Summary
Cannabidivarin (CBDV) and cannabidiol (CBD) have recently emerged among cannabinoids for their potential antiepileptic properties, as shown in several animal models. We report the case of a patient affected by symptomatic partial epilepsy who used cannabis as self‐medication after the failure of countless pharmacological/surgical treatments. Clinical and video electroencephalogram (EEG) evaluations were periodically performed, and the serum levels of CBDV, CBD, and Δ9‐tetrahydrocannabinol were repeatedly measured. After cannabis administration, a dramatic clinical improvement, in terms of both decrease in seizure frequency and recovery of cognitive functions, was observed, which might parallel high CBDV plasma concentrations. To widen the spectrum of CBDV possible mechanisms of action, electrophysiological methods were applied to investigate whether it could exert some effects on γ‐aminobutyric acid (GABA)A receptors. Our experiments showed that, in human hippocampal tissues of four patients affected by drug‐resistant temporal lobe epilepsy (TLE) transplanted in Xenopus oocytes, there is decrease of current rundown (i.e., reduction of use‐dependent GABAA current) after prolonged exposure to CBDV. This result has been confirmed using a single case of Rasmussen encephalitis (RE). Our patient's electroclinical improvement supports the hypothesis that cannabis could actually represent an effective, well‐tolerated antiepileptic drug. Moreover, the experimental data suggest that CBDV may greatly contribute to cannabis anticonvulsant effect through its possible GABAergic action.
Keywords: Epilepsy, Cannabidivarin, Antiepileptic, GABA
The potential role of cannabis in treating several diseases, including epilepsy, has been known for centuries.1, 2 On the basis of in vitro and in vivo studies, phytocannabinoids other than the well‐known Δ9‐tetrahydrocannabinol (Δ9‐THC) are thought to have a major anticonvulsant effect, and in particular cannabidiol (CBD) has received great attention in the scientific community and media.3, 4, 5 Its propyl analogue, cannabidivarin (CBDV), has been recently proposed to have an antiepileptic action in animal models.6 However, data from well‐designed clinical trials for both compounds are still lacking.7 We report the case of a patient affected by drug‐resistant focal epilepsy treated with homemade cannabis‐containing infusions who underwent clinical, video electroencephalogram (EEG) and laboratory monitoring. Considering the patient's global improvement and the laboratory findings suggesting a possible beneficial effect of CBDV, we used the technique of membrane microtransplantation from brain tissues to Xenopus laevis oocytes8 to investigate whether CBDV may affect γ‐aminobutyric acid (GABA)‐ergic transmission.
Case Report
Clinical, EEG, and neuroimaging findings
We report the case of a 19‐year‐old boy referred to our Epilepsy Centre for a symptomatic, probably immune‐mediated partial epilepsy begun at the age of 5. His medical history was characterized by progressive unilateral neurological deficits, namely, right hemiparesis with dystonia and motor aphasia, and recurrent partial seizures with heterogeneous subjective manifestations, right motor signs, and speech and consciousness impairment. EEG recordings showed abundant epileptiform activity on the left centrotemporal regions. Magnetic resonance imaging (MRI) scans documented a lesion involving left putamen, external capsule, and insula along with atrophy of the head of the ipsilateral caudate nucleus, first interpreted as a perinatal hypoxic/ischemic lesion (Fig. 1). Cardiological evaluation, autoantibody screening (including anti‐Hu, Yo, Ri, Ma2, CV2, amphiphysin, N‐methyl‐d‐aspartate receptor [NMDAR], voltage‐gated potassium channel receptor [VGKCR], GABAB‐ receptor [GABAB‐R], and α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptor [AMPAR] antibodies), and cerebrospinal fluid analyses were normal; only homozygous prothrombin G20210A mutation was detected. After the failure of all available antiepileptic drugs (used in several associations) and the recurrence of convulsive status epilepticus requiring intensive care unit management, vagus nerve stimulation and surgical treatments (specifically, removal of left insula, anterior temporal lobe, hippocampus, and amygdala) were unsuccessfully attempted. Although histological findings were rather nonspecific, age at onset, clinical and radiological features (unilateral neurological deficits, unilateral seizure onset, and basal ganglia involvement), and the dramatic evolution were highly suggestive of a focal variant of Rasmussen encephalitis (RE) primarily involving the temporal lobe; therefore, oral and intravenous steroids were administered without improvement (for more details, see the Supporting materials section).
