Abstract
The aim was to investigate if hydrogen sulfide (H2S) induces the formation of the NLRP3 inflammasome and subsequent IL‐1β and IL‐18 secretion in human peripheral blood mononuclear cells (PBMCs) and in the human monocyte cell line THP1. Bacterial production of H2S has been suggested to participate in the inflammatory host response in periodontitis pathogenesis. H2S is a toxic gas with pro‐inflammatory properties. It is produced by bacterial degradation of sulfur‐containing amino acids, for example, cysteine. We hypothesize that H2S affects the inflammatory host response by inducing formation of the NLRP3 inflammasome and thereby causes the secretion of IL‐1ß and IL‐18. PBMCs from eight healthy blood donors, the human monocyte cell line THP1 Null, and two variants of the THP1 cell line unable to form the NLRP3 inflammasome were cultured in the presence or absence of 1 mM sodium hydrosulfide (NaHS) in 24‐well plates at 37°C for 24 hr. Supernatants were collected and the IL‐1β and IL‐18 concentrations were measured with DuoSet ELISA Development kit. PBMCs exposed to NaHS produced more IL‐1ß and IL‐18 than unexposed control cells (p = .023 and p = .008, respectively). An increase of extracellular potassium ions (K+) inhibited the secretion of IL‐1ß and IL‐18 (p = .008). Further, NaHS triggered the secretion of IL‐1ß and IL‐18 in human THP1‐Null monocytes (p = .0006 and p = .002, respectively), while the NaHS‐dependent secretion was reduced in the monocyte cell lines unable to form the NLRP3 inflammasome. Hence, the results suggest that NaHS induces the formation of the NLRP3 inflammasome and thus the secretion of IL‐1ß and IL‐18. Enhanced NLRP3 inflammasome‐dependent secretion of IL‐1β and IL‐18 in human mononuclear leukocytes exposed to NaHS in vitro is reported. This may be a mode for H2S to contribute to the inflammatory host response and pathogenesis of periodontal disease.
Keywords: hydrogen sulfide, IL‐1ß, IL‐18, monocytes, NLRP3 inflammasome, periodontitis
1. INTRODUCTION
Periodontitis, a bacteria‐induced inflammation, is the result of a disruption of the homeostasis between the bacteria and the host. This dysbiosis is characterized by a high bacterial diversity and the growth of anaerobic, Gram‐negative, and proteolytic bacteria in the subgingival pocket (Kilian, Chapple, Hannig, et al., 2016). The new biofilm produces, among other metabolites, hydrogen sulfide (H2S), suggested to be part of the pathogenesis of periodontal disease because it is well known for its toxicity. H2S is produced by bacterial degradation of sulfur‐containing amino acids, for example, cysteine (Kuester & Williams, 1964) by many different bacterial strains (Basic, Blomqvist, Carlén, & Dahlén, 2015; Persson, Claesson, & Carlsson, 1989), and its presence has been reported in gingival crevicular fluid (Persson, 1992).
Although, it is generally accepted that the formation of the subgingival biofilm is essential for the induction of periodontitis, it remains unclear whether and how the biofilm and/or its end products contribute to the inflammatory host response that is characteristic for the disease. Monocytes/macrophages play an important role in the immune response of the host because they are able to produce a large variety of cytokines, for example, IL‐1β. The possible role of H2S on host cells in the periodontium is ambiguous because previous studies have reported both pro‐ and anti‐inflammatory properties of H2S (Whiteman & Winyard, 2011; Zhang & Bhatia, 2008). H2S can induce pro‐inflammatory cytokine IL‐8 production from gingival and oral epithelial cells in vitro (Chen, Kajiya, Giro, et al., 2010), but it has also been shown to cause cell death in lymphocytes and reduce their IL‐2 production. (Mirandola, Gobbi, Sponzilli, et al., 2007).
