Summary
Objective
The aim of this study is to determine whether the use of 7 tesla (T) MRI in clinical practice leads to higher detection rates of focal cortical dysplasias in possible candidates for epilepsy surgery.
Methods
In our center patients are referred for 7 T MRI if lesional focal epilepsy is suspected, but no abnormalities are detected at one or more previous, sufficient‐quality lower‐field MRI scans, acquired with a dedicated epilepsy protocol, or when concealed pathology is suspected in combination with MR‐visible mesiotemporal sclerosis—dual pathology. We assessed 40 epilepsy patients who underwent 7 T MRI for presurgical evaluation and whose scans (both 7 T and lower field) were discussed during multidisciplinary epilepsy surgery meetings that included a dedicated epilepsy neuroradiologist. We compared the conclusions of the multidisciplinary visual assessments of 7 T and lower‐field MRI scans.
Results
In our series of 40 patients, multidisciplinary evaluation of 7 T MRI identified additional lesions not seen on lower‐field MRI in 9 patients (23%). These findings were guiding in surgical planning. So far, 6 patients underwent surgery, with histological confirmation of focal cortical dysplasia or mild malformation of cortical development.
Significance
Seven T MRI improves detection of subtle focal cortical dysplasia and mild malformations of cortical development in patients with intractable epilepsy and may therefore contribute to identification of surgical candidates and complete resection of the epileptogenic lesion, and thus to postoperative seizure freedom.
Keywords: Focal cortical dysplasia, Malformation of cortical development, Magnetic resonance imaging, Ultra‐high field, 7 T
Key Points.
The increased field strength in 7 T MRI provides an increase in signal‐to‐noise ratio that can be used to improve image contrast and resolution
Seven T scans were acquired with a 3D protocol, including T 1, T 2, and T 2 * weighted images, FLAIR, and white matter suppression sequences with 0.5‐ to 0.8‐mm resolution
Seven T MRI allowed detection of focal cortical dysplasia and mild malformation of cortical development in 23% of patients previously considered “MRI‐negative”
Improved lesion detection contributes to identification of surgical candidates and complete resection of epileptogenic lesions
In patients with pharmacoresistant focal epilepsy, resection of the epileptogenic zone can be considered. Focal cortical dysplasia (FCD) is the most frequent etiology (42%) in patients younger than 18 years of age selected for surgery. In adults FCD is the third most common etiology (13%) after hippocampal sclerosis (43%) and tumors (30%).1
Focal cortical dysplasias are a subgroup of circumscript malformations of cortical development (MCDs) and are characterized by abnormal migration, maturation, and differentiation of neurons.2 Onset of epilepsy occurs typically before the age of 12 years, and roughly 76% of patients have medically refractory seizures.3 Resective surgery can be highly effective, with reported seizure freedom rates ranging from 33% to 80% in mixed FCD type cohorts of patients operated in the last two decades.1, 4, 5, 6, 7, 8, 9, 10 In a large study of 211 patients with FCD, International League Against Epilepsy (ILAE) type I and II operated 1998–2010, 65% remained seizure free (Engel class I) up to latest follow‐up (2–12 years).8 Seizure freedom rate markedly increases in cases of complete resection,4, 8, 11, 12, 13 averaging 77% in a literature review by Lerner et al.1 Appropriate detection and delineation of a FCD on MRI contributes to identification of surgical candidates and complete resection of the epileptogenic lesion, and thus to postoperative seizure freedom.14, 15, 16
However, the structural abnormalities in FCD are often very subtle and may escape detection on conventional structural neuroimaging. This makes FCD the most common pathology (45–51%) in patients who undergo epilepsy surgery for focal refractory epilepsy and have a “nonlesional MRI.”1, 17 In a review of surgical series from 2000 to 2008, 37% of FCD type I lesions and 15% of FCD type II lesions were not detected with 1.5 or 3 tesla (T) MRI.1 Detection seems even more problematic for the most subtle architectural abnormalities in the FCD spectrum, classified as mild malformations of cortical development (mMCD), of which 58% were not visible on MRI in a series published in 2008.18
Studies comparing 1.5 and 3 T MRI in epilepsy surgery candidates have shown that imaging at 3 T can identify FCDs that escaped detection at 1.5 T.19, 20, 21, 22 In a cohort of patients with FCD type II, 3 T MRI showed a lesion in 20% of patients with nonlesional 1.5 T.22
We hypothesize that a similar effect can be gained from 7 T, by virtue of increased signal‐to‐noise ratio, allowing higher resolution and contrast.23 Dysplastic lesions previously detected using lower‐field MRI are reported to be detected with more confidence and visualized in greater detail at 7 T.24 One previous study reported an improved detection rate of 7 T MRI compared to lower‐field MRI, although half of the patients in whom a new lesion was detected at 7 T underwent previous scanning in presurgical evaluation at only 1.5 T, and not at 3 T, the field strength currently considered optimal.25
In our study we aim for verification of the clinical value in a larger group of patients, of whom most underwent previous 3 T MRI, and using a full three‐dimensional (3D) scanning protocol with submillimeter resolutions in fluid attenuated inversion recovery (FLAIR), T 1, T 2, and T 2 *‐weighted, and white matter suppression sequences. We wanted to determine whether the use of 7 T MRI in clinical practice led to higher detection rates of focal cortical dysplasia in candidates for epilepsy surgery, and how this influenced surgical decision making.
Materials and Methods
Patients
The University Medical Center Utrecht (UMCU) is a tertiary referral center for patients with refractory epilepsy and for epilepsy surgery. A multidisciplinary team consisting of neurologists, neurophysiologists, neurosurgeons, neuroradiologists, nuclear medicine physicians, and neuropsychologists from different centers evaluates surgical candidacy of each patient, determines the type and order of ancillary investigations, and decides on the selection or rejection for surgery and on surgical strategy during epilepsy surgery meetings (ESMs). Patients are referred for 7 T MRI if lesional focal epilepsy is suspected but no abnormalities are detected at one or more previous, sufficient‐quality MRI scans, acquired with a dedicated epilepsy protocol (3 T in most cases),26 or when concealed pathology is suspected in combination with MR‐visible mesiotemporal sclerosis (dual pathology).
