Abstract
Background
We explored the expression pattern, prognostic potential, and functional role of microRNA-588 (miR-588) in human breast cancer (BC).
Material/Methods
The expression pattern of miR-588 was assessed by qPCR in BC cell lines and human BC carcinomas. The correlations between miR-588 and BC patients’ clinicopathological characteristics, as well as BC patients’ overall survival, were statistically assessed. In in vitro culture, MCF-7 and MDA-MB-231 cells were infected with lentivirus to overexpress endogenous miR-588. The subsequent effects of miR-588 upregulation on BC cell proliferation and cisplatin chemosensitivity were examined.
Results
miR-588 was found to be significantly downregulated in both BC cell lines and carcinoma tissues of BC patients. Low expression of miR-588 was closely correlated with BC patients’ poor prognosis of TNM stage, lymph node metastasis, and estrogen receptor status. In addition, patients with low miR-588-expressing carcinomas had much shorter overall survival. In MCF-7 and MDA-MB-231 cells, lentiviral infection induced significant miR-588 upregulation, and miR-588 upregulation had an anti-tumor effect in BC cells by significantly inhibiting cancer proliferation and increasing cisplatin chemosensitivity.
Conclusions
miR-588 is downregulated in BC and its aberrant expression is closely associated with patients’ poor prognosis and overall survival, thus suggesting a biomarker role. miR-588 also has anti-tumor function in BC, making it a potential therapeutic target for BC treatment.
MeSH Keywords: Carcinoma, Acinar Cell; Triple Negative Breast Neoplasms; Tumor Markers, Biological
Background
Human breast cancer (BC) is one of major gynecological carcinomas in modern society. According to a survey in 2012, more than 1.7 million women are diagnosed with BC, and BC-related deaths increase by nearly half a million deaths every year [1,2]. In the United States, almost 1 in 8 women will be diagnosed with BC at some time point in life [3]. In China, the incidence of BC has been considerably increasing in recent decades, with more than 1.6 million women diagnosed with BC and nearly 1.2 million women dying of BC in 2014 [4]. The underlying pathological mechanisms contributing to BC carcinogenesis and development are extremely complex and heterogeneous [5–7]. While surgery is the best option, post-operational relapse and metastasis remain the major concerns for patients with BC [8,9]. Therefore, it is critical to fully understand the underlying signaling pathways of BC, and to identify novel biomarkers and therapeutic targets for BC early diagnosis and treatment.
MicroRNAs (miRNAs) are groups of short (19~20 nucleotides long), single-stranded, non-coding RNAs that physically bind the 3′ un-translated regions (3′ UTR) of targeted genes to post-transcriptionally induce gene and protein inhibition, thus playing important roles in almost all aspects of biological development in animals and humans [10–12]. Various studies have shown that that miRNAs may be aberrantly expressed, either upregulated or downregulated, thus acting as prognostic biomarkers or functional cancer regulators, in human BC [13–16]. For instance, Lorio et al. demonstrated that miR-125b, miR-145, miR-21, and miR-155 were aberrantly deregulated in BC and were correlated with BC patient clinicopathological characteristics, such as estrogen and progesterone receptor expression, tumor stage, vascular invasion, and proliferation index [17]. Also, Mar-Aguilar et al. showed that 7 miRNAs – miR-10b, miR-21, miR-125b, miR-145, miR-155, miR-191, and miR-382 – were significantly dysregulated in the circulation system of BC patients and that a combination of miR-145, miR-155, and miR-382 may be used as specific biomarker to identify BC [18].
A recent study demonstrated that microRNA-588 (miR-588) was predominantly downregulated in human lung squamous cell carcinoma and was closely correlated with lung cancer patient tumor stage and lymph node invasion [19]. In addition, it was shown that miR-588 can act as a tumor suppressor in human lung cancer by reversely regulating its downstream target gene of progranulin [19]. However, the expression pattern, prognostic potential, and function of miR-588 have never been elucidated in human BC.