The pro‐inflammatory cytokine IL‐1β has been implicated as a key cytokine in periodontal disease (Graves, 2008; Kornman, 1997; Yilmaz & Lee, 2015). An inactive form of the protein (pro‐IL‐1β) is cleaved to its bioactive form, IL‐1β, via the formation of a multiprotein complex, called the NLRP3 inflammasome (Pétrilli et al., 2007). This is conducted via the cleavage and activation of pro‐caspase‐1 to its active form caspase‐1. In the same way as pro‐IL‐1β, pro‐IL‐18 is cleaved to IL‐18. A variety of substances, such as ATP, peptidoglycans, and crystals can induce the formation of the NRLP3 inflammasome and consequently the release of IL‐1β and IL‐18 (Pétrilli et al., 2007). A previous report has shown that NaHS also is able to induce the production and secretion of IL‐1β in the human monocyte cell line U937 (Zhi, Ang, Zhang, Moore, & Bhatia, 2007), but if this is mediated through the formation of the NLRP3 inflammasome remains unclear.
The aim of this study was to investigate the effect of hydrogen sulfide on the inflammatory response by studying human mononuclear leukocytes in vitro and their secretion of IL‐1β and IL‐18.
2. MATERIAL AND METHODS
2.1. Isolation of cells from human peripheral blood
Blood from eight and 10 unidentified donors respectively, collected at the Sahlgrenska University Hospital in Gothenburg, Sweden, was used in this study. The subjects that donate the blood consent that the blood may be used in research but no information of the subjects is available for the researchers. The subjects are defined as “healthy” because they fulfill the requirements to donate blood to the hospital blood bank, but the status of their oral health is not known. The peripheral blood mononuclear cells (PBMCs) were extracted by centrifugation using Ficoll‐Pague Plus (GE Healthcare Bio‐Sciences AB, Uppsala, Sweden). The cells were suspended in a solution containing Dulbecco´s Modified Eagle´s Medium, 5 % heat‐inactivated human AB serum (Sigma‐Aldrich Sweden AB, Stockholm Sweden), 100 U/mL penicillin, and 100 μg/mL of streptomycin (Invitrogen, Lidingö, Sweden). Using 0.4% Trypan Blue (Sigma‐Aldrich Sweden AB), the cell viability was determined, and the cells were counted in a Bürker chamber.
2.2. The human monocyte cell line THP1
The model cell line THP1‐Null (InvivoGen, San Diego, CA, USA) expresses high levels of NLRP3, ASC, and pro‐caspase‐1. In contrast, the ASC‐deficient THP1‐defASC cell line and the NLRP3‐deficient human monocytes THP1‐defNLRP3 (also InvivoGen) are both unable to form the NLRP3 inflammasome. The cells were cultured in RPMI 1640 growth medium (Invitrogen, Sweden) with 10% heat‐inactivated fetal bovine serum (Invitrogen, Sweden), 50 U/mL penicillin, and 50 μg/mL of streptomycin (Invitrogen, Sweden). The cell viability was determined using 0.4 % Trypan Blue, and the cells were counted.
2.3. H2S exposure
The cells (2 × 106 PBMCs/THP1 cells/mL) were exposed to 1 μL/mL lipopolysaccharides (LPS, from Escherichia coli, Sigma‐Aldrich Sweden AB) for 3 hr at 37°C (humidified atmosphere, 5% CO2) in 96‐well plates to induce the production of pro‐IL‐1β. This step was, however, skipped for the last experiment without pre‐exposure to LPS. NaHS was used as H2S source, and the cells were exposed to 1 mM for 24 hr. In order to block the activation of the NLRP3 inflammasome, 130 mM of potassium chloride (KCl; Merck KGaA, Darmstadt, Germany) was added (Pétrilli et al., 2007). To test the method, 200 μg/mL aluminum potassium sulfate dodecahydrate (Merck) was used as a positive control. The cells exposed to NaHS were compared with unexposed controls.
2.4. The secretion of cytokines
The secretion of IL‐1β and IL‐18 by PBMCs/THP1 cells was measured with the use of the DuoSet ELISA Development Kit (R&D Systems, Abingdon, UK) according to the manufacturer´s instructions. Briefly, the plates were coated with capture antibody overnight. The supernatants were incubated with a cytokine‐specific biotinylated detection antibody and marked with streptavidin‐conjugated horseradish‐peroxidase. After the addition of substrate, the absorbance was recorded with an ELISA microplate reader (BioPlex 200 instrument equipped with BioManager analysis software (Bio‐Rad Laboratories AB, Solna, Sweden)).
2.5. Statistical analyses
Statistical analyses were performed in GraphPad Prism 6.0. The results from PBMCs were analyzed with Wilcoxon Signed‐Rank Test while the results from THP1 cells were compared using Mann–Whitney U Test.