For this series we consecutively included 40 patients who underwent 7 T MRI for presurgical evaluation between March 2009 and February 2016 and whose scans (both 7 T and lower‐field scans) were discussed in the ESMs. Two young patients, ages 7 and 8 years, scanned in this period were excluded from analysis. In one, the examination had to be stopped because of inconsolable fear. In the other, severe movement artefacts in all sequences made accurate assessment impossible. In the 40 included patients, at least one sequence was of sufficient quality for reliable visible assessment, although some degree of movement artefacts in one or more sequences was common, especially with pediatric patients. For the review and analysis of patient data, institutional ethical board approval was acquired. All patients gave informed consent for the 7 T MRI examination.
MRI acquisition
Seven T MRI scans were performed on a Phillips Achieva 7 T MRI system (Philips Healthcare, Best, the Netherlands) with a 16‐channel receive coil or, after May 2011, a 32‐channel receive head coil combined with dual channel transmit coil (Nova Medical, Wilmington, MA, U.S.A.). From mid‐2015 on, dielectric pads, containing calcium titanate (Leiden University Medical Center, Leiden, the Netherlands), were routinely used to reduce artefacts and signal loss in the temporal regions.27 Pads were placed to the sides of the subject's head. Patients all used ear plugs to protect from acoustical noise.
In our center, 3 T MRI scans were performed with 3 T Philips Achieva with eight‐channel sensitivity encoding (SENSE) head coil. The 3 T is the default machine for epilepsy patients; only on specific indication (e.g., vagal nerve stimulator in situ) and in the past 1.5 T was performed, on a Phillips Achieva 8ch or Philips Ingenia dStream.
Scan protocols
The 7 T scanning protocol consists of T 1, T 2, FLAIR, and T 2 *‐weighted sequences and a gradient echo white matter suppression (WMS) sequence, all with 0.5‐ to 0.8‐mm isotropic resolution with 50% overlap of slices; acquisition time was circa 45 min. Since 2008, there have been minor adjustments in the protocol, mainly the addition of the WMS sequence and modification of the T 2 * echo times. Parameters of our 1.5, 3, and 7 T MRI sequences performed in our center are described in Table S1. In case of severe motion artefacts, precluding reliable assessment, sequences are repeated to generate at least T 1, T 2, and/or FLAIR sequences of sufficient quality, at the cost of T 2 * and WMS sequences.
All sequences are routinely reformatted in 0.5‐mm (interpolated) multiplanar reconstructions to have axial, coronal, and sagittal images for all sequences, allowing for optimal appreciation of cortical architecture. This protocol covers all sequences recommended in various guidelines for the detection of epileptogenic lesions.26
MRI assessment
MRIs were routinely reviewed (prior to the ESM) by visual analysis on a high‐resolution workstation by a dedicated epilepsy radiologist who takes part in the ESMs and who also revised all scans performed outside the UMCU. During bimonthly multidisciplinary meetings, patients’ full histories, electroencephalograms (EEGs), and results of other investigations are discussed. MRI scans are shown on a projector screen in the presence of a neuroradiologist and collectively reassessed by the entire ESM team. The resulting conclusion is taken as final and was used in this study. Scans were sometimes reviewed on more than one occasion. The reported collective assessment of the lower‐field MRI always took place prior to the meeting during which the 7 T MRI was evaluated.
A radiological diagnosis of possible FCD was made in case of presence of distinctive imaging features,28 most notably blurring of the gray‐white matter border and transmantle sign.
Hippocampal atrophy in combination with signal changes was indicative of mesiotemporal sclerosis.29 Covert dual pathology was suspected if the clinical presentation could not be explained by mesiotemporal sclerosis alone. MRI scans were considered nonlesional if no possibly epileptogenic lesions were seen. Patients with aspecific or radiological findings strongly divergent from patient and electrophysiological characteristics, for example, arachnoid cysts, age‐related or gliotic white matter lesions, and enlarged perivascular spaces, were considered MRI‐negative. No postprocessing techniques were used or evaluated for the purpose of this study.
Surgery
All surgical procedures were carried out in the UMCU by two neurosurgeons with expertise in epilepsy surgery. All patients in this series were operated either after chronic electrocorticography (ECOG) or with guidance of acute (intraoperative) ECOG. The surgeons strive to resect tissues en‐bloc as much as possible, to allow optimal histopathological examination.
Histopathology
Resected brain tissue was fixed in 10% buffered formalin and embedded in paraffin. Paraffin‐embedded tissue was sectioned at 5 μm and used for histology and immunohistochemistry. Sections were processed for hematoxylin and eosin staining as well as for immunohistochemical staining for a number of neuronal and glial markers. Surgical samples were evaluated and classified according to the ILAE guidelines.2, 30
Results
Patient characteristics, ancillary tests, and surgical procedures
Included in this study were 40 patients in whom 7 T MRI was performed on clinical indication and whose scans were assessed in multidisciplinary ESMs. Age at time of 7 T scan was 7–48 years (median 18).
Thirty‐five (88%) were previously scanned at 3 T, 4 (10%) had at least a 1.5 T, and 1 previously had a 1.0 T MRI. Eighteen patients (43%) underwent both 3 T and a 1.5/1/0.5 T MRI. Other ancillary tests performed were fluorodeoxyglucose positron emission tomography (FDG‐PET) (in 38, 95%) and magnetoencephalography (in 29, 73%). So far, single positron CT (SPECT) has been performed in 15 patients, including one failed examination (38%) (ictal subtraction with MRI co‐registration: n = 12). Three patients were still awaiting SPECT when study data were collected.
