Abstract
Oestrogen is considered to be the ‘female’ hormone, whereas testosterone is considered the ‘male’ hormone. However, both hormones are present in both sexes. Thus sexual distinctions are not qualitative differences, but rather result from quantitative divergence in hormone concentrations and differential expressions of steroid hormone receptors. In males, oestrogen is present in low concentrations in blood, but can be extraordinarily high in semen, and as high as 250 pg ml−1 in rete testis fluids1,2, which is higher than serum oestradiol in the female3. It is well known that male reproductive tissues express oestrogen receptors4–7, but the role of oestrogen in male reproduction has remained unclear. Here we provide evidence of a physiological role for oestrogen in male reproductive organs. We show that oestrogen regulates the reabsorption of luminal fluid in the head of the epididymis. Disruption of this essential function causes sperm to enter the epididymis diluted, rather than concentrated, resulting in infertility. This finding raises further concern over the potential direct effects of environmental oestrogens on male reproduction and reported declines in human sperm counts8,9.
Classic cellular responses to the hormone oestrogen are mediated through nuclear oestrogen receptors (ER), which function as ligand-dependent transcription factors. Efferent ductules of the testis are known to express high amounts of ER-α10,11, higher even than uterine tissue, and both the α and β forms of ER are present in efferent ductules and the epididymis10. These ductules form a series of small tubules that transport sperm from the testis to the epididymis12. In humans, one third of the epididymal head consists of efferent ductules13. In addition to ciliated cells that stir the luminal fluid, their epithelia contain non-ciliated cells that resemble proximal tubule cells in the kidney. The non-ciliated cells have a reabsorptive function that results in the uptake of water, ions and proteins from the ductal lumen12,14. Ductules in the rat reabsorb nearly 90% of the rete testis fluid, coupling water and active ion transport in an electroneutral environment, in which Na+ and water are reabsorbed at equal rates, thereby increasing the concentration of sperm as they enter the epididymis15,16. This method of concentrating sperm improves their survival and maturation during epididymal storage and ensures that a large number of sperm are released upon ejaculation, increasing the randomness of fertilization and providing genetic variation14. These data and the observation that efferent ductules contain the highest concentrations of ER in the male led us to hypothesize that oestrogen participates in the regulation of fluid reabsorption in the male reproductive tract.
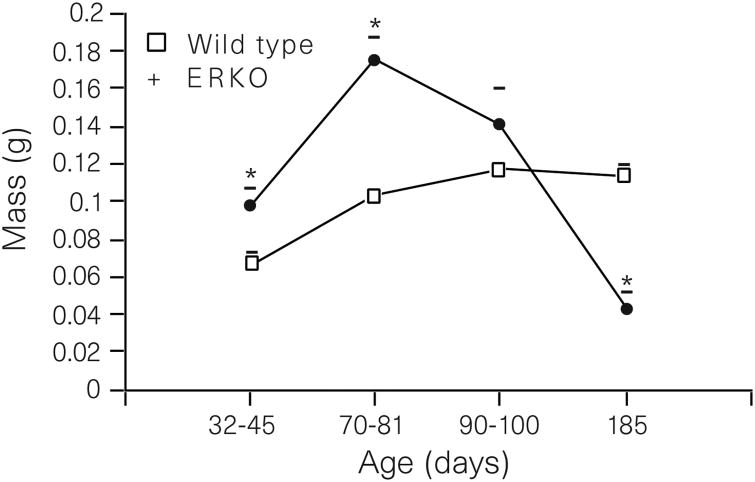
To test this hypothesis, we used the ER-α gene knockout mouse (ERKO)17,18. The ERKO male is infertile18, but its testes appear normal until puberty, when they begin to degenerate as early as 20–40 days of age. By 150 days, the testes are atrophic19. Sperm from the ERKO male are abnormal and sperm concentrations are significantly reduced in the epididmysis19. The reproductive tract in ERKO males contains a dilated rete protruding into the testis (Fig. 1a, b). Downstream from the rete, the efferent ductules are also swollen (Fig. 1c, d), with luminal areas more than twice the size of those of wild-type males at 90 days of age. From these observations, it appears that luminal fluid is not being removed by the ductal epithelium or that there is an excess of fluid secreted by the testis, causing fluids to accumulate in seminiferous tubules, rete and efferent ductules. Epithelial cells of the wild-type (Fig. 1e) contain endocytotic vesicles and large PAS+ lysosomal granules, organelles common to cells active in the uptake of luminal fluids. However, these structures are greatly reduced or missing in the epithelium of ERKO (Fig. 1f), which is decreased in height by 45%. Based on these data, we hypothesized that an increase in testis weight would occur as testicular secretions accumulated in the lumen of the seminiferous tubule. As postulated, a transient increase in testis weight in ERKO males is seen between 32 and 81 days of age and a decrease by 185 days (Fig. 2), suggesting that long-term atrophy of testes from ERKO is caused by back-pressure of the luminal fluids20,21.