Figure 1.
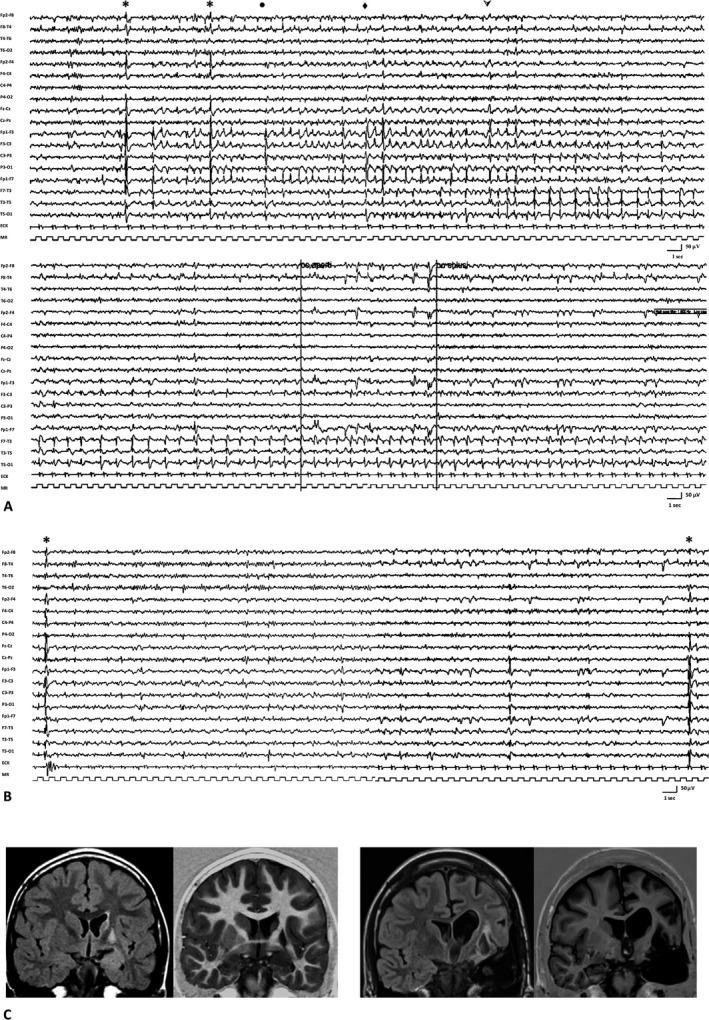
(A) EEG recording performed before the introduction of cannabis: the tracing shows a subcontinuous paroxysmal epileptiform activity characterized by discharges of spikes in the left hemisphere with rapid contralateral transmission, followed by a brief voltage attenuation on the left frontocentral and anterior temporal regions, and then by a pseudoperiodic slow spike‐and‐wave activity propagating over the midposterior temporal areas. The clinical correlate consisted of a seizure characterized by intense right perioral myoclonus (*), followed by ipsilateral tonic deviation of the mouth (●), dystonic posturing of the right limbs (♦), and partial consciousness impairment ( ).The neurological examination showed severe ideomotor slowing and an evident worsening of the preexistent aphasia, with increased difficulty in verbal comprehension and word reaching. (B) EEG recording performed 1 year after the introduction of cannabis: the tracing shows diffuse discharges of spikes, clinically associated with isolated perioral myoclonus (*), and focal theta‐delta activity intermingled with spikes and spike‐and‐waves located on the frontal and anterior and middle temporal regions. An improvement in cognitive and, more specifically, language functions was observed. (C) MRI scan (FLAIR and IR sequences). On the left: MRI scan before surgery, showing the presence of a lesion involving the left insula with concomitant slight hyperintensity and bulging of the mesial temporal area and atrophy of the head of the ipsilateral caudate nucleus. On the right: MRI scan after left temporal lobectomy, showing diffuse white matter alterations and left lateral ventricle dilatation.