Thirteen patients (33%) underwent resective surgery, all guided by ECOG (chronic grid registration n = 10, chronic with grid and depth electrodes n = 1, acute grid registration n = 2). At a follow‐up ranging from 1 month to 5 years (mean 17 months, median 9 months), 11/12 (91%) patients were seizure free (Engel score 1A, n = 10; Engel 1D, n = 1). One patient was scored Engel 4B at 6 months (one patient has not yet been evaluated postoperatively).
Seven patients (18%) await invasive EEG monitoring. Twelve (30%) patients were rejected for resective surgery, of whom one underwent multiple subpial transections in the motor cortex and one received a vagal nerve stimulator. Surgery is postponed in one patient with likely FCD because of unexpected and prolonged seizure freedom. In three patients (8%) the presurgical evaluation was stopped because of expected unacceptable motor deficit or a significant decrease in burden of disease. Four (10%) patients are still under evaluation and await noninvasive tests: SPECT (n = 2) and functional MRI and EEG (fMRI‐EEG) (n = 2).
Findings of 7 T MRI
Table 1 provides an overview of 7 T and lower‐field MRI findings in all patients as well as the consequent treatment strategies and histological diagnoses if available.
Table 1.
Epilepsy surgery meeting conclusions in patients with nonlesional or unclear lower‐field MRI
| ESM conclusion based on lower‐field MRI | ESM conclusion based on 7 T | Surgery | Histology |
|---|---|---|---|
| 38 Considered nonlesional | 8 Lesional | 5 Resective surgery (with ECOG) |
2 FCD ILAE type IIa 1 FCD ILAE type IIb 1 mMCD type 2 1 Proliferative oligodendroglial hyperplasia with mild malformation cortical development |
|
1 Resective surgery with ACOG planned 1 ECOG planned |
2 Yet unknown (FCD suspected) | ||
| 1 Still under evaluation | Yet unknown (FCD suspected) | ||
| 30 Nonlesional | 6 Resective surgery (ECOG guided) |
2 mMCD type 2 1 FCD ILAE type IIa 1 Normal histology 1 Normal histology (sample error suspected) 1 Normal histology (suboptimal assessment due to tissue fragmentation) |
|
| 6 ECOG or S‐EEG planned | – | ||
| 12 Rejected for resective surgery (1 MST, 1 VNS) | – | ||
| 3 No surgery (declined/clinical improvement) | – | ||
| 3 Still under evaluation | – | ||
| 2 Suspicion of dual pathology | Additional lesion | Resective surgery | mMCD type 2, no MTS |
| No additional lesions | Resective surgery | MTS + FCD ILAE type IIIa |
ECOG, electrocorticography; ESM, epilepsy surgery meeting; FCD, focal cortical dysplasia; ILAE, International League Against Epilepsy; mMCD, mild malformation of cortical development; MST, multiple subpial transections; MTS, mesiotemporal (hippocampal) sclerosis; S‐EEG, stereo EEG; VNS, vagal nerve stimulator.
Table 2 summarizes the findings of relevant ancillary tests in patients with new findings at 7 T (Patients 1–9, Table 2) and in those patients who underwent surgery following a clinically indicated 7 T without newly identified epileptogenic lesions (Patients 10–16, Table 2). A table of all 40 patients is provided as Table S2.
Table 2.
Patients with new lesions at 7 T or MRI‐negative with abnormal histopathology
| Pat. no. | Sex/age | Age at debut | Semiology | Surface EEG ictal epileptiform discharges | Surface EEG interictal epileptiform discharges | ECOG | MEG | FDG PET | SPECT | 1.5 T MRI | 3 T MRI | 7 T MRI | Surgery | Histopathology (ILEA type) | Outcome (Engel Score) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ♀/44 | 26 | Focal impaired awareness seizures; hyperkinetic seizures | R frontal parasagittal | R frontal parasagittal | Continuous spiking, R frontal parasagittal | Not performed | No abnormalities | Not performed | Not performed | No abnormalities | Subtle blurring, hyperintensity on DIR R frontal parasagittal | R Partial lobectomy frontal parasagittal | FCD IIb | 1A+ 5 years |
| 2 | ♂/7 | 4 | Focal impaired awareness seizures, R hand automatisms | R frontal | R frontal parasagittal | Continuous spiking R frontal parasagittal | Not performed | No abnormalities | Not performed | Not performed | No abnormalities | R frontal small area with subtle blurring of G/W boundary, subtle white matter hyperintensity (T2) | R frontal lesionectomy | FCD IIa | 1A+ ½ year |
| 3 | ♀/40 | 24 | Focal impaired awareness seizures, dysphasia and alexia; focal to bilateral tonic‐clonic seizures | L temporal | L temporal | Interictal diffuse L temporal, ictal neocortical L mid‐temporal | Spikes neocortex L medial temporal gyrus | No abnormalities | Not performed | No abnormalities a | No abnormalities | Subcortical hyperintensities (FLAIR + T2), blurring of GW‐junction L anterior superior temporal gyrus | Lesionectomy L medial temporal gyrus | mMCD with oligodendroglial hyperplasia | 1A+ 1 year |
| 4 | ♀/22 | 8 | Focal impaired awareness seizures; visual aura; focal to bilateral tonic‐clonic seizures | Onset mid‐frontal | Mid‐frontal | Not performed | Multifocal, at distance from visual cortex | L Occipital hypermetabolism | Not performed | No abnormalities | No abnormalities | Subtle white matter signal changes, transmantle sign‐like configuration L inferior occipital gyrus. | Under evaluation | n/a | n/a |
| 5 | ♂/21 | 15 | Focal impaired awareness seizures, stops activity, staring, L‐sided version, automatisms | L frontal | L frontal | Continuous spiking L medial frontal gyrus | L frontal epileptic activity | L temporal hypometabolism | L frontal (consistent with 7 T MRI) and less pronounced L parietal | Not performed | No abnormalities a | L frontal operculum/inferior frontal sulcus abnormal gyration and subtle transmantle hyperintensity (WMS), enhanced venous vasculature in sulcus (T2*) | Corticectomy L medial frontal gyrus | mMCD type 2, sample error likely, no examination of center of MRI lesion | 1A+ 4 months |