Figure 1.

Seminiferous tubules, rete testis and efferent ductules in ERKO and wild-type mice. a, Wild-type seminiferous tubules exhibit normal spermatogenesis and a small rete testis (RT). b, ERKO rete testis and seminiferous tubules (ST) are dilated. Spermatogenesis appears abnormal in several tubules. c, Wild-type efferent ductules (DE) have narrow lumen. d, ERKO efferent ductules have dilated lumen. e, Wild-type efferent-ductule epithelium contains endocytotic vesicles (EV) and numerous PAS+ lysosomes (L). f, ERKO efferent-ductule epithelium is reduced in height and non-ciliated cells (N) contain fewer lysosomes and endocytotic vesicles; C, cilia. Scale bars: a, b, 100 μm; c, d, 50μm; e, f, 10μm.
Figure 2.
Testicular mass between 32 and 185 days of age (mean ± s.e.m.). ERKO mass increases at 32-45 and 70-80 days. By 185 days, the ERKO testes are atrophic. Differences were determined by the unpaired Student's t-test (P < 0.05); N = 5-8 for wild-type mice (WT) and N = 7-10 for ERKO.
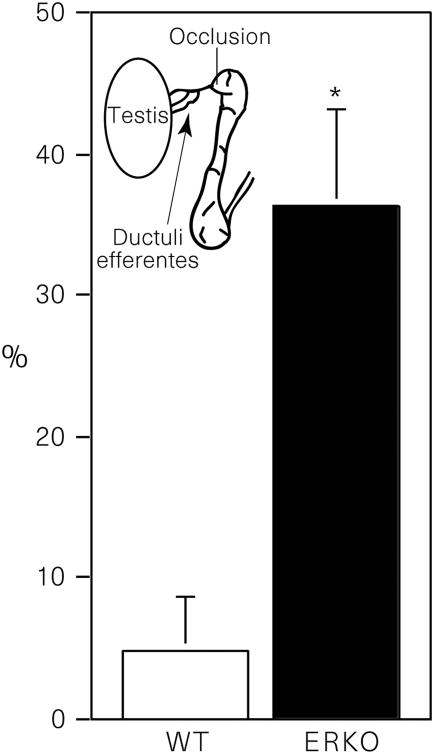
We further reasoned that if the efferent ductules in ERKO mice were dysfunctional, then occlusion of the initial segment epididymis, immediately distal to the ductules, would cause fluid to accumulate more rapidly in their testes than in the wild type. Therefore, we surgically cauterized the initial segment of the epididymis, occluding the terminal end of the efferent ductules. The contralateral side served as the control. Testes were removed 48 h after surgery and the difference in weight between the occluded and sham-operated side represented the build-up of luminal fluid caused by a lack of reabsorption in the efferent ductules. After ductal occlusion, testes of ERKO weigh 30% more than testes of wild-type mice (Fig. 3). However, these data do not remove the possibility that the increase in weight results from an abnormal rate of fluid secretion by the seminiferous epithelium in the testis of ERKO. Therefore, we also unilaterally occluded the rete testis in a group of ERKO and wild-type males and compared differences in testicular weight changes at 24 h. Instead of showing increases in fluid secretion, the testis in ERKO secretes significantly less fluid (P < 0.05) in 24h than does the wild-type: 22.4 ± 3.0% increase versus 37.9 ± 5.3%, respectively. In the ERKO male, efferent ductules do not appear capable of reabsorbing luminal fluids received from the testis. Thus the ERKO mouse provides evidence that oestrogens may be responsible for the regulation of fluid transport, which increases the concentration of sperm before entering the epididymis.