Material and Methods
Ethics approval
The ethics approval to perform this study was granted by the Clinical Study and Ethics Committees at Sichuan Cancer Hospital in Chengdu, China. Consent forms were signed by all patients included in the study. All procedures were performed in accordance with the principles of the Declaration of Helsinki.
Breast cancer cell lines and clinical tissues
In our study, 7 breast cancer cell lines – MCF-7, MDA-MB-231, MDA-MB-361, MDA-MB-436, UACC-732, UACC-812, SK-BR-3, and HCC-1428 – were purchased from the American Type Culture Collection (ATCC, USA). Also, a nonmetastatic human mammary epithelial cell line, MCF-10A, was purchased from the China Center for Typical Culture Collection (CCTCC, Wuhan, China). All cells were maintained in 6-well plates in Dulbecco’s modified Eagle’s medium (DMEM, Thermo Fisher Scientific, USA) supplemented with 10% fetal calf serum (FCS, Thermo Fisher Scientific, USA), 0.1 mM non-essential amino acids (Invitrogen, USA), 1X nonessential amino acid (Thermo Fisher Scientific, USA), 100 units/ml penicillin (Thermo Fisher Scientific, USA), and 0.1 mg/ml streptomycin (Thermo Fisher Scientific, USA) in a tissue-culture environment at 37°C with 5% CO2.
A total of 174 patients diagnosed with breast cancer were included in this study. All patients received mastectomy surgery in the Department of Breast Surgery and the Intensive Care Unit at Sichuan Cancer Hospital in Chengdu between June 2008 and September 2012. During surgery, breast carcinomas, as well as adjacent non-carcinoma breast epithelial tissues, were retrieved, immediately snap-frozen in liquid nitrogen, and then preserved at −75°C until future assessment.
RNA extraction and quantitative real-time PCR (qPCR)
Total RNA was extracted from breast cell lines or clinical tissues using a PureLink® miRNA Isolation Kit (Invitrogen, USA) according to the manufacturer’s recommendation. Reverse transcription was performed using a TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems, USA) according to the manufacturer’s recommendation. Quantitative real-time PCR (qPCR) of characterizing miR-588 expression was conducted using TaqMan Advanced miRNA Assays (Applied Biosystems, USA) on an ABI automated 7900HT Fast Real-time PCR System (Applied Biosystems, USA) according to the manufacturer’s directions. Endogenous U6 snRNA expression level was used as internal control. Relative miR-588 expression was characterized using the 2−ΔΔCt method.
MiR-588 upregulation assay
A lentiviral plasmid expressing the oligonucleotides of human miR-588 mimics, Lenti-588, and a control lentiviral plasmid expressing non-specific oligonucleotides, Lenti-NC, were purchased from SunBio (Shanghai SunBio Technology, China). Two breast cancer cell lines, MCF-7 and MDA-MB-231, were infected with Lenti-588 or Lenti-NC (1X1013 units/ml), along with polybrene (6 μg/ml) at multiplicity of infection (MOI) of 30~35 for 48 h. Cells were then selected under blasticidine (20 μg/ml) for 5 to 7 days. After infection was stabilized, cancer cells were collected, re-plated in normal culture medium, and passaged at least 3~5 times. The efficacy of lentiviral infection was then checked by qPCR.
Breast cancer proliferation assay
The in vitro growth of infected breast cancer cells was assessed using a Vybrant® MTT Cell Proliferation Assay Kit (Invitrogen, USA) according to the manufacturer’s directions. Briefly, MCF-7 and MDA-MB-231 were collected off 6-well plates and re-cultured in a 96-well plate (5000 cells/well). Every 24 h, 10 mL of MTT stock solution was mixed into the culture medium in each well for 4 h, followed by reaction to SDS-HCl solution for 30 mins. After removing all medium contents, the 96-well plate was examined by a BioTek Synergy II microplate reader (BioTek, USA). Breast cancer cell proliferation rates were characterized as the luminescence reading for each well, at optical density (OD) of 490 nm.