).The neurological examination showed severe ideomotor slowing and an evident worsening of the preexistent aphasia, with increased difficulty in verbal comprehension and word reaching. (B) EEG recording performed 1 year after the introduction of cannabis: the tracing shows diffuse discharges of spikes, clinically associated with isolated perioral myoclonus (*), and focal theta‐delta activity intermingled with spikes and spike‐and‐waves located on the frontal and anterior and middle temporal regions. An improvement in cognitive and, more specifically, language functions was observed. (C) MRI scan (FLAIR and IR sequences). On the left: MRI scan before surgery, showing the presence of a lesion involving the left insula with concomitant slight hyperintensity and bulging of the mesial temporal area and atrophy of the head of the ipsilateral caudate nucleus. On the right: MRI scan after left temporal lobectomy, showing diffuse white matter alterations and left lateral ventricle dilatation.
Empirical use of cannabis
In January 2014, the patient was experiencing dozens of seizures a day, characterized by right perioral myoclonia followed by stiffening of the right arm and increased difficulty in word reaching, with prolonged postictal weakness. Interictal video EEG showed a subcontinuous paroxysmal activity involving the left hemisphere, especially frontocentral and anterior temporal regions (Fig. 1). At that time, his parents decided to try cannabis and started giving him homemade oral infusions obtained by simmering 300–400 mg of cannabis inflorescence in 250 ml of milk twice a day. His previous therapy (topiramate 300 mg/day, levetiracetam 4,000 mg/day, carbamazepine 800 mg/day, and clonazepam 4 mg/day) was unchanged.
Follow‐up and outcome
Seizures decreased in frequency soon after the treatment was started. During the following months, different strains of cannabis were used with variable clinical effects. However, it was unmistakably observed that each time cannabis was withdrawn, focal motor seizures involving the right hemisoma relapsed after about 4 days from discontinuation. When the same strain was used for several months, the patient's general conditions steadily improved, in terms of both motor and cognitive performances. In particular, periodic neurological examinations showed an increase in verbal fluency/comprehension and praxic skills. He was also administered a simplified version of the Neuropsychological Assessment Battery for adolescents with epilepsy recommended by the Italian League Against Epilepsy (including Wechsler Adult Intelligence Scale, Trail Making Test, and Wisconsin Card Sorting Test) and Aachen Aphasia Test, which confirmed his improvement. The patient's participation in school activities, which had been intended only to reach socialization goals, dramatically changed, and he could even graduate thanks to a simplified lesson plan and the help of a learning support teacher. According to the patient's seizure diary, major motor seizures disappeared, although isolated perioral myoclonic jerks occurred several times a day. Weekly/monthly prolonged video EEG monitoring with polygraphic study documented the drastic reduction of the usual subcontinuous paroxysmal epileptiform activity over the left frontotemporal regions, although abundant slow abnormalities intermingled with spikes/spike‐and‐waves persisted. During follow‐up, no side effects were reported. In the attempt to find a consistent correlation with the patient's clinical evolution, the serum levels of THC, CBD, and CBDV were periodically measured. Interestingly, when the patient's conditions improved, high concentrations of CBDV were detected in his blood (Fig. 2A). Serum levels of the other antiepileptic drugs were repeatedly measured as well and were always within range, without remarkable changes compared with the levels detected before cannabis introduction.
Figure 2.