Figure 3.
Change in testis mass 48 h after occlusion of the initial-segment epididymis. Values shown are mean (± s.e.m.) percentage difference between the occluded and sham-operated side. Differences between wild-type (WT) and ERKO means were determined by the unpaired Student's t-test (P < 0.05).
To assess the function of oestrogen in adult tissues without developmental influence, we treated wild-type mice for 3 days with an anti-oestrogen compound and used in vitro methods of analysis to determine fluid reabsorption. ICI 182,780 (ICI Pharmaceuticals) is a pure anti-oestrogen that inhibits increases in uterine weight stimulated by 17β-oestradiol and binds both ER-α and ER-β22,23. Small segments of adult efferent ductules were isolated for organ culture and the tubular ends were ligated with fine suture, preventing the inflow of culture medium. Using this in vitro model, we compared the function of ductules in wild-type, ERKO and ICI-treated males over a 24-h period. In ductules from wild-type mice, the epithelium is capable of rapidly removing luminal fluid, which results in the collapse of the ductule walls (Fig. 4). After 24 h of ligation, the luminal area of ductules from wild-type decreased 82% (Fig. 5). In contrast, the luminal area of ductules from ERKO increased 46%, and ductules from wild-type mice treated with ICI showed only a slight decrease of 14%. Thus efferent ductules from ERKO are incapable of reabsorbing luminal fluid, while wild-type ductules remove most of the fluid within 3 h. Blockage of ER function, by treating ductules from wild-type with an anti-oestrogen, significantly inhibits fluid reabsorption.
Figure 4.

Ligated efferent ductules in vitro. a, Wild-type ductule lumen (between arrows) collapse by 3 h. b, ERKO ductule lumen (between arrows) is wider than wild type and appears to increase in diameter after incubation. c, Wild-type ductule treated with an anti-oestrogen (ICI 182,780) initially has a normal lumen but has a slight reduction in diameter after incubation. Scale bars, 100μm.
Figure 5.
The effects of in vitro ligation on changes in luminal area of efferent ductule segments 24 h after ligation. Mean (± s.e.m.) percentage difference between 0 h and 24 h in luminal areas for isolated ductules from wild-type controls, ERKO and ICI-treated mice.
Thus ER-α is important both for normal growth of the male reproductive tract and for adult function of the efferent ductules. However, because wild-type ICI-treated ductules in vitro did not swell like the tissues from ERKO mice, the mechanisms of oestrogen action are not fully understood. For example, it is not known whether the role of ER-β is the same as that of ER-α in efferent ductules. Recent data in HeLa cells have shown that anti-oestrogens and 17β-oestradiol produce opposite effects on ER-β, depending upon the type of response element complex formed24. Thus ER-β function in ductules of ERKO males could account for the in vitro observations. Regardless of the precise mechanisms, developmental disruption of ER-α in mice causes a total loss of net fluid reabsorption. In the human, mutational dysfunction in ER-α and P450 aromatase (the enzyme that converts androgens to oestrogens) decreases sperm counts and results in poor sperm viability25-27.Our data suggest that oestrogen deficiency or oestrogen insensitivity in man might also result in the accumulation of fluid in efferent ductules and subsequent atrophy of the testis. Tamoxifen, which is commonly known to be a mixed oestrogen agonist/antagonist in different tissues, has been used to increase sperm counts in oligo-spermic men28. In conclusion, our data describe a physiological endpoint of oestrogen action in the male reproductive system. This finding is important given recent concerns over reported declines in human sperm counts and speculation that exposure to environmental oestrogens maybe a cause of this8,9,29. The extensive presence of ER (both α and β) at other sites in the male reproductive system and throughout the body10,11,23 makes it possible that new and unexpected functions may be found for the ‘female’ hormone in men.