| 6 | ♂/14 | 4 | Focal impaired awareness seizures, hyperkinetic | L frontal | Ictal onset L frontal | Continuous spiking depth of L superior frontal sulcus (depth electrode) | L frontal spikes | No seizure focus | Not performed | No abnormalities a | No abnormalities | L frontal abnormally deep sulcus with blurring GM‐junction, cortical thickening and subtle transmantle sign | Lesionectomy L middle frontal gyrus | FCD IIb | 1A+ 1½ months |
| 7 | ♂/14 | 10 | Focal impaired awareness seizures, nocturnal seizures with automatisms; bilateral tonic‐clonic seizures | Not performed | L centro‐parietal | Not performed | L parietal epileptic activity | L parietal hypometabolism | Not performed | Not performed | No abnormalities | L parietal radial band of poorly demarcated white matter signal changes | Lesionectomy with ACOG planned | n/a | n/a |
| 8 | ♀/28 | 15 | Focal impaired awareness seizures, arrest of activity; focal to bilateral tonic‐clonic seizures | L temporal | Bilateral fronto‐centro‐temporal | Planned | Not performed | L temporo‐parietal, angular gyrus hypometabolism | Not performed | No abnormalities a | No abnormalities a | Abnormal intraparenchymal venous pattern posterior L medial temporal gyrus. | Grid planned | n/a | n/a |
| 9 | ♂/15 | 11 | Focal impaired awareness seizures, L‐sided head version, irresponsiveness, dysphasia, amnesia; focal to bilateral tonic‐clonic seizures | Non‐lateralizing | Multifocal | Diffuse L temporo‐parietal | L baso‐temporal, up to posterior temporal | No abnormalities | Not performed | MTS L + dubious blurring GWM‐junction and decreased volume L temporal | MTS L + dubious blurring GWM‐junction and decreased volume L temporal a | MTS L + abnormal gyration L anterior temporal and enhanced venous vasculature (T2*) | NVS after initial grid registration. Ultimately selective L anterior lobectomy + hippocampectomy | mMCD type 2, no MTS | 4B+ ½ year |
| 10 | ♀/28 | 0 | Focal impaired awareness seizures, automatisms, expressive dysphasia | L mid‐temporal sharp waves | L mid‐temporal spikes | L anterior temporal neocortical + hippocampus | Not performed | L temporal hypometabolism | Not performed | Possible MTS L | MTS L + unclear demarcation of GWM‐junction temporal lobe | MTS L + unclear demarcation of GWM‐junction temporal lobe. No indication of dual pathology | L anterior temporal lobectomy + amygdalahi pocampectomy | MTS ILAE class 2 + FCD IIIa | 1A− 1 year |
| 11 | ♀/15 | 9 | Focal aware seizures, R‐sided version eyes/head; bilateral tonic‐clonic seizures | L fronto‐central | No distinct epileptiform activity | Ictal onset L fronto‐central | L fronto‐central spikes | No abnormalities | Not performed | No abnormalities | No abnormalities | No abnormalities | Lesionectomy L pre‐central, pre‐central sulcus | Normal tissue (suspect for sample error, FCD in bottom of sulcus) | 1A− 2 years |
| 12 | ♂/14 | 7 | Focal impaired awareness seizures, left tonic; focal to bilateral tonic‐clonic seizures | R fronto‐central | R posterior temporal | Continuous spiking R baso‐ temporo‐occipital | R temporal spikes | R baso‐temporo‐occipital hypo‐metabolism | Not performed | No abnormalities a | No abnormalities | No abnormalities (severe signal loss in temporal lobes) | R baso‐temporo‐occipital lesionectomy | FCD IIa | 1A− 1 year |
| 13 | ♂/18 | 14 | Focal impaired awareness seizures hyperkinetic; focal to bilateral tonic‐clonic seizures | No distinct epileptiform activity | No distinct epileptiform activity | No interictal activity, ictal medial frontal gyrus | No abnormalities | R frontal hypometabolism | Not performed | No abnormalities a | Not performed | No abnormalities | Corticectomy R medial frontal gyrus | mMCD type 2 | 1D+ 5 year |
| 14 | ♀/22 | 11 | Focal impaired awareness seizures, sensory dysphasia, derealization, sensations in left foot; focal to bilateral tonic‐clonic seizures | R posterior baso‐temporal | R posterior temporal | Bursts R posterior temporal, sporadic spikes R hippocampus | R hemisphere, no clear localization | Dubious hypometabolism R operculum | Not performed | Not performed | No abnormalities | No abnormalities | Resection posterior baso‐temporal, fusiform gyrus | Normal, but suboptimal assessment due to fragmentation | 1A+ 1/2 year |
| 15 | ♂/33 | 20 | Focal impaired awareness seizures, auditory phenomena, R‐sided head version, confusion; focal to bilateral tonic‐clonic seizures | L posterior temporal | Aspecific irregularities bilateral fronto‐temporal | Sporadic spikes L lateral temporal | Not conclusive | L baso‐temporal‐parietal, hypometabolism in large area | Not performed | Not performed | No abnormalitiesa | No abnormalities | Corticectomy L superior and middle temporal gyrus | No abnormalities | 1A+ 1 month |
| 16 | ♂/16 | 12 | Focal impaired awareness seizures, myoclonia all extremities, L‐sided version of eyes followed by diminished vision; bilateral tonic‐clonic seizures | R temporo‐occipital | R temporo‐occipital | Continuous spikes R lateral temporal, sporadic spikes R parietal and lateral and medial temporal | Baso‐temporal/lateral and R posterior insula | R temporo‐occipital hypometabolism | Not performed | No abnormalitiesa | No abnormalities | No abnormalities | R temporal lobectomy | mMCD type 2 | Follows |
DIR, double inversion recovery sequence; ECOG, electrocorticography; EEG, electroencephalogram; Engel score: 1A, completely seizure free; 1D, only seizures after discontinuation of antiepileptic drugs; +, on antiepileptic drugs; −, antiepileptic drugs discontinued; FDG‐PET, fluorodeoxyglucose (18F) positron emission tomography; FLAIR, fluid‐attenuated inversion recovery sequence; GW‐junction, gray and white matter junction L, left‐sided; MEG, magnetoencephalogram; MTS, mesiotemporal sclerosis; R, right‐sided.