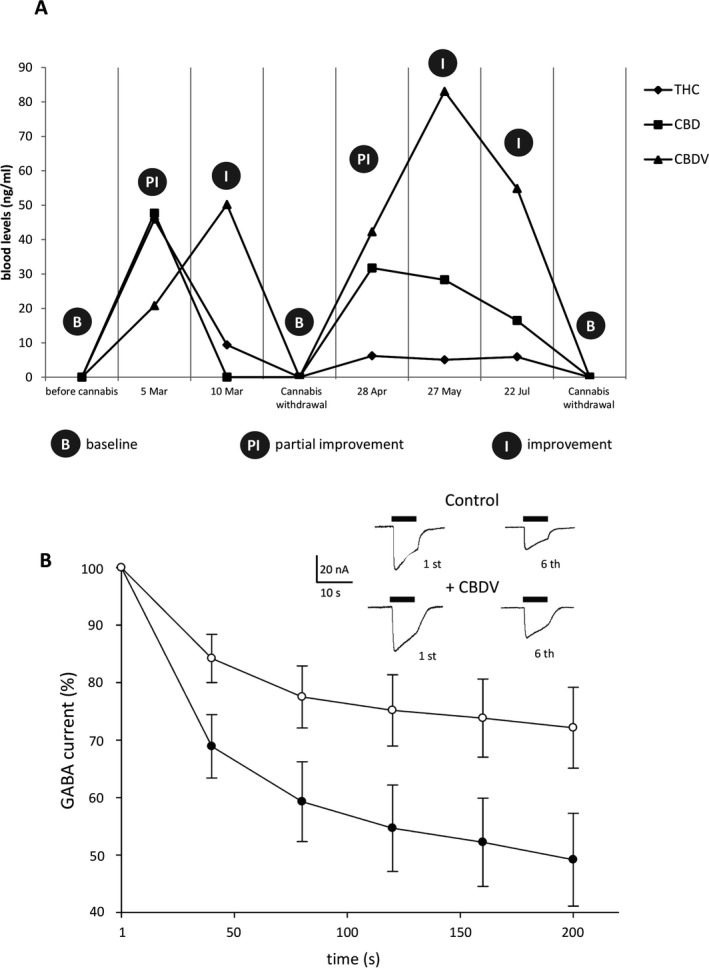
(A) Cannabinoids serum levels and electroclinical correlations. B: baseline, defined as the coexistence of subcontinuous motor seizures (right perioral myoclonus, ipsilateral tonic deviation of the mouth, dystonic posturing of the right limbs, and partial consciousness impairment) and severe ideomotor slowing with mixed aphasia; PI: partial improvement, defined as the coexistence of perioral myoclonia, subclinical ictal discharges, and motor aphasia; I: improvement, defined as the persistence of isolated perioral myoclonic jerks and the improvement of language fluency. Plasma concentrations of Δ9‐tetrahydrocannabinol (THC), cannabidiol (CBD), and cannabidivarin (CBDV) were quantified by liquid chromatography tandem mass spectrometry (LC‐MS/MS) according to Ferreiròs et al. with slight modifications. After solid phase extraction (SPE), chromatography was performed on KInetex C18 column (50 × 2.1 mm, 2.6 particle size) with a gradient of 0.01% formic acid in water and 0.01% formic acid in methanol. THC and CBDV were monitored using positive mode electrospray ionization and multiple reaction monitoring (MRM). The transitions were m/z 315 → 193 and 287.2 → 165.2 for THC and CBDV, respectively. The limit of quantification (LOQ) was 0.5 ng/ml for both analyses. (B) Normalized time‐course of the averaged (mean ± SEM) GABA current rundown in oocytes transplanted with hippocampal membranes in control condition (filled symbol) and after 50 nm CBDV application (empty symbol). Data refer to 30 oocytes, 4 patients. In all experiments the holding potential was −60 mV. Current amplitude normalized to the first response of rundown protocol. Inset: Representative GABA current traces in one transplanted oocyte showing the first and sixth response to GABA 500 μm (black bars) before and after 50 nm CBDV application as indicated. Data refer to 4 TLE patients (4 males, mean age 32.7 ± 9.5 years; mean epilepsy onset 12.7 ± 1 years). All patients had mainly complex partial seizures resistant to maximal doses of different antiepileptic drugs and had hippocampal sclerosis (HS) with neuronal loss within all hippocampal subfields, including CA1 and CA4 (HS, ILAE type 1). For the clinical part of the study, informed consent was obtained. For the experiments, TLE tissues with HS were provided by Amsterdam University (AMC) and used in accordance with the Declaration of Helsinki.