Methods
Histology and morphometry
Testis and mid-proximal efferent ductules were fixed and processed for light microscopy30. Luminal area, ductal circumference and epithelial height were taken in 5 areas per ductule cross-section in 5 ductules per mouse.
Initial-segment epididymal occlusions and rete testis ligation
Under surgical anaesthesia, initial-segment epididymidis was occluded by cauterization or rete testis was ligated with 0000 suture. The contralateral testis was sham operated. The animals were allowed to recover and testes were taken at either 48 h (initial-segment occlusions) or 24 h (rete ligation) and weighed. For the initial segment occlusions, N = 7 for both wild-type and ERKO. For rete testis occlusions, N = 14 for wild-type mice and N = 12 for ERKO.
In vitro ligation of efferent ductules
One group of wild-type mice was given single daily injections of ICI 182,780 (1 mg per kg) for 3 days before removal of the ductules. Efferent ductules were microdissected into 1.5-mm lengths and incubated for 24 h in M199 culture medium containing dihydrotestosterone (4 × 10−7 M), 17β-oestradiol (1 × 10−9 M), bovine lipoprotein (0.16 mg ml−1) or all components plus ICI 182,780 (1 × 10 −6 M) at 34 °C in humidified 95% air/5% CO2. After 24 h, segments were ligated on both ends to prevent entry and exit of fluids. Digital images of the ductules were analysed at 0, 3 or 12 and 24 h after ligation (Figs 4, 5). Ductal segments damaged by microdissection or stretching were discarded. Only segments with rapid ciliary beat and clear lumens were ligated. N = 3 mice for each treatment group, and 3–12 ductal segments per animal were analysed. Differences between means were determined by a one-way analysis of variance (P < 0.0001) followed by Bonferroni multiple comparisons test (P < 0.001).
Acknowledgments
We thank E. Jassim and C. Finnigan-Bunick for technical assistance, P. Cooke, V. K. Ganjam and D. J. Miller for reviews of the manuscript and A. Wakeling and Zeneca Pharmaceuticals for providing ICI 182, 780.
References
- 1.Ganjam VK, Amann RP. Steroid content of fluids and sperm entering and leaving the bovine epididymis, in epididymal tissue, and in accessory sex gland secretions. Endocrinology. 1976;99:1618–1630. doi: 10.1210/endo-99-6-1618. [DOI] [PubMed] [Google Scholar]
- 2.Free MJ, Jaffe RA. Collection of rete testis fluid from rats without previous efferent duct ligation. Biol Reprod. 1979;20:269–278. doi: 10.1095/biolreprod20.2.269. [DOI] [PubMed] [Google Scholar]
- 3.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 4.Cooke PS, Young P, Hess RA, Cunha GR. Estrogen receptor expression in developing epididymis, efferent ductules, and other male reproductive organs. Endocrinology. 1991;128:2874–2879. doi: 10.1210/endo-128-6-2874. [DOI] [PubMed] [Google Scholar]
- 5.Greco T, Duello T, Gorski J. Estrogen receptors; estradiol; and diethylstilbestrol in early development: the mouse as a model for the study of estrogen receptors and estrogen sensitivity in embryonic development of male and female reproductive tracts. Endocr Rev. 1993;14:59–71. doi: 10.1210/edrv-14-1-59. [DOI] [PubMed] [Google Scholar]
- 6.Schleicher G, Drews U, Stumpf WE, Sar M. Differential distribution of dihydrotestosterone and estradiol binding sites in the epididymis of the mouse. An autoradiographic study. Histochemistry. 1984;81:139–147. doi: 10.1007/BF00490107. [DOI] [PubMed] [Google Scholar]
- 7.West N, Brenner R. Estrogen receptor in the ductuli efferentes epididymis and testis of rhesus and cynomolgus macaques. Biol Reprod. 1990;42:533–538. doi: 10.1095/biolreprod42.3.533. [DOI] [PubMed] [Google Scholar]
- 8.Sharpe RM, Skakkebaek NE. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993;341:1392–1395. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- 9.Auger J, Kunstmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med. 1995;332:281–285. doi: 10.1056/NEJM199502023320501. [DOI] [PubMed] [Google Scholar]