3. RESULTS
3.1. Cytokine secretion by human peripheral blood leukocytes
The secretion of IL‐1ß and IL‐18 after NaHS exposure compared with unexposed peripheral blood cells is seen in Figure 1 for eight blood donors. PBMCs exposed to NaHS produced significantly more IL‐1ß and IL‐18 (p = .023 and 0.008, respectively). The secretion of both IL‐1ß and IL‐18 (both p = .008) was inhibited when the extracellular concentration of KCl was increased.
Figure 1.
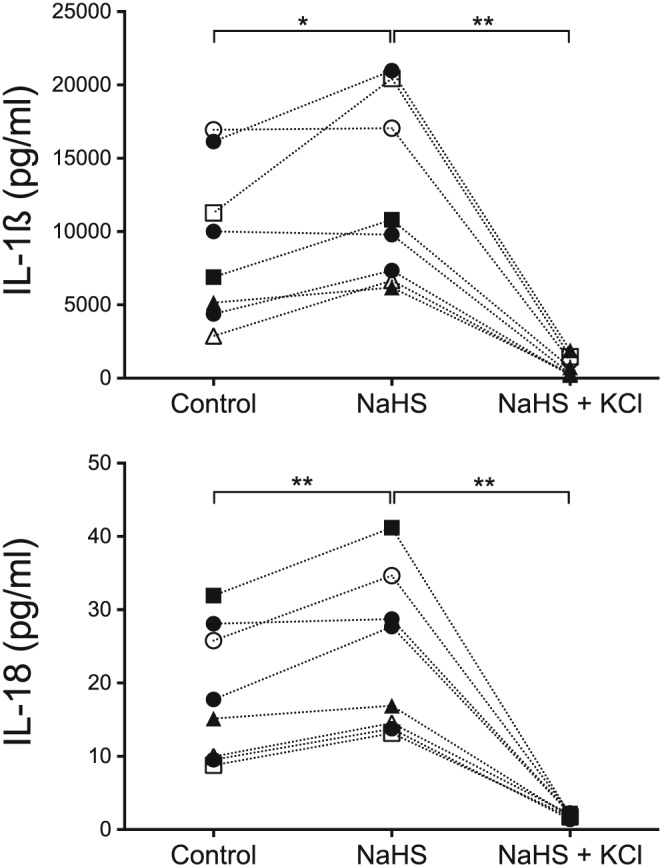
The IL‐1ß and IL‐18 secretion of peripheral blood mononuclear cells, measured from eight donors exposed to 1 mM hydrogen sulfide ions (NaHS) for 24 hr. The cells were pre‐exposed to lipopolysaccharides prior to NaHS. A Wilcoxon Signed‐Rank Test revealed a statistically significant difference (p = .023) in IL‐1ß secretion when the cells were exposed to NaHS compared with unexposed control cells. IL‐18 secretion was also statistically significant (p = .008). A statistical difference (p = .008 for both IL‐1ß and IL‐18) was also seen between the cells exposed to NaHS and KCl compared with only NaHS. The different symbols in the figures illustrate eight different blood donors
3.2. Cytokine secretion from THP1 cells
The IL‐1ß secretion in human THP1 Null monocytes was triggered when the cells were exposed to NaHS (p = .0006) compared with unexposed cells (Figure 2). A similar result was seen for IL‐18 secretion (p = .002). The two monocyte cell lines that are unable to form the NLRP3 inflammasome, THP1‐defASC and THP1‐defNLRP3, did not produce more IL‐1ß and IL‐18 when exposed to NaHS than unexposed control cells.
Figure 2.