Breast cancer chemosensitivity assay
Infected breast cancer cell chemosensitivity to cisplatin was assessed using an alamarBlue® Cell Viability Assay (Invitrogen, USA) according to the manufacturer’s directions. Briefly, MCF-7 and MDA-MB-231 were collected off 6-well plates and re-cultured in a 96-well plate (5000 cells/well). Various concentrations of cisplatin were added into tested wells for 24 h. For MCF-7 cells, cisplatin was used at concentrations of 0, 1, 5, 10, 20, and 50 mM. For MDA-MB-231, cisplatin was used at concentrations of 0, 10, 20, 50, 100, and 200 mM. Cisplatin was removed from culture medium 24 h later and 10 mL alamarBlue® reagent was mixed into culture medium for 2 h. After removing all medium contents, the 96-well plate was examined using a BioTek Synergy II microplate reader (BioTek, USA). Relative viabilities of breast cancer cells were characterized as the luminescence reading for each well, at optical density (OD) of 570 nm.
Statistical analysis
All experiments were performed in biological triplicates and the averaged data are presented in mean values ± standard errors. Statistical analysis was conducted using version 11.0 SPSS software (SPSS, USA). The statistical assessment of the correlation of miR-588 and breast cancer patient clinicopathological characteristics was performed using the Pearson’s chi-square distribution. Breast cancer patient overall survival was assessed using the Kaplan-Meier model and compared between patients expressing low and high miR-588 using the log-rank test. Univariate and multivariate analyses were performed using Cox proportional hazard regression models. All other statistical assessments were performed using an unpaired t test. A P value <0.05 demonstrated statistical significance.
Results
MiR-588 is downregulated in breast cancer
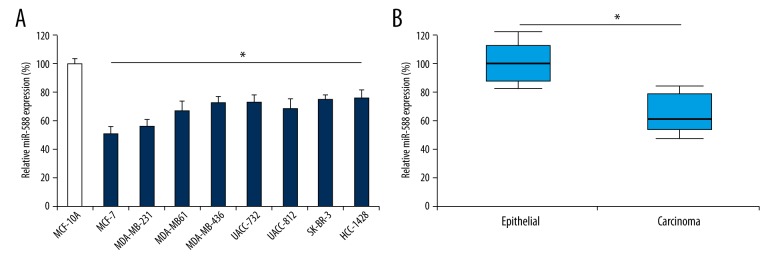
The expression pattern of miR-588 in breast cancer was assessed in both in vitro cancer cell lines and in vivo clinical tissues. The result of qPCR demonstrated that, in all assessed breast cancer cell lines, their endogenous miR-588 expressions were significantly lower than in a nonmetastatic human mammary epithelial cell line, MCF-10A (Figure 1A, *P<0.05). Furthermore, in clinical tissues from 174 patients with breast cancer, miR-588 expression was also discovered to be significantly lower in primary breast carcinomas than in adjacent non-carcinoma epithelial tissues (Figure 1B, *P<0.05).
Figure 1.
Expression pattern of miR-588 in breast cancer. (A) Expression of miR-588 was assessed by qPCR in breast cancer cell lines MCF-7, MDA-MB-231, MDA-MB-361, MDA-MB-436, UACC-732, UACC-812, SK-BR-3, and HCC-1428, and compared to its expression in MCF-10A cells (*P<0.05). (B) Expression of miR-588 was also assessed in human breast tissues between non-carcinoma breast epithelial tissues and carcinoma tissues (*P<0.05).
MiR-588 downregulation is closely correlated with poor prognosis of patients with breast cancer
As we discovered miR-588 was downregulated in both breast cancer cell lines and carcinoma tissues of breast cancer patients, we wondered whether the aberrant expression pattern of miR-588 was linked to the clinical outcome of cancer patients. Thus, we divided 174 patients into 2 arms, according to the mean expression level of miR-588 calculated from all carcinoma tissues. One arm included 90 patients whose breast carcinomas had low miR-588 expressions (< mean). The other arm included 84 patients whose breast carcinomas had high miR-588 expressions (> mean). Then, we used the Pearson’s chi-square test to assess the correlation of miR-588 expression with breast cancer patient clinicopathological characteristics (Table 1). We found that breast patient age, tumor size, and histological grades were not associated with miR-588 expression. On the other hand, we found that cancer patient TNM stage, lymph node metastasis, and estrogen receptor statues were strongly correlated with differential miR-588 expression levels (Table 1, *P<0.05).