MRI scan performed outside University Medical Center Utrecht.
Thirty‐eight patients underwent 7 T MRI because lower‐field MRI was interpreted as showing no relevant epilepsy‐related lesions. In 8 of those, a focal abnormality, suspect of FCD, could be identified on 7 T. Six of them have been operated, with histology confirming FCD type IIa in 2, FCD IIb in 1, mMCD type 2 in 2, and mMCD with oligodendroglial hyperplasia in 1 case (exemplary cases in Figs. 1, 2, 3). In Patient 5, mMCD type 2 was diagnosed; however, close relation to language areas precluded en‐bloc resection of the complete MRI abnormality.30 Possibly deeper tissues would have been compatible with FCD I or II type (Fig. 3). In another patient, FCD is also suspected, but surgery with acute ECOG is postponed because of current seizure freedom. One patient is still under evaluation. All patients with new lesions had previous good‐quality 3 T MRI scans.
Figure 1.
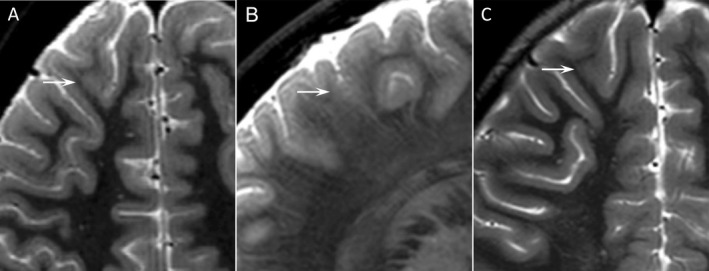
Transverse (A) and sagittal 7 T T 2 (B), transverse 3 T T 2 (C). Patient 2. Male, 7 years, focal impaired awareness seizures. Seven T MRI showed blurring of gray‐white matter junction suspect for FCD. The lesion was only retrospectively identified on 3 T, where thick slices (4 mm + 1 mm gap) lead to many false mimics from partial‐volume effects of adjacent gyri. Lesionectomy in the medial frontal gyrus was performed. Histology confirmed FCD ILAE type IIa.
Figure 2.
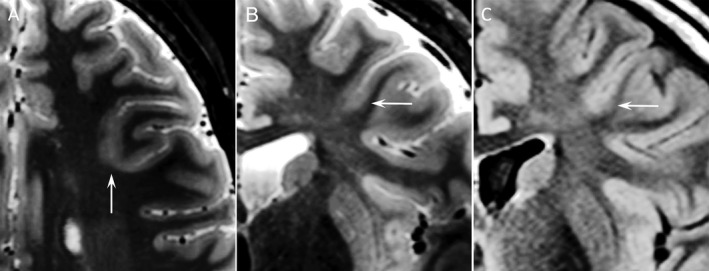
Transverse (A) and coronal 7 T T 2 (B), 3 T FLAIR (C). Patient 6. Male, 14 years, focal impaired awareness seizures. At 7 T, blurring of gray‐white matter junction and cortical thickening compatible with FCD. In retrospect also recognizable on 3 T but initially overlooked because of many (similar looking) partial‐volume effects and signal variation in adjacent gyri. ECOG registration plus depth electrodes in the MRI lesion was performed followed by lesionectomy of the bottom of a sulcus in the medial frontal gyrus. Histology confirmed FCD type IIb.
Figure 3.
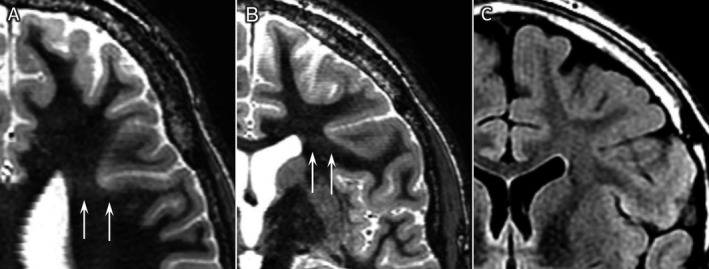
Transverse (A) and coronal 7 T white matter suppression sequence (B), coronal 3 T FLAIR (C). Patient 5. Male, 21 years, focal impaired awareness seizures. On 7 T, WMS images were very subtle white matter signal changes adjoining a deep sulcus (resembling subtle transmantle sign) in a region with a dense gyral pattern, suggestive of subtle cortical dysplasia. No abnormalities had been identified on 3 T images. Histology was classified as mMCD type 2; however, because of close relation to language areas en‐bloc resection of the complete MRI abnormality was not possible, and deeper tissues could have been compatible with FCD I or II types.