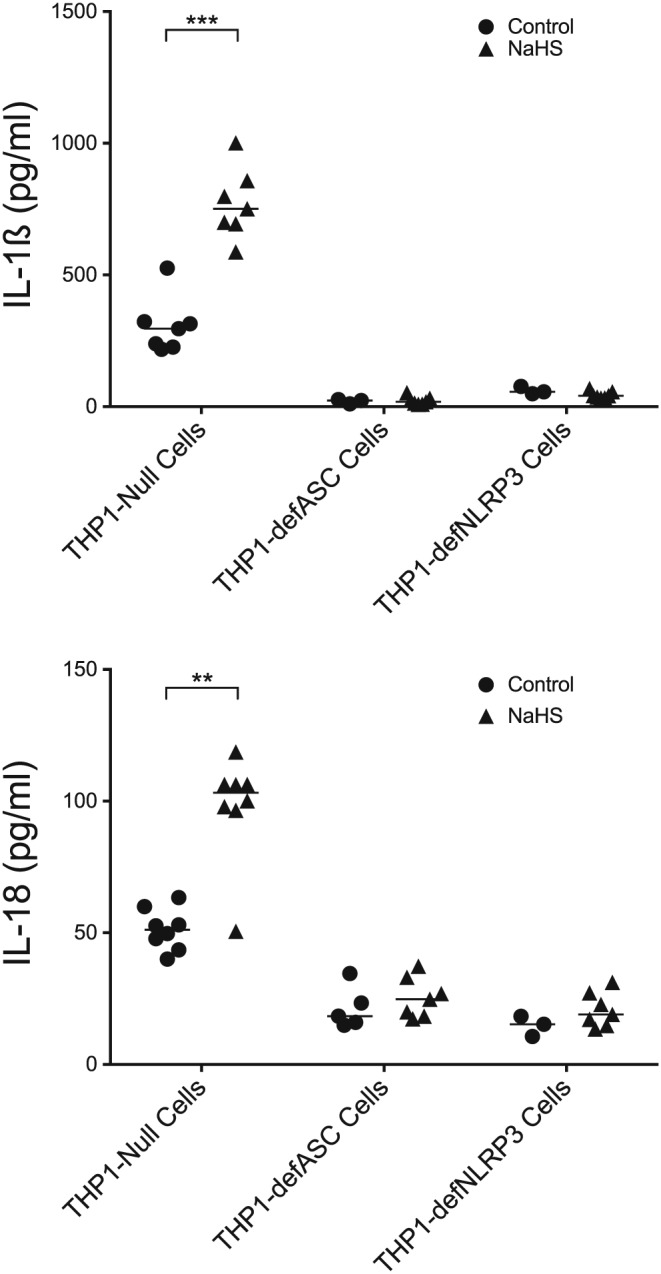
The IL‐1ß and IL‐18 secretion of three THP1 cell lines exposed to 1 mM NaHS for 24 hr. Prior to NaHS, the cells were exposed to lipopolysaccharides. The THP1‐Null cells showed a statistically higher IL‐1ß and IL‐18 secretion when exposed to NaHS (Mann–Whitney U test, p = .0006 for IL‐1ß and p = .002 for IL‐18) compared with unexposed cells. When the other two cell lines were tested, both unable to form the NLRP3‐inflammasome, there was no difference in IL‐1ß and IL‐18 secretion when exposed to NaHS compared to control. The median of the group is shown as a vertical line
3.3. Cytokine secretion from cells not exposed to lipopolysaccharides
The IL‐1ß secretion was also measured from cells not pre‐exposed to LPS. Human PBMCs from 10 donors were exposed to NaHS (Figure 3). A significantly higher IL‐1ß secretion was seen when the cells were exposed to NaHS compared with unexposed control cells (p = .020) or when KCl was added (p = .004).
Figure 3.
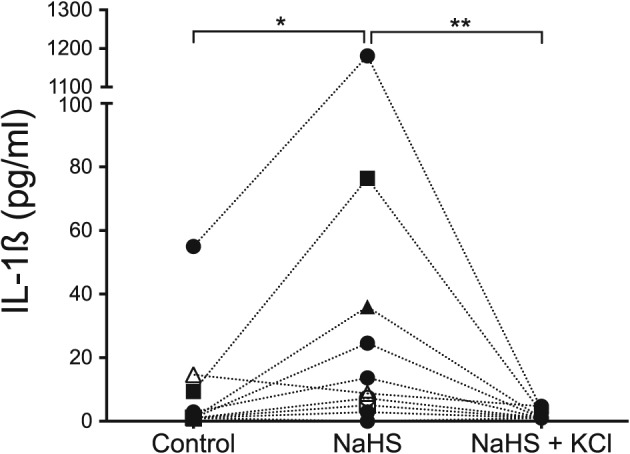
Peripheral blood mononuclear cells from 10 donors not pre‐exposed to lipopolysaccharides, but only exposed to 1 mM NaHS for 24 hr. The exposure to NaHS resulted in a statistically significant difference in IL‐1ß secretion compared with control cells (p = .020, Wilcoxon Signed‐Rank Test). A reduction in IL‐1ß secretion was seen when the cells were exposed to increased extracellular KCl concentrations (p = .004)
Cellular viability was also assessed after 24 hr of incubation. The results showed similar viability for cells exposed to NaHS (88 ± 4%) as for unexposed control cells (92 ± 3%).