From Clinical Cues to Experimental Approach
The potential mechanisms underlying CBDV clinical effects are several and only partially known.6, 9 Considering some recent data emphasizing the possible role of GABA‐mediated action of cannabis, we decided to test CBDV effect on GABAergic transmission in human tissue.10, 11 However, because of the unavailability of the patient's own surgical specimens (temporal lobe tissue was already fixed and embedded in paraffin) and the rarity of his pathology, we performed our experiments on the hippocampal specimens resected from four drug‐resistant temporal lobe epilepsy (TLE) patients (for details, see Fig. 2B) and on specimens obtained from a single patient affected by RE (female, 25 years old).
Patients and methods
TLE tissues with hippocampal sclerosis (HS) and RE tissues were transplanted in Xenopus oocytes. Membrane and oocyte preparation/injection procedures were performed as previously detailed.8, 10 GABA current rundown (I%) was defined as the decrease, calculated in percentage, of the current peak amplitude after six 10‐s applications of 500 μm GABA at 40‐s intervals;9 the percentage of the original current amplitude remains. Oocytes were incubated with CBDV (50 nm) for 120–150 min after the control rundown protocol. For an internal control, other cells were incubated with oocytes Ringer's solution8, 10 in the same conditions. All results are given as mean ± standard error (SEM). Two data sets were considered statistically different when p < 0.05 (analysis of variance [ANOVA] test). All drugs were purchased from Sigma Italia, with the exception of GABA (Tocris, Bristol, UK) and CBDV (THC Pharm, Frankfurt, Germany).
Electrophysiological results
Considering the proven effect of cannabinoids on GABA‐evoked currents11 (IGABA), we focused our attention on the modulation exerted by CBDV on GABAA‐Rs, previously reported to be impaired in these patients.10 This impairment was recorded as a strong use‐dependent desensitization (i.e., rundown) of the GABAA receptor mediated currents that was recovered by blocking the protein kinase C (PKC) pathway.12 In agreement with previous results,10 application of GABA (500 μm) to transplanted oocytes elicited bicuculline‐sensitive inward currents (IGABA amplitude range: −10 nA to −100 nA). When oocytes were injected with hippocampal membranes of TLE patients and RE tissues, we found a consistent IGABA rundown following repetitive application of GABA (I% TLE = 45% ± 12; 49 oocytes; 4 patients; Fig. 2B; I% RE = 49% ± 7.9; 10 oocytes; for RE, see Fig. S1). In these cells, the simultaneous application of CBDV (from 50 nm to 50 μm) and GABA did not alter the IGABA rundown (data not shown). In contrast, prolonged exposure to CBDV (from 30 min to 3 h)12 decreased IGABA rundown, with a maximal effect obtained 2 h after 50 nm CBDV treatment (I% TLE = 65% ± 11; 35 oocytes; 4 patients, p < 0.05; Fig. 2B; I% RE = 72% ± 6.7; 10 oocytes; p < 0.05; see Fig. S1). Concentrations lower than 5 nm did not exert effects; at 10 nm we observed a small, but not statistically significant, effect, while CBDV doses higher than 50 nm (up to 50 μm) did not induce a further improvement of rundown (data not shown). The described effect was not detected in brain tissues obtained from 4 nonepileptic controls (data not shown). Because it has been previously shown13 that GABAAR rundown was affected by levetiracetam (LEV), we performed experiments incubating oocytes with both CBDV 50 nm and LEV 1 μm. Our results clearly indicate that the two compounds did not show a cumulative effect (from I% = 47% ± 5.2 to 63.5 ± 4.1 for CBDV; from 49% ± 5.0 to 66.1 ± 5.3 for CBDV plus LEV; 8 oocytes each set of experiments).