Two patients underwent 7 T MRI because of clinical suspicion of dual pathology in combination with mesiotemporal sclerosis (MTS) visualized at 3 T MRI. In one patient (Patient 10, Table 2) with left‐sided MTS and associated dysplasia in the temporal pole, there was a clinical and electrophysiological suspicion of an additional contralateral (right‐sided) seizure onset. Seven T MRI did not indicate the presence of such a lesion, and a left‐sided anterior temporal lobectomy plus amygdalohippocampectomy was performed. Histopathology confirmed MTS + FCD IIIa.
In the other patient (Patient 9, Table 2), 3 T MRI suggested MTS, but semiology and electrophysiology indicated temporal neocortical pathology. Seven T MRI revealed an abnormal gyration pattern in combination with enhanced venous vasculature on the T 2 * sequence, indicating a possible developmental abnormality in the anterior temporal lobe. Selective left anterior temporal lobectomy with hippocampectomy was performed, and the hippocampus was found to have normal histology, whereas in the temporal lobe mild architectural abnormalities compatible with mMCD type 2 were confirmed.
After identification of new lesions at 7 T, we reviewed lower‐field‐strength images once more. Even though these were inconspicuous at first, in hindsight, with knowledge of the 7 T MRI findings, lesions could be recognized in 3 T images in 3 of 9 patients (33%).
Six patients were operated even though presurgical assessment of 7 T MRI did not reveal any relevant lesions. They underwent grid‐ECOG‐guided corticectomy, and in 3 patients histopathological analysis revealed a dysplastic lesion (2 mMCD type II, Patients 13 and 16; 1 FCD IIa, Patient 12; Table 2). With knowledge of the histological diagnosis, even in hindsight no lesion could be seen at 7 T; however, temporal signal loss typical to ultra‐high‐field acquisitions with insufficient correction methods made assessment of the region of resection impossible in one of these patients (Patient 12; Table 2). In another patient (Patient 11; Table 2), normal histology was seen, even though highly localized continuous spiking patterns in ECOG registration were strongly suggestive of a small bottom of the sulcus FCD. The difficult access to this lesion precluded en‐bloc resection, and the deeply located lesion was removed with a surgical aspirator, most likely leading to sample error in the pathological diagnosis. Indeed, retrospectively subtle features indicative of FCD were identified on the 7 T MRI.
Discussion
Of 40 patients with a clinical presurgical indication for 7 T MRI because of refractory focal epilepsy and presumed normal or nonexplanatory lower‐field MRI, in 9 (23%) 7 T MRI revealed a lesion, guiding further surgical decision making. Six of these 9 patients have been operated upon, with confirmation of FCD ILAE type IIA (n = 2), FCD IIB (n = 1), mMCD type II (n = 2), or mMCD with oligodendroglial hyperplasia (n = 1). One patient is planned for surgical treatment under suspicion of FCD, and in one patient with suspected FCD surgery was postponed because of prolonged seizure freedom. One patient is still being evaluated.
A recent study by de Ciantis25 evaluated the diagnostic yield of 7 T MRI in 21 patients with intractable focal epilepsy and unrevealing 1.5 or 3 T MRI. Structural lesions were identified in 6 (29%) of 21 patients, 4 of whom underwent epilepsy surgery and whose histopathology confirmed FCD (ILAE type IIa, n = 2; IIb, n = 1; IIIa, n = 1). In that study, however, only half of the 6 presumed MR‐negative patients in whom 7 T revealed a new lesion underwent previous MRI at 3 T, whereas in our series all 9 patients with new lesions underwent 3 T MRI before. Our findings therefore truly reflect the added value of 7 T over the current standard, 3 T MRI, in the presurgical evaluation.
Visualization of FCD
Several MRI features characterize FCD28 but with great variety in conspicuity. Especially in mMCD and FCD type I, changes can be very subtle18 and indistinguishable from signal averaging and partial volume effects. The same phenomena can hinder appreciation of blurring of the gray‐white matter junction, a telltale feature of FCD. Submillimeter resolution and slice thickness greatly reduce the detrimental signal averaging and partial volume effects. Moreover, multiaxial images are needed to be able to optimally assess the highly convoluted cerebral cortex. Subtle signal changes can be obvious in one plane but completely obscured by the cortical anatomy in another plane. In some cases, even, the sole identifier can be an abnormal gyration pattern, only recognizable in high‐quality 3D image sets.18, 28, 31
With current coil and processing techniques, it is not possible to achieve submillimeter high resolutions in all essential sequences (T 1, T 2, FLAIR, T 2 *)26, 32 at 3 T while maintaining tissue contrast and staying within acceptable acquisition times. At 7 T this is currently possible and is its most important strength. On the other hand, advances in coil design and data processing could possibly also achieve this at lower field strengths in the future.33
Inability of 7 T MRI to visualize dysplasia
In 30 out of 38 (79%) patients with nonlesional lower‐field MRI, 7 T MRI did not show epilepsy‐related lesions either. Possibly, there is no structural substrate, but it is known that a portion of these patients do prove to have histological abnormalities eventually.18 In our series 3 patients had confirmed FCD or mild malformation of cortical development in the absence of any reported MR abnormalities at 7 T, even after retrospective review. In 1 patient, even though histology was normal, probably because of sample error, based on ECOG findings FCD is likely. In retrospect there are signs of a bottom of sulcus FCD on the 7 T MRI.
There are several possible reasons why assessment of the high‐resolution images does not always provide the pivotal information hoped for.
First, experience in 7 T MRI assessment is still limited compared to that in 1.5 and 3 T MRI, the clinical standard for many years. The added detail is sometimes a double‐edged sword; partial volume effects are greatly reduced and much smaller structures are visible. But as a result the images reveal a great number of structures for which it can be problematic to differentiate between physiological clinically meaningless variation and true pathology.