4. DISCUSSION
This study investigated if NaHS induced the formation of the NLRP3 inflammasome and thus subsequent secretion of IL‐1β and IL‐18 in human peripheral blood mononuclear cells and in the human monocyte cell line THP1. An enhanced NLRP3 inflammasome‐dependent secretion of IL‐1β and IL‐18 in human mononuclear leukocytes exposed to NaHS in vitro was found.
The production and secretion of IL‐1β and IL‐18 is conducted as a result of two signals. The first signal stimulates the production of pro‐IL‐1β and pro‐IL‐18 in the nucleus and can be induced by LPS. The second signal, investigated in this study with the use of NaHS, leads to the formation of the NLRP3 inflammasome and thereby causes the release of IL‐1β and IL‐18 from the cell.
We also tested the effect of NaHS on IL‐1β secretion in PBMCs without pre‐exposure to LPS, to study if H2S could contribute to both production and secretion of the cytokines (Figure 3). The cells responded similarly to the cells that were pre‐exposed to LPS, but the secretion was lower than when the cells were pre‐exposed to LPS before adding NaHS. A reduced IL‐1β secretion without LPS treatment is in agreement with a previous report where ragweed pollen extract exposure in THP1 macrophages was studied (Varga et al., 2013). A previous study showed that NaHS exposure increased Akt phosphorylation, regulated by PI3K (Cai et al., 2007). This was confirmed in endothelial cells along with phosphorylation of ERK and p38 (Altaany, Yang, & Wang, 2013; Papapetropoulos, Pyriochou, Altaany, et al., 2009). Further, another study disclosed that exposure to NaHS, though the (ERK)‐NF‐κB signaling pathway, contributed to the production of IL‐1β (Zhi et al., 2007). This was conducted by IkBα degradation and consequently NF‐κB p65 activation. The study reported that the activation was highest after 30 min of incubation and at the concentration of 0.1 mM NaHS. There are, however, other reports claiming the opposite, that NaHS does not activate NF‐kB (Sulen, Gullaksen, Bader, et al., 2016; Whiteman et al., 2010). Nevertheless, it seems that H2S may substitute the LPS signal 1, possibly via mitogen‐activated protein kinases.
THP1 cells were used to investigate if the secretion of IL‐1β and IL‐18 was mediated through the formation of the NLRP3 inflammasome. The results showed a statistically significant increase in cytokine secretion in the THP1‐Null cell line when the cells were exposed to NaHS, illustrating that monocytes do secrete IL‐1β and IL‐18 in the presence of NaHS (Figure 2). However, two variants of the THP1 cell line that are unable to form the NLRP3 inflammasome did not produce IL‐1β and IL‐18 upon exposure to NaHS in our study. This strongly suggests that the secretion of the cytokines was mediated through the formation of the NLRP3 inflammasome.
Many different substances such as ATP, peptidoglycans, nigericin, or monosodium urate crystals may induce the second signal that activates the NLRP3 inflammasome, but the mechanism involved is still unknown. Pétrilli and coworkers (Pétrilli et al., 2007) suggested that the substances that activate the NLRP3 inflammasome have the ability to lower the intracellular potassium ion (K+) concentration, because the effect of all of the different substances are blocked if the efflux of K+ is inhibited. In our study, the cytokine production was inhibited when the extracellular concentration of K+ was increased. A previous report on porcine and bovine retinae showed the inhibitory effect of H2S donors on K+‐evoked [3H] D‐aspartate release (Opere, Monjok, Kulkarni, Njie, & Ohia, 2009). Furthermore, a study showed an inhibitory effect of H2S on potassium channels of trigeminal ganglion neurons (Feng, Zhou, Meng, et al., 2013) and another the effect of H2S on Kv 7.4 channels that resulted in vasodilation (Martelli, Testai, Breschi, et al., 2013). All these studies together suggest that the triggers of the NLRP3 inflammasome, including H2S, reduce the intracellular K+ concentration and thereby cause the formation of the NLRP3 inflammasome.