- 10.Hess RA, et al. Estrogen receptor (α & β) expression in the excurrent ducts of the adult male rat reproductive tract. J Androl. 1997;18:602–611. [PubMed] [Google Scholar]
- 11.Fisher JS, et al. Immunolocalisation of oestrogen receptor-α within the testis and excurrent ducts of the rat and marmoset monkey from perinatal life to adulthood. J Endocrinol. 1997;153:485–495. doi: 10.1677/joe.0.1530485. [DOI] [PubMed] [Google Scholar]
- 12.Ilio K, Hess R. Structure and function of the ductuli efferentes: A review. Microsc Res Tech. 1994;29:432–467. doi: 10.1002/jemt.1070290604. [DOI] [PubMed] [Google Scholar]
- 13.Yeung CH, Cooper TG, Bergmann M, Schulze H. Organization of tubules in the human caput epididymidis and the ultrastructure of their epithelia. Am J Anat. 1991;191:261–279. doi: 10.1002/aja.1001910306. [DOI] [PubMed] [Google Scholar]
- 14.Robaire B, Hermo L. In: The Physiology of Reproduction. Knobil E, Neill J, editors. Raven; New York: 1988. pp. 999–1080. [Google Scholar]
- 15.Chan HC, Zhou WL, Fu WO, Ko WH, Wong PY. Different regulatory pathways involved in ATP-stimulated chloride secretion in rat epididymal epithelium. J Cell Physiol. 1995;164:271–276. doi: 10.1002/jcp.1041640207. [DOI] [PubMed] [Google Scholar]
- 16.Clulow J, Jones R, Hansen L. Micropuncture and cannulation studies of fluid composition and transport in the ductuli efferentes testis of the rat: comparisons with the homologous metanephric proximal tubule. Exp Physiol. 1994;79:915–928. doi: 10.1113/expphysiol.1994.sp003817. [DOI] [PubMed] [Google Scholar]
- 17.Lubahn DB, et al. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korach KS, et al. Estrogen receptor gene disruption: molecular characterization and experimental and clinical phenotypes. Recent Prog Horm Res. 1996;51:159–186. [PubMed] [Google Scholar]
- 19.Eddy EM, et al. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology. 1996;137:4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- 20.Smith G. The effects of ligation and the vasa efferentia and vasectomy on testicular function in the adult rat. Endocrinology. 1962;23:385–399. doi: 10.1677/joe.0.0230385. [DOI] [PubMed] [Google Scholar]
- 21.Hess R, Moore B, Forrer J, Linder R, Abuel-Atta A. The fungicide benomyl [methyl 1-butylcarbamoyl-2-benzimidazole carbamate] causes testicular dysfunction by inducing the sloughing of germ cells and occlusion of efferent ductules. Fundam Appl Toxicol. 1991;17:733–745. doi: 10.1016/0272-0590(91)90181-3. [DOI] [PubMed] [Google Scholar]
- 22.Wakeling A, Dukes M, Bowler J. A potent specific pure antiestrogenwith clinical potential. Cancer Res. 1991;51:3867–3873. [PubMed] [Google Scholar]
- 23.Kuiper GG, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 24.Paech K, et al. Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 25.Carani C, et al. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med. 1997;337:91–95. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 26.Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab. 1995;80:3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 27.Smith EP, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. Erratum, N. Engl. J. Med. 332, 131 (1995) [DOI] [PubMed] [Google Scholar]
- 28.Krause W, Holland-Moritz H, Schramm P. Treatment of idiopathic oligozoospermia with tamoxifen—a randomized controlled study. Int J Androl. 1992;15:14–18. doi: 10.1111/j.1365-2605.1992.tb01110.x. [DOI] [PubMed] [Google Scholar]
- 29.Sharpe RM. Declining sperm counts in men—is there an endocrine cause? J Endocrinol. 1993;136:357–360. doi: 10.1677/joe.0.1360357. [DOI] [PubMed] [Google Scholar]
- 30.Hess RA, Moore BJ. In: Methods in Reproductive Toxicology. Chapin RE, Heindel JJ, editors. Academic; San Diego: 1993. pp. 52–85. [Google Scholar]