Abstract
Background
Failure to adhere to infection control guidelines, especially during assisted monitoring of blood glucose, has caused multiple hepatitis B outbreaks in assisted living facilities (ALFs). In conjunction with the response to such an outbreak at an ALF (“Facility X”) where most residents had neuropsychiatric disorders, we evaluated seroprotection rates conferred by hepatitis B vaccine and assessed the influence of demographic factors on vaccine response.
Methods
Residents were screened for hepatitis B and C infection, and those susceptible were vaccinated against hepatitis A and hepatitis B with one dose of TWINRIX™ (GSK) given at 0, 1, and 7 months. Blood samples were collected 1–2 months after receipt of the third vaccine dose to test for antibody to hepatitis B surface antigen (anti-HBs).
Results
Of the 27 residents who had post-vaccination blood specimens collected, 22 (81%) achieved anti-HBs concentrations ≥10 mIU/mL. Neither age nor neuropsychiatric comorbidity was a significant determinant of seroprotection. Geometric mean concentration was lower among residents aged 60–74 years (74.3 mIU/mL) than among residents aged 46–59 years (105.3 mIU/mL) but highest among residents aged ≥75 years (122.5 mIU/mL). The effect of diabetes on vaccination response could not be examined because 16/17 (94%) diabetic residents had HBV infection by the time of investigation.
Conclusions
Adult vaccine recipients of all ages, even those over 60 years of age, demonstrated a robust capacity for achieving hepatitis B seroprotection in response to the combined hepatitis A/hepatitis B vaccine. The role for vaccination in interrupting HBV transmission during an outbreak remains unclear, but concerns about age-related response to hepatitis B vaccine may be insufficient to justify foregoing vaccination of susceptible residents of ALFs.
Keywords: Hepatitis B, HBV, Vaccination, Age, Neuropsychiatric
1. Introduction
Hepatitis B virus (HBV) is transmitted by percutaneous or mucosal exposure to blood or bodily fluids from an infected person. Assisted living facilities (ALFs) provide long-term residential care to a growing number of adults needing assistance with activities of daily living. A substantial proportion of ALF residents have diabetes, many of whom will undergo assisted monitoring of blood glucose (AMBG) [1]. Performing AMBG requires use of fingerstick devices and blood glucose meters, which frequently become contaminated with blood [2,3]. Infection control guidelines for safe blood glucose monitoring have been available since 1990, and guidelines targeting long-term care settings were published in 2005 [4]. Lapses in infection control during AMBG (e.g., reuse of fingerstick devices) have resulted in outbreaks of HBV infection among residents of long-term care facilities across the United States [4–15]. Among 30 HBV outbreaks that occurred in long-term care facilities (i.e., nursing homes or ALFs) and were reported to the Centers for Disease Control and Prevention (CDC) during 1996–2012, 26 (87%) were linked to breaches in infection control practice during AMBG [14]. In response to these outbreaks, in 2011 the Advisory Committee on Immunization Practices (ACIP) published new guidelines recommending that all previously unvaccinated adults under age 60 with diabetes be vaccinated against hepatitis B [16]. Because of scant data on the effectiveness of hepatitis B vaccination among adults aged ≥60 years the recommendation for this age group was for vaccination at the discretion of the treating clinician after assessing their risk and the likelihood of an adequate immune response to vaccination [16].
Hepatitis B vaccination is an effective measure to prevent HBV infection and is recommended for adults who are at high risk for infection [17]. Previously identified high-risk groups also include residents and staff of facilities housing developmentally disabled persons [18–22]. Four of the 30 HBV outbreaks described above occurred in long-term care facilities in which most residents had neuropsychiatric disorders. Of these, only two were linked to infection control lapses during AMBG [11,13,14].
The effectiveness of hepatitis B vaccination for interrupting HBV transmission during an outbreak remains unclear because vaccination typically requires administration of three doses over a 6-month period, and completion of the series can be undermined by resident transfers between facilities. Even assuming completion of the vaccination series, adults aged ≥60 years might be less likely to achieve seroprotection (hepatitis B antibody [anti-HBs] ≥10 mIU/mL [16]) after receipt of hepatitis B vaccine than has been demonstrated among adults aged <40 years [23–28]. Therefore, we evaluated the immunogenicity of bivalent vaccine against hepatitis A and B administered to residents during a HBV outbreak at ALF “Facility X” and assessed the influence of demographic factors on vaccine response.
2. Materials and methods
2.1. Study population
In January 2010, the Virginia Department of Health (VDH) received reports of two HBV infections (one acute case detected through routine hemodialysis screening, and one chronic case report) among residents of a single 160-bed ALF. Both patients had diabetes and received AMBG. Most residents of this ALF had neuropsychiatric disorders. VDH began an investigation with assistance from CDC to determine the number of ALF residents infected with HBV, identify modes of transmission, and implement control and prevention measures which included HBV vaccination [13]. The protocol for post-vaccination response testing was reviewed and approved by VDH and CDC Institutional Review Boards; written informed consent was signed by all participating patients or their conservators if the patient was unable to provide informed consent on his or her own behalf.
2.2. Serologic testing and vaccine administration