Despite that it is generally acknowledged that H2S is a toxic gas, our understanding of its functions is poor. It is proposed that H2S has the ability to split essential disulfide bonds in proteins and can bind to metal ions (Beauchamp, Bus, & Popp, 1984). Further, H2S has been suggested to inhibit cytochrome oxidase (Nicholls & Kim, 1982), catalase, and myeloperoxidase (Claesson, Granlund‐Edstedt, Persson, & Carlsson, 1989). It has also been shown to induce apoptosis in human gingival fibroblasts in vitro (Zhang, Dong, & Chu, 2010). These factors are believed to explain the toxicity of H2S. Our study suggests one more way by which H2S may affect the host cells and thereby contribute to disease development (Figure 4). H2S may induce and/or maintain the host immune response by the secretion of the pro‐inflammatory cytokines IL‐1β and IL‐18 in macrophages. This may play an important role in the pathogenesis of periodontal disease, characterized by a proteolytic biofilm in the periodontal pocket.
Figure 4.
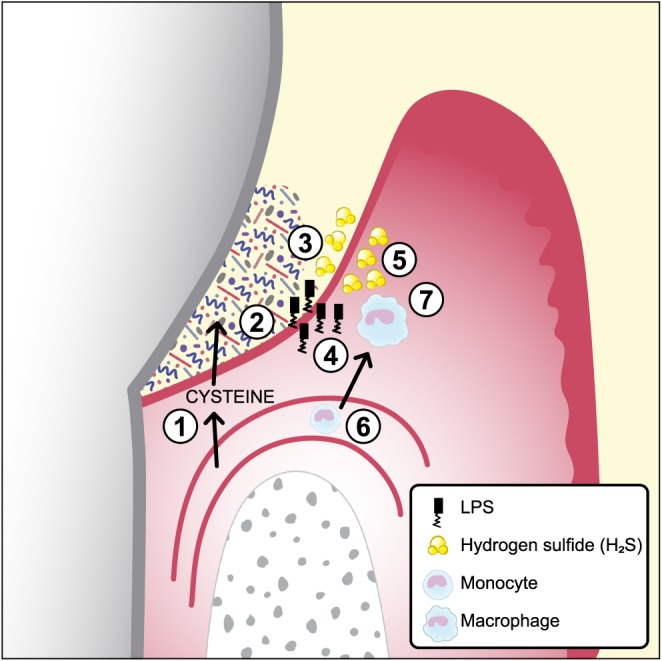
- Serum exudate from blood vessels containing serum proteins, peptides, and amino acids including cysteine.
- The exudate (gingival crevicular fluid) continues through the thin pocket epithelium (junctional epithelium) into the subgingival pocket.
- The subgingival plaque, containing numerous, mainly Gram‐negative, anaerobic bacteria with proteolytic capacity, degrade proteins, peptides, and amino acids including cysteine.
- Growing Gram‐negative anaerobes release lipopolysaccharides that penetrate the junctional epithelium into gingival connective tissues.
- Growing Gram‐negative anaerobes (Fusobacterium spp., Porphyromonas gingivalis, Treponema spp., and others) produce metabolites, for example, hydrogen sulfide (H2S).
- The inflammatory lesion attracts monocytes that migrate into the connective tissue and differentiate to macrophages.
- The effect of lipopolysaccharides and H2S on macrophages and the subsequent secretion of the pro‐inflammatory cytokines IL‐1β and IL‐18
The elevated secretion of IL‐1β and IL‐18 when exposed to NaHS shows the pro‐inflammatory capacity of H2S on human PBMCs from healthy blood donors (Figures 1 and 3). Because the cells were not separated further, it is unclear whether the production came from monocytes and/or lymphocytes. The ratio between these two was not further investigated in this study. Despite the small sample size of eight and 10 blood donors, a statistically significant increase of IL‐1β and IL‐18 secretion was seen in the presence of NaHS. However, there were distinct individual variations between the donors, which suggests that the sensitivity to NaHS exposure among the donors varies. Because the periodontal status of the donors is unknown, we can only speculate that the cells with higher IL‐1β and IL‐18 responses are from donors more prone to develop periodontal disease. This aspect is investigated in an ongoing clinical trial on periodontitis patients and healthy controls.