Interestingly, CBDV effect was not blocked by selective PKC antagonist GF 109203X 1 μm 12 (I% = 60.5% ± 9.0, 19 oocytes; 4 patients) and by transient receptor potential vanilloid (TRPV) channel antagonist capsazepine 10 μm 9 (see Table S1), but it was blocked by broad spectrum kinase inhibitor staurosporine13 (from I% = 42 ± 13 to I% = 44 ± 11, 10 oocytes, 2 TLE patients; see Table S1).
Discussion
We report the case of a patient with a probable focal variant of RE who tried cannabis in addition to his medical therapy. The periodic electroclinical evaluations during cannabis treatment showed a variable decrease in both seizure frequency and epileptiform paroxysmal activity over time but, most importantly, a consistent, unambiguous worsening of the patient's conditions soon after cannabis withdrawal. Interestingly, the repeated analyses of cannabinoid serum levels revealed that, paralleling electroclinical improvements, the concentrations of CBDV were always particularly high, which suggested that CBDV might play a crucial role and greatly contribute to cannabis anticonvulsive effect. As expected, it was not possible to identify a “threshold efficacy level” for either CBD or CBDV. In addition, our data did not allow us to exclude that it was not a single compound but the different molecules combined together to have the most effective antiepileptic action. This hypothesis is in accordance with recent in vivo studies testing enriched cannabis extracts, which showed not only that cannabis anticonvulsant properties are most likely independent from THC but also that CBD and CBDV may have an additive effect, as proved by isobolographic methods.14 Interactions between cannabinoids and the other antiepileptic drugs (AEDs)15 used by the patient (namely, LEV, topiramate, clobazam, clonazepam) could have also influenced the clinical outcome, either through pharmacodynamic mechanisms or reciprocal changes in pharmacokinetic properties. Indeed, although periodic controls did not reveal significant alterations in AED concentrations after the introduction of cannabis, potential interferences with cannabinoid level, bioavailability, and metabolism cannot be ruled out at present.
Based on the reported clinical experience and surprising beneficial effect of cannabis, we tried to better define the general action of CBDV. In a set of experiments, we showed a new possible mechanism of action elicited by CBDV on GABAA current rundown, a well‐known phenomenon described in drug‐resistant epileptic patients.10, 12 Notably, we can clearly state that CBDV and LEV did not act in a synergistic fashion on rundown improvement. In addition, this effect was not mediated by TRPV channels activity,9 but it was modulated by a kinase or a PKC isoform other than the PKC α, β1,δ, ζ.13
Although further investigations are needed, these findings may be relevant for future pharmacological developments. Moreover, considering the specific epileptic syndrome of our patient and the acknowledged anti‐inflammatory properties of cannabis, immune‐modulating effects contributing to the clinical improvement should be taken into consideration.
In conclusion, our case supports the tolerability and potential efficacy of cannabis in the treatment of drug‐resistant epilepsy and highlights the role of CBDV as an antiepileptic drug. Accordingly, for the first time we propose GABAA‐Rs as a possible new target for CBDV.
The reported clinical experience and the following experimental approach confirm the need for both well‐designed randomized clinical trials (RCTs) and targeted experimental studies.
Disclosure
The authors have no conflict of interest to declare. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Figure S1. GABA current rundown in RE tissue.
