. Rapid detection of anti-Helicobacter pylori IgG in urine using immunochromatography. Aliment Pharmacol Ther 2001;15:699–702. 10.1046/j.1365-2036.2001.00968.x [DOI] [PubMed] [Google Scholar]
- 24. Fujisawa T, Kaneko T, Kumagai T, et al. Evaluation of urinary rapid test for Helicobacter pylori in general practice. J Clin Lab Anal 2001;15:154–9. 10.1002/jcla.1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu DC, Kuo CH, Lu CY, et al. Evaluation of an office-based urine test for detecting Helicobacter pylori: a prospective pilot study. Hepatogastroenterology 2001;48:614–7. [PubMed] [Google Scholar]
- 26. Miwa H, Akamatsu S, Tachikawa T, et al. On-site diagnosis of H. pylori infection by urine. Diagn Microbiol Infect Dis 2001;39:95–7. 10.1016/S0732-8893(01)00205-X [DOI] [PubMed] [Google Scholar]
- 27. Adachi K, Kawamura A, Ono M, et al. Comparative evaluation of urine-based and other minimally invasive methods for the diagnosis of Helicobacter pylori infection. J Gastroenterol 2002;37:703–8. 10.1007/s005350200115 [DOI] [PubMed] [Google Scholar]
- 28. Wong WM, Wong BC, Xia HH, et al. An evaluation of a rapid urine test for the diagnosis of Helicobacter pylori infection in the chinese population. Aliment Pharmacol Ther 2002;16:813–7. 10.1046/j.1365-2036.2002.01235.x [DOI] [PubMed] [Google Scholar]
- 29. Kuo FC, Wang SW, Wu IC, et al. Evaluation of urine ELISA test for detecting Helicobacter pylori infection in Taiwan: a prospective study. World J Gastroenterol 2005;11:5545–8. 10.3748/wjg.v11.i35.5545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Muhsen K, Athamna A, Athamna M, et al. Evaluation of a urine-based enzyme-linked immunosorbent assay test for the detection of Helicobacter pylori infection among 3- to 5-year-old Israeli Arab healthy children. J Pediatr Gastroenterol Nutr 2006;43:398–401. 10.1097/01.mpg.0000232017.40907.e4 [DOI] [PubMed] [Google Scholar]
- 31. Nguyen LT, Uchida T, Tsukamoto Y, et al. Evaluation of rapid urine test for the detection of Helicobacter pylori infection in the Vietnamese population. Dig Dis Sci 2010;55:89–93. 10.1007/s10620-009-0720-9 [DOI] [PubMed] [Google Scholar]
- 32. Quach DT, Hiyama T, Shimamoto F, et al. Value of a new stick-type rapid urine test for the diagnosis of Helicobacter pylori infection in the Vietnamese population. World J Gastroenterol 2014;20:5087–91. 10.3748/wjg.v20.i17.5087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuo FC, Wang SW, Wu IC, Ic W, et al. Evaluation of urine ELISA test for detecting Helicobacter pylori infection in Taiwan: a prospective study. World J Gastroenterol 2005;11:5545–8. 10.3748/wjg.v11.i35.5545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sterne JA, Gavaghan D, Egger M. Publication and related Bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000;53:1119–29. [DOI] [PubMed] [Google Scholar]
- 35. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut 2012;61:646–64. 10.1136/gutjnl-2012-302084 [DOI] [PubMed] [Google Scholar]
- 36. Wang YK, Kuo FC, Liu CJ, et al. Diagnosis of Helicobacter pylori infection: current options and developments. World J Gastroenterol 2015;21:11221–35. 10.3748/wjg.v21.i40.11221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miftahussurur M, Yamaoka Y. DiagnosticMethods ofHelicobacter pyloriInfection for Epidemiological Studies: Critical Importance of Indirect Test Validation. Biomed Res Int 2016;2016:1–14. 10.1155/2016/4819423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hamza TH, van Houwelingen HC, Heijenbrok-Kal MH, et al. Associating explanatory variables with summary receiver operating characteristic curves in diagnostic meta-analysis. J Clin Epidemiol 2009;62:1284–91. 10.1016/j.jclinepi.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 39. Schlattmann P, Verba M, Dewey M, et al. Mixture models in diagnostic meta-analyses--clustering summary receiver operating characteristic curves accounted for heterogeneity and correlation. J Clin Epidemiol 2015;68:61–72. 10.1016/j.jclinepi.2014.08.013 [DOI] [PubMed] [Google Scholar]
- 40. Chen KF, Chaou CH, Jiang JY, et al. Diagnostic accuracy of Lipopolysaccharide-Binding protein as biomarker for Sepsis in adult patients: a systematic review and Meta-Analysis. PLoS One 2016;11:e0153188 10.1371/journal.pone.0153188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bücker R, Azevedo-Vethacke M, Groll C, et al. Helicobacter pylori colonization critically depends on postprandial gastric conditions. Sci Rep 2012;2:994 10.1038/srep00994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gong Y, Wei W, Jingwei L, et al. Helicobacter pylori infection status correlates with serum parameter levels responding to Multi-organ functions. Dig Dis Sci 2015;60:1748–54. 10.1007/s10620-015-3522-2 [DOI] [PubMed] [Google Scholar]
- 43. Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25 10.1186/1471-2288-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leeflang MM, Deeks JJ, Takwoingi Y, et al. Cochrane diagnostic test accuracy reviews. Syst Rev 2013;2:82 10.1186/2046-4053-2-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-013248supp001.jpg (104.2KB, jpg)
bmjopen-2016-013248supp002.jpg (271KB, jpg)
bmjopen-2016-013248supp005.pdf (216.5KB, pdf)
bmjopen-2016-013248supp003.jpg (119.4KB, jpg)
bmjopen-2016-013248supp004.jpg (41.9KB, jpg)