5. CONCLUSION
The production of pro‐inflammatory cytokines is essential in the induction and development of inflammatory diseases such as periodontal disease. We have studied the effect of the bacterial waste product H2S, by the use of NaHS, on cytokine secretion in vitro. Our results show higher secretion of IL‐1ß and IL‐18 from mononuclear leukocytes and THP1 cells when exposed to NaHS compared with unexposed controls. The NLRP3 inflammasome is essential for NaHS induced IL‐1ß and IL‐18 secretion in monocytes. The results of our study suggest a possible model (Figure 4) on how bacterial activity, through the end products of the biofilm, may contribute to the inflammatory host response and disease development. Further in vivo studies are needed to examine the function of H2S in the pathogenesis of periodontal disease.
ACKNOWLEDGMENTS
Special thanks to Anna‐Karin Östberg for technical assistance and interpretation of data. This study was supported by TUA‐Grant (TUAGBG‐89621, TUAGBG‐365041), the Swedish Dental Society, and the Gothenburg Dental Society. We are grateful to My Ewander for her expert assistance in preparing Figure 4.
Basic A, Alizadehgharib S, Dahlén G, Dahlgren U. Hydrogen sulfide exposure induces NLRP3 inflammasome‐dependent IL‐1β and IL‐18 secretion in human mononuclear leukocytes in vitro . Clin Exp Dent Res. 2017;3:115–120. https://doi.org/10.1002/cre2.69
REFERENCES
- Altaany, Z. , Yang, G. , & Wang, R. (2013). Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. Journal of Cellular and Molecular Medicine, 17(7), 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basic, A. , Blomqvist, S. , Carlén, A. , & Dahlén, G. (2015). Estimation of bacterial hydrogen sulfide production in vitro. Journal of Oral Microbiology, 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp, R. O. Jr. , Bus, J. S. , & Popp, J. A. (1984). A critical review of the literature on hydrogen sulfide toxicity. Critical Reviews in Toxicology, 13(1), 25–97. [DOI] [PubMed] [Google Scholar]
- Cai, W. J. , Wang, M. J. , Moore, P. K. , Jin, H. M. , Yao, T. , & Zhu, Y. C. (2007). The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovascular Research, 76(1), 29–40. [DOI] [PubMed] [Google Scholar]
- Chen, W. , Kajiya, M. , Giro, G. , Ouhara, K. , Mackler, H.E. , Mawardi, H. , … Kawai, T. (2010). Bacteria‐derived hydrogen sulfide promotes IL‐8 production from epithelial cells. Biochemical and Biophysical Research Communications, 391(1), 645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson, R. , Granlund‐Edstedt, M. , Persson, S. , & Carlsson, J. (1989). Activity of polymorphonuclear leukocytes in the presence of sulfide. Infection and Immunity, 57(9), 2776–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, X. , Zhou, Y. L. , Meng, X. , Qi, F.H. , Chen, W. , Jiang, X. , & Xu, G.Y. (2013). Hydrogen sulfide increases excitability through suppression of sustained potassium channel currents of rat trigeminal ganglion neurons. Molecular Pain, 9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves, D. (2008). Cytokines that promote periodontal tissue destruction. Journal of Periodontology, 79(8 SUPPL), 1585–1591. [DOI] [PubMed] [Google Scholar]
- Kilian, M. , Chapple, I. L. C. , Hannig, M. , Marsh, P.D. , Meuric, V. , Pedersen, A.M.L. , … Zaura, E. (2016). The oral microbiome – an update for oral healthcare professionals. British Dental Journal, 221(10), 657–666. [DOI] [PubMed] [Google Scholar]
- Kornman, K. S. (1997). The interleukin‐1 genotype as a severity factor in adult periodontal disease. Journal of Clinical Periodontology, 24(1), 72–77. [DOI] [PubMed] [Google Scholar]
- Kuester, E. , & Williams, S. T. (1964). Production of hydrogen sulfide by Streptomycetes and methods for its detection. Applied Microbiology, 12, 46–52. [PMC free article] [PubMed] [Google Scholar]