Second, thorough evaluation of 7 T examinations is relatively time consuming. With constructed slice thickness of 0.5 mm, a completed scan protocol easily produces up to 4,000 slices. Possibly, lesions are missed if no extra time is reserved for the clinical evaluation. The number of slices and thus evaluation time could be reduced by increasing slice thickness and reducing reconstruction planes, but this would likely negate the advantage of performing 7 T MRI.
Third, with the increase in field strength come more artefacts.23 Magnetic susceptibility effects and field inhomogeneities cause artefacts and signal loss. Temporal lobes are particularly problematic with varying, but occasionally severe signal loss, making assessment impossible. Several techniques to counter these artefacts are already successfully used, and more are under development. A rather simple but effective tool we routinely use since 2015 are the dielectric calcium titanate pads with passive RF shimming qualities, which have shown to greatly reduce signal loss in the temporal lobes.27 With growing clinical and technical experience, one would expect the diagnostic value of 7 T MRI to increase.
Strengths and weaknesses
We chose to evaluate 7 T MRI in daily clinical practice, with the conclusion of the multidisciplinary meetings as final assessment. This design reflects how the added information from 7 T MRI affects decision making and how its use may benefit patient care. Naturally, this setting does not allow for a formal and perfectly objective comparison of the diagnostic accuracy between 7 T and lower field strength, which would require systematic scoring by multiple observers in a controlled environment. For the decision for surgical candidacy, clinical information and ancillary tests are combined for more robust assessments. We are convinced that lesion detection on MRI is more sensitive with fewer false positives when taking supporting information and other tests into account. Omitting this aspect in evaluating the value of 7 T can lead to results that are not fully applicable to clinical practice. However, 7 T MRI scans are offered later in the diagnostic work‐up, and consequently in 17 of 40 patients (43%) additional ancillary test were available during the 7 T review. This was true for 5 of 9 (55%) patients with new 7 T MRI findings. The added information available during multidisciplinary assessment of the 7 T scans may have contributed to a better detection rate. Moreover, acquisition time is 45–50 min at 7 T, compared to 25 min for standard protocols at 1.5 or 3 T in our center. Hence, the longer acquisition is an additional benefit (leading to either an increase in signal‐to‐noise or higher resolution) on top of the increase in field strength.
Conclusion
Our study demonstrates that the use of ultra‐high‐field MRI with an extensive scanning protocol can improve lesion detection compared to the current clinical standards. It seems worthwhile to further invest in improving MRI techniques for patients with focal refractory epilepsy. In our series of 40 patients with refractory focal epilepsy, multidisciplinary evaluation of 7 T MRI identified clinically relevant additional malformation of cortical development lesions in 9 patients (23%). Seven T MRI delivers on the promise to improve detection of focal cortical dysplasia and mild malformation of cortical development in patients with intractable epilepsy.
Disclosure of Conflict of Interest
Fredy Visser is an employee of Philips Medical. The remaining authors have no conflicts of interest.
Ethics Approval
This study is purely retrospective and is approved as such by the Utrecht institutional Medical Ethical Committee and therefore did not require formal ethical evaluation. All patients have given informed consent for the 7 T MRI examination. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Table S1. Scan parameters.
Table S2. Characteristics and findings ancillary tests of all 40 patients.
Acknowledgments
Tim Veersema received funding by the Dutch Epilepsy Foundation (grant 12‐12) for this study as part of a research project on patients with refractory epilepsy due to malformations of cortical development. The authors thank Wim Spliet, Wim van Hecke, and Angelika Mühlebner for performing histopathology and providing feedback considering correspondence with imaging findings.
Biography
Tim Veersema is a physician and PhD candidate at University Medical Center Utrecht.

References
- 1. Lerner JT, Salamon N, Hauptman JS, et al. Assessment and surgical outcomes for mild type I and severe type II cortical dysplasia: a critical review and the UCLA experience. Epilepsia 2009;50:1310–1335. [DOI] [PubMed] [Google Scholar]
- 2. Blümcke I, Thom M, Aronica E, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia 2011;52:158–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pang T, Atefy R, Sheen V. Malformations of cortical development. Neurologist 2008;14:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jin B, Wang J, Zhou J, et al. A longitudinal study of surgical outcome of pharmacoresistant epilepsy caused by focal cortical dysplasia. J Neurol 2016;263:2403–2410. [DOI] [PubMed] [Google Scholar]
- 5. Kwon HE, Eom S, Kang H, et al. Surgical treatment of pediatric focal cortical dysplasia. Neurology 2016;87:945–951. [DOI] [PubMed] [Google Scholar]
- 6. Mrelashvili A, Witte RJ, Wirrell EC, et al. Seizure freedom in children with pathology‐confirmed focal cortical dysplasia. Pediatr Neurol 2015;53:513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xue H, Cai L, Dong S, et al. Clinical characteristics and post‐surgical outcomes of focal cortical dysplasia subtypes. J Clin Neurosci 2016;23:68–72. [DOI] [PubMed] [Google Scholar]