Table 1.
Correlation of miR-588 expression with breast cancer patients’ clinicopathological characteristics.
| Clinicopathological characteristics | n | MiR-588 expression pattern | P value | |
|---|---|---|---|---|
| Low (n=90) n (%) | High (n=84) n (%) | |||
| Age (years) | ||||
| ≤55 | 60 | 31 (34.4%) | 29 (34.5%) | 0.319 |
| >55 | 114 | 59 (65.5%) | 55 (65.5%) | |
| TNM stage | ||||
| I/II | 91 | 33 (36.7%) | 58 (69.0%) | 0.019* |
| III | 83 | 57 (63.3%) | 26 (31.0%) | |
| Tumor size (cm) | ||||
| ≤5 | 127 | 63 (70.0%) | 64 (76.2%) | 0.417 |
| >5 | 47 | 27 (30.0%) | 20 (23.8%) | |
| Histology | ||||
| 1 | 36 | 22 (24.4%) | 14 (16.7%) | 0.254 |
| 2 | 103 | 49 (54.4%) | 54 (64.3%) | |
| 3 | 35 | 19 (21.1%) | 16 (19.4%) | |
| Lymph node metastasis | ||||
| Negative | 84 | 29 (32.2%) | 55 (65.5%) | 0.014* |
| Positive | 90 | 61 (67.8%) | 29 (34.5%) | |
| Estrogen receptor status | ||||
| Negative | 91 | 32 (35.6%) | 59 (70.2%) | 0.009* |
| Positive | 83 | 58 (64.4%) | 25 (29.8%) | |
TNM – tumor (T), the extent of spread to the lymph nodes (N), and the presence of metastasis (M) (*P<0.05).
We then focussed on the key prognostic factor of breast cancer patients – postoperative overall survival. By separating patient data into 2 arms, we discovered that cancer patient overall survival was much shorter in those with low miR-588 expression than in those with high miR-588 expression (Figure 2, P<0.001, log-rank test). We used Cox proportional hazard regression models to analyze the correlation of breast cancer patient overall survival with clinicopathological characteristics. The analyses, using both univariate and multivariate parameters, demonstrated that breast cancer patient TNM stage, lymph node metastasis, estrogen receptor status, and miR-588 expression were all independently correlated with patient overall survival (Table 2, *P<0.05).
Figure 2.
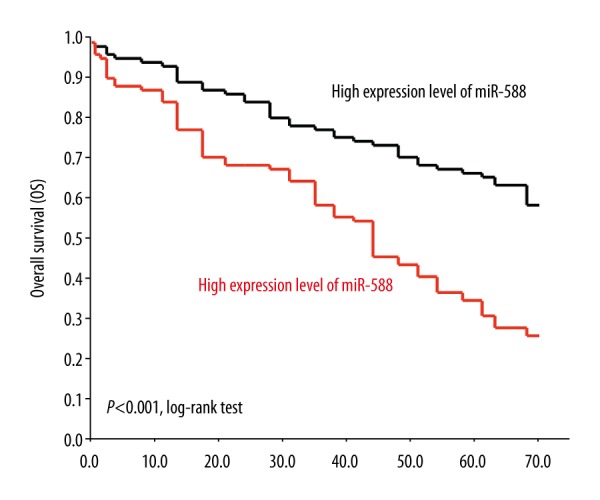
MiR-588 downregulation is correlated with breast cancer patient short survival. Breast cancer patient overall survival (OS) was surveyed according to endogenous miR-588 expression in their primary tumors. Using the log-rank test, patients with low expression levels of miR-588 showed significantly shorter survival than patients with high expression levels of miR-588 (P<0.001).
Table 2.