- Martelli, A. , Testai, L. , Breschi, M. C. , Lawson, K. , McKay, N.G. , Miceli, F. , … Calderone, V. (2013). Vasorelaxation by hydrogen sulphide involves activation of Kv7 potassium channels. Pharmacological Research, 70(1), 27–34. [DOI] [PubMed] [Google Scholar]
- Mirandola, P. , Gobbi, G. , Sponzilli, I. , Pambianco, M. , Malinverno, C. , Cacchioli, A. , … Vitale, M. (2007). Exogenous hydrogen sulfide induces functional inhibition and cell death of cytotoxic lymphocytes subsets. Journal of Cellular Physiology, 213(3), 826–833. [DOI] [PubMed] [Google Scholar]
- Nicholls, P. , & Kim, J. K. (1982). Sulphide as an inhibitor and electron donor for the cytochrome c oxidase system. Canadian Journal of Biochemistry, 60(6), 613–623. [DOI] [PubMed] [Google Scholar]
- Opere, C. A. , Monjok, E. M. , Kulkarni, K. H. , Njie, Y. F. , & Ohia, S. E. (2009). Regulation of [3H] d‐aspartate release from mammalian isolated retinae by hydrogen sulfide. Neurochemical Research, 34(11), 1962–1968. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos, A. , Pyriochou, A. , Altaany, Z. , Yang, G. , Marazioti, A. , Zhou, Z. , … Wang, R. (2009). Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proceedings of the National Academy of Sciences of the United States of America, 106(51), 21972–21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson, S. (1992). Hydrogen sulfide and methyl mercaptan in periodontal pockets. Oral Microbiology and Immunology, 7(6), 378–379. [DOI] [PubMed] [Google Scholar]
- Persson, S. , Claesson, R. , & Carlsson, J. (1989). The capacity of subgingival microbiotas to produce volatile sulfur compounds in human serum. Oral Microbiology and Immunology, 4(3), 169–172. [DOI] [PubMed] [Google Scholar]
- Pétrilli, V. , Papin, S. , Dostert, C. , Mayor, A. , Martinon, F. , & Tschopp, J. (2007). Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death and Differentiation, 14(9), 1583–1589. [DOI] [PubMed] [Google Scholar]
- Sulen, A. , Gullaksen, S. E. , Bader, L. , McClymont, D.W. , Skavland, J. , Gavasso, S. , & Gjertsen, BT. (2016). Signaling effects of sodium hydrosulfide in healthy donor peripheral blood mononuclear cells. Pharmacological Research, 113, 216–227. [DOI] [PubMed] [Google Scholar]
- Varga, A. , Budai, M. M. , Milesz, S. , Bácsi, A. , Tozsér, J. , & Benko, S. (2013). Ragweed pollen extract intensifies lipopolysaccharide‐induced priming of NLRP3 inflammasome in human macrophages. Immunology, 138(4), 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman, M. , & Winyard, P. G. (2011). Hydrogen sulfide and inflammation: The good, the bad, the ugly and the promising. Expert Review of Clinical Pharmacology, 4(1), 13–32. [DOI] [PubMed] [Google Scholar]
- Whiteman, M. , Li, L. , Rose, P. , Tan, C. H. , Parkinson, D. B. , & Moore, P. K. (2010). The effect of hydrogen sulfide donors on lipopolysaccharide‐induced formation of inflammatory mediators in macrophages. Antioxidants and Redox Signaling, 12(10), 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz, Ö. , & Lee, K. L. (2015). The inflammasome and danger molecule signaling: At the crossroads of inflammation and pathogen persistence in the oral cavity. Periodontology 2000, 69(1), 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , & Bhatia, M. (2008). Hydrogen sulfide: A novel mediator of leukocyte activation. Immunopharmacology and Immunotoxicology, 30(4), 631–645. [DOI] [PubMed] [Google Scholar]
- Zhang, J. H. , Dong, Z. , & Chu, L. (2010). Hydrogen sulfide induces apoptosis in human periodontium cells. Journal of Periodontal Research, 45(1), 71–78. [DOI] [PubMed] [Google Scholar]
- Zhi, L. , Ang, A. D. , Zhang, H. , Moore, P. K. , & Bhatia, M. (2007). Hydrogen sulfide induces the synthesis of proinflammatory cytokines in human monocyte cell line U937 via the ERK‐NF‐κB pathway. Journal of Leukocyte Biology, 81(5), 1322–1332. [DOI] [PubMed] [Google Scholar]