Table S1. Effect of different blockers on the improvement of current rundown (I%) by CBDV.
Appendix S1. Case report.
Acknowledgments
The authors would like to thank Dr. M. Albini and Dr. M. Fanella for their contribution in data collection. The authors thank all the patients who made this study possible. This work was supported by grants AICE‐FIRE and UCB Pharma (E.P.), Ri.MED Foundation (P.C.), and the Italian Ministry of Health for Institutional Research (C.R.). G.R. was supported by PhD in Clinical/Experimental Neuroscience and Psychiatry at Sapienza University, Rome.
Biographies
Alessandra Morano, MD, Department of Neurology and Psychiatry, University of Rome Sapienza.

Pierangelo Cifelli, MD, PhD, Department of Physiology and Pharmacology, University of Rome Sapienza.

References
- 1. O'Shaughnessy WB. On the preparations of the Indian hemp, or Gunjah. Prov Med J Retrosp Med Sci 1843;5:363–369. [Google Scholar]
- 2. Koppel BS, Brust JCM, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders. Neurology 2014;82:1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Devinsky O, Cilio MR, Cross H, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014;55:791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maa E, Figi P. The case for medical marijuana in epilepsy. Epilepsia 2014;55:783–786. [DOI] [PubMed] [Google Scholar]
- 5. Mathern GW, Beninsig L, Nehlig A. Fewer specialists support using medical marijuana and CBD in treating epilepsy patients compared with other medical professionals and patients: result of Epilepsia's survey. Epilepsia 2015;56:1–6. [DOI] [PubMed] [Google Scholar]
- 6. Hill AJ, Mercier MS, Hill TD, et al. Cannabidivarin is anticonvulsant in mouse and rat. Br J Pharmacol 2012;167:1629–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Friedman D, Devinsky O. Cannabinoids in the treatment of epilepsy. N Engl J Med 2015;373:11. [DOI] [PubMed] [Google Scholar]
- 8. Eusebi F, Palma E, Amici M, et al. Microtransplantation of ligand‐gated receptor‐channels from fresh or frozen nervous tissue into Xenopus oocytes: a potent tool for expanding functional information. Prog Neurobiol 2009;88:32–40. [DOI] [PubMed] [Google Scholar]
- 9. Iannotti FA, Hill CL, Leo A, et al. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci 2014;5:1131–1141. [DOI] [PubMed] [Google Scholar]
- 10. Palma E, Ragozzino DA, Di Angelantonio S, et al. Phosphatase inhibitors remove the run‐down of gamma‐aminobutyricacid type A receptors in the human epileptic brain. Proc Natl Acad Sci USA 2004;101:10183–10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Golovko T, Min R, Lozovaya N, et al. Control of inhibition by the direct action of cannabinoids on GABAA receptors. Cereb Cortex 2014;25:2440–2455. [DOI] [PubMed] [Google Scholar]
- 12. Roseti C, Fucile S, Lauro C, et al. Fractalkine/CX3CL1 modulates GABAA currents in human temporal lobe epilepsy. Epilepsia 2013;54:1834–1844. [DOI] [PubMed] [Google Scholar]
- 13. Palma E, Ragozzino D, Di Angelantonio S, et al. The antiepileptic drug levetiracetam stabilizes the human epileptic GABAA receptors upon repetitive activation. Epilepsia 2007;48:1842–1849. [DOI] [PubMed] [Google Scholar]
- 14. Hill TD, Cascio MG, Romano B, et al. Cannabidivarin‐rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor‐independent mechanism. Br J Pharmacol 2013;170:679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patsalos PN, Perucca E. Clinically important drug interactions in epilepsy: interactions between antiepileptic drugs and other drugs. Lancet Neurol 2003;2:473–481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. GABA current rundown in RE tissue.
Table S1. Effect of different blockers on the improvement of current rundown (I%) by CBDV.
Appendix S1. Case report.