Correlation of breast cancer patients’ overall survival with their clinicopathological characteristics.
| Clinicopathological characteristics | Comparison | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| P value | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | ||
| Age (years) | ≤55 vs. >55 | 0.457 | 1.255 (0.987–1.583) | ||
| TNM stage | I/II vs. III | 0.015* | 2.561 (2.259–2.891) | 0.010* | 1.975 (1.698–2.286) |
| Tumor size (cm) | ≤5 vs. >5 | 0.336 | 0.782 (0.592–1.049) | ||
| Histology | 1 vs. 2 vs. 3 | 0.188 | 0.446 (0.278–0.685) | ||
| Lymph node metastasis | Negative vs. positive | 0.009* | 4.556 (4.286–4.894) | 0.014* | 3.279 (2.985–3.586) |
| Estrogen receptor status | Negative vs. positive | 0.007* | 4.123 (3.784–4.578) | 0.010* | 2.874 (2.488–3.281) |
| MiR-588 expression | Low vs. high | 0.015* | 2.572 (2.091–2.989) | 0.019* | 2.032 (1.985–2.352) |
TNM – tumor (T), the extent of spread to the lymph nodes (N), and the presence of metastasis (M) (*P<0.05).
MiR-588 upregulation inhibited proliferation and increased chemosensitivity in breast cancer
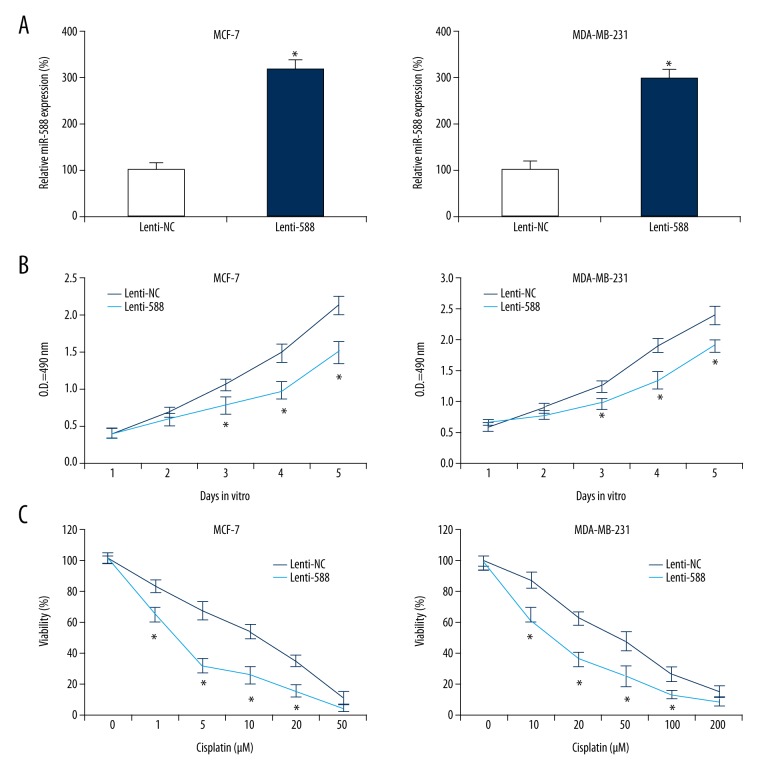
Finally, we wondered whether miR-588 might have functional role in breast cancer. Two breast cancer cell lines, MCF-7 and MDA-MB-231, were infected with lentivirus of Lenti-588 or overexpressed endogenous miR-588. In controlled infection, cells were infected with a non-specific lentivirus, Lenti-NC. QPCR showed that, in infected MCF-7 and MDA-MB-231 cells, miR-588 was significantly upregulated by Lenti-588 (Figure 3A, *P<0.05).
Figure 3.
Effect of miR-588 on breast cancer proliferation and chemosensitivity. (A) MCF-7 and MDA-MB-231 cells were infected with lentivirus of Lenti-588 to overexpress endogenous miR-588 expressions. In parallel control cells, they were infected with a non-specific lentivirus, Lenti-NC. QPCR was then used to assess the efficacy of lentiviral infection (*P<0.05). (B) Infected MCF-7 and MDA-MB-231 cells were equally plated in 96-well plates for 5 days in vitro. Daily growth rate of cancer cells was assessed using a proliferation assay at optical density (OD) of 490 nm (*P<0.05). (C) In 96-well plates containing infected MCF-7 and MDA-MB-231 cells, various concentrations of cisplatin were added. For MCF-7 cells, cisplatin concentrations were 0, 1, 5, 10, 20, and 50 mM. For MDA-MB-231 cells, cisplatin concentrations were 0, 10, 20, 50, 100, and 200 mM. At 24 h later, the relative viability of breast cancer cells in each well was assessed at optical density (OD) of 570 nm, and normalized to the OD value in the well without cisplatin treatment (*P<0.05).