- 8. Fauser S, Essang C, Altenmüller D‐M, et al. Long‐term seizure outcome in 211 patients with focal cortical dysplasia. Epilepsia 2015;56:66–76. [DOI] [PubMed] [Google Scholar]
- 9. Kim DW, Kim S, Park S‐H, et al. Comparison of MRI features and surgical outcome among the subtypes of focal cortical dysplasia. Seizure 2012;21:789–794. [DOI] [PubMed] [Google Scholar]
- 10. Kim DW, Lee SK, Chu K, et al. Predictors of surgical outcome and pathologic considerations in focal cortical dysplasia. Neurology 2009;72:211–216. [DOI] [PubMed] [Google Scholar]
- 11. Téllez‐Zenteno JF, Ronquillo LH, Moien‐Afshari F, et al. Surgical outcomes in lesional and non‐lesional epilepsy: a systematic review and meta‐analysis. Epilepsy Res 2010;89:310–318. [DOI] [PubMed] [Google Scholar]
- 12. West S, Nolan SJ, Cotton J, et al. Surgery for epilepsy Cochrane Database Syst Rev. 2015:7: CD010541. [DOI] [PubMed] [Google Scholar]
- 13. Rowland NC, Englot DJ, Cage TA, et al. A meta‐analysis of predictors of seizure freedom in the surgical management of focal cortical dysplasia. J Neurosurg 2012;116:1035–1041. [DOI] [PubMed] [Google Scholar]
- 14. Leach JL, Miles L, Henkel DM, et al. Magnetic resonance imaging abnormalities in the resection region correlate with histopathological type, gliosis extent, and postoperative outcome in pediatric cortical dysplasia. J Neurosurg Pediatr 2014;14:68–80. [DOI] [PubMed] [Google Scholar]
- 15. Bien CG, Szinay M, Wagner J, et al. Characteristics and surgical outcomes of patients with refractory magnetic resonance imaging‐negative epilepsies. Arch Neurol 2009;66:1491–1499. [DOI] [PubMed] [Google Scholar]
- 16. Phi JH, Cho B‐K, Wang K‐C, et al. Longitudinal analyses of the surgical outcomes of pediatric epilepsy patients with focal cortical dysplasia. J Neurosurg Pediatr 2010;6:49–56. [DOI] [PubMed] [Google Scholar]
- 17. Wang ZI, Alexopoulos AV, Jones SE, et al. The pathology of magnetic‐resonance‐imaging‐negative epilepsy. Mod Pathol 2013;26:1051–1058. [DOI] [PubMed] [Google Scholar]
- 18. Krsek P, Maton B, Korman B, et al. Different features of histopathological subtypes of pediatric focal cortical dysplasia. Ann Neurol 2008;63:758–769. [DOI] [PubMed] [Google Scholar]
- 19. Knake S, Triantafyllou C, Wald LL, et al. 3 T phased array MRI improves the presurgical evaluation in focal epilepsies: a prospective study. Neurology 2005;65:1026–1031. [DOI] [PubMed] [Google Scholar]
- 20. Zijlmans M, de Kort GAP, Witkamp TD, et al. 3 T versus 1.5 T phased‐array MRI in the presurgical work‐up of patients with partial epilepsy of uncertain focus. J Magn Reson Imaging 2009;30:256–262. [DOI] [PubMed] [Google Scholar]
- 21. Phal PM, Usmanov A, Nesbit GM, et al. Qualitative comparison of 3‐T and 1.5‐T MRI in the evaluation of epilepsy. Am J Roentgenol 2008;191:890–895. [DOI] [PubMed] [Google Scholar]
- 22. Mellerio C, Labeyrie M‐A, Chassoux F, et al. 3 T MRI improves the detection of transmantle sign in type 2 focal cortical dysplasia. Epilepsia 2014;55:117–122. [DOI] [PubMed] [Google Scholar]
- 23. van der Kolk AG, Hendrikse J, Zwanenburg JJM, et al. Clinical applications of 7T MRI in the brain. Eur J Radiol 2013;82:708–718. [DOI] [PubMed] [Google Scholar]
- 24. Colon AJ, van Osch MJP, Buijs M, et al. Detection superiority of 7T MRI protocol in patients with epilepsy and suspected focal cortical dysplasia. Acta Neurol Belg 2016;116:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Ciantis A, Barba C, Tassi L, et al. 7T MRI in focal epilepsy with unrevealing conventional field strength imaging. Epilepsia 2016;57:445–454. [DOI] [PubMed] [Google Scholar]
- 26. Mouthaan BE, Rados M, Barsi P, et al. Current use of imaging and electromagnetic source localization procedures in epilepsy surgery centers across Europe. Epilepsia 2016;57:770–776. [DOI] [PubMed] [Google Scholar]
- 27. Teeuwisse WM, Brink WM, Webb AG. Quantitative assessment of the effects of high‐permittivity pads in 7 tesla MRI of the brain. Magn Reson Med 2012;67:1285–1293. [DOI] [PubMed] [Google Scholar]
- 28. Madan N, Grant PE. New directions in clinical imaging of cortical dysplasias. Epilepsia 2009;50(Suppl 9):9–18. [DOI] [PubMed] [Google Scholar]
- 29. Knake S, Wehner T, Grant PE. Structural imaging of mesial temporal lobe epilepsy In Rosenow F, Ryvlin P, Lüders HO. (Eds.). The mesial temporal lobe epilepsies. Montrouge, France: Editions John Libbey Eurotext, 2010:165–173. [Google Scholar]
- 30. Blümcke I, Aronica E, Miyata H, et al. International recommendation for a comprehensive neuropathologic workup of epilepsy surgery brain tissue: a consensus Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia 2016;57:348–358. [DOI] [PubMed] [Google Scholar]
- 31. Mellerio C, Roca P, Chassoux F, et al. The power button sign: a newly described central sulcal pattern on surface rendering MR images of type 2 focal cortical dysplasia. Radiology 2015;274:500–507. [DOI] [PubMed] [Google Scholar]
- 32. Wellmer J, Quesada CM, Rothe L, et al. Proposal for a magnetic resonance imaging protocol for the detection of epileptogenic lesions at early outpatient stages. Epilepsia 2013;54:1977–1987. [DOI] [PubMed] [Google Scholar]
- 33. Blamire AM. The technology of MRI—the next 10 years? Br J Radiol 2008;81:601–617. [DOI] [PubMed] [Google Scholar]
- 34. Visser F, Zwanenburg JJM, Hoogduin JM, et al. High‐resolution magnetization‐prepared 3D‐FLAIR imaging at 7.0 tesla. Magn Reson Med 2010;64:194–202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Scan parameters.
Table S2. Characteristics and findings ancillary tests of all 40 patients.