Infected MCF-7 and MDA-MB-231 cells were re-cultured in 96-well plates. Their growth was assessed by a proliferation assay for 5 days in vitro, demonstrating that, in both MCF-7 and MDA-MB-231 cells, miR-588 upregulation significantly inhibited their in vitro proliferation (Figure 3B, *P<0.05).
Moreover, infected MCF-7 and MDA-MB-231 cells were assessed by a cisplatin chemosensitivity assay. MCF-7 cells were treated with cisplatin for 24 h at concentrations between 1 and 50 mM. MDA-MB-231 cells were treated with cisplatin for 24 h at concentrations between 10 and 200 mM. Then, a viability measurement demonstrated that, in MCF-7 and MDA-MB-231 cells, miR-588 upregulation significantly increased their cisplatin chemosensitivity (Figure 3C, *P<0.05).
Discussions
We investigated the expression pattern, prognostic potential, and functional role of miR-588 in human breast cancer. First, we utilized quantitative qPCR to assess the gene expression of miR-588 in 8 BC cell lines. We found that, as compared to MCF-10A, a benign breast epithelial cell line, miR-588, was downregulated in all tested BC cell lines. Among the assessed BC cell lines, MDA-MB-231 and MDA-MB-436 were triple-negative breast cancer cells. Our results show that miR-588 was downregulated in all triple-negative and non-triple-negative BC cells, suggesting that miR-588 downregulation was predominant among different BC subtypes. In addition, we compared miR-588 expression between breast carcinoma and non-carcinoma tissues in human patients. QPCR showed similar miR-588 downregulation pattern in in vivo breast carcinomas as in in vitro BC cell lines. These results are in line with a recent study showing that miR-588 was significantly downregulated in human lung cancer [19], suggesting that miR-588 may be aberrantly and predominantly downregulated in various cancer types.
Secondly, we assessed the correlation of miR-588 expression with clinicopathological characteristics of BC patients. Based on mean value of endogenous miR-588 expression in their carcinoma samples, BC patients were divided into 2 groups, 1 with low miR-588 expression and 1 with high miR-588 expression. Then, using the Pearson chi-square test, we discovered that low expression level of miR-588 in BC patient carcinomas was closely correlated with poor prognosis, including advanced TNM stage, positive lymph node metastasis status, and positive estrogen receptor status. A previous study showed that, in patients with lung squamous cell carcinoma, miR-588 was also associated with clinical stage and lymph node metastasis [19]. Thus, it would be interestingly to learn, through further screening of other carcinoma samples, whether miR-588 may be used a broader biomarker for predicting other types of cancers in addition to breast and lung cancers.
The most significant finding of our study may be that the low expression level of miR-588 was closely correlated with poor overall survival of BC patients. In a previous study, although miR-588 was identified to be associated with clinical properties of lung cancer patients, the possible correlation of miR-588 with survival data was not assessed [19]. In our study, we used Cox proportional hazard regression models and confirmed that miR-588 was truly an independent prognostic factor for predicting cancer patient overall survival. Thus, our study is the first to present the clinical relevance of cancer patient survival on miR-588 expression among any known human cancers.
We used lentiviral infection technology to assess the possible regulatory role of miR-588 in BC. After generating BC cells with stably overexpressed miR-588, we discovered that miR-588 upregulation had significant anti-cancer effects by inhibiting BC proliferation and increasing cisplatin chemosensitivity. Functional regulation of miR-588 was also found in human lung cancer, as miR-588 upregulation inhibited lung cancer cell migration and invasion [19]. Our study is the first to present solid evidence of a tumor-regulatory role of miR-588 in breast cancer.
Conclusions
Overall, our study presented for the first time that miR-588 was predominantly downregulated in human BC. We also showed that miR-588 might be a useful biomarker, as well as a novel therapeutic target in BC, since its upregulation had significant tumor-suppressive effects on BC cells.
Footnotes
Source of support: Departmental sources
References
- 1.Tao Z, Shi A, Lu C, et al. Breast Cancer: Epidemiology and etiology. Cell Biochem Biophys. 2015;72:333–38. doi: 10.1007/s12013-014-0459-6. [DOI] [PubMed] [Google Scholar]
- 2.Smigal C, Jemal A, Ward E, et al. Trends in breast cancer by race and ethnicity: update 2006. Cancer J Clin. 2006;56:168–83. doi: 10.3322/canjclin.56.3.168. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 4.Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol. 2014;15:e279–89. doi: 10.1016/S1470-2045(13)70567-9. [DOI] [PubMed] [Google Scholar]
- 5.McNamara KM, Moore NL, Hickey TE, et al. Complexities of androgen receptor signalling in breast cancer. Endocr Relat Cancer. 2014;21:T161–81. doi: 10.1530/ERC-14-0243. [DOI] [PubMed] [Google Scholar]
- 6.Sato F, Saji S, Toi M. Genomic tumor evolution of breast cancer. Breast Cancer. 2016;23:4–11. doi: 10.1007/s12282-015-0617-8. [DOI] [PubMed] [Google Scholar]
- 7.Videira M, Reis RL, Brito MA. Deconstructing breast cancer cell biology and the mechanisms of multidrug resistance. Biochim Biophys Acta. 2014;1846:312–25. doi: 10.1016/j.bbcan.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Telli ML, Sledge GW. The future of breast cancer systemic therapy: The next 10 years. J Mol Med (Berl) 2015;93:119–25. doi: 10.1007/s00109-014-1238-y. [DOI] [PubMed] [Google Scholar]
- 9.Ebctcg, McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–35. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhaskaran M, Mohan M. MicroRNAs: History, biogenesis, and their evolving role in animal development and disease. Vet Pathol. 2014;51:759–74. doi: 10.1177/0300985813502820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3–14. doi: 10.1016/j.addr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milenkovic D, Jude B, Morand C. miRNA as molecular target of polyphenols underlying their biological effects. Free Radic Biol Med. 2013;64:40–51. doi: 10.1016/j.freeradbiomed.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 13.Khoshnaw SM, Green AR, Powe DG, Ellis IO. MicroRNA involvement in the pathogenesis and management of breast cancer. J Clin Pathol. 2009;62:422–28. doi: 10.1136/jcp.2008.060681. [DOI] [PubMed] [Google Scholar]
- 14.Gotte M. MicroRNAs in breast cancer pathogenesis. Minerva Ginecol. 2010;62:559–71. [PubMed] [Google Scholar]
- 15.Shi M, Guo N. MicroRNA expression and its implications for the diagnosis and therapeutic strategies of breast cancer. Cancer Treat Rev. 2009;35:328–34. doi: 10.1016/j.ctrv.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Iorio MV, Casalini P, Piovan C, et al. Breast cancer and microRNAs: Therapeutic impact. Breast. 2011;20(Suppl 3):S63–70. doi: 10.1016/S0960-9776(11)70297-1. [DOI] [PubMed] [Google Scholar]
- 17.Iorio MV, Ferracin M, Liu CG, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–70. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 18.Mar-Aguilar F, Mendoza-Ramirez JA, Malagon-Santiago I, et al. Serum circulating microRNA profiling for identification of potential breast cancer biomarkers. Dis Markers. 2013;34:163–69. doi: 10.3233/DMA-120957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian L, Lin L, Du Y, et al. MicroRNA-588 suppresses tumor cell migration and invasion by targeting GRN in lung squamous cell carcinoma. Mol Med Rep. 2016;14:3021–28. doi: 10.3892/mmr.2016.5643. [DOI] [PMC free article] [PubMed] [Google Scholar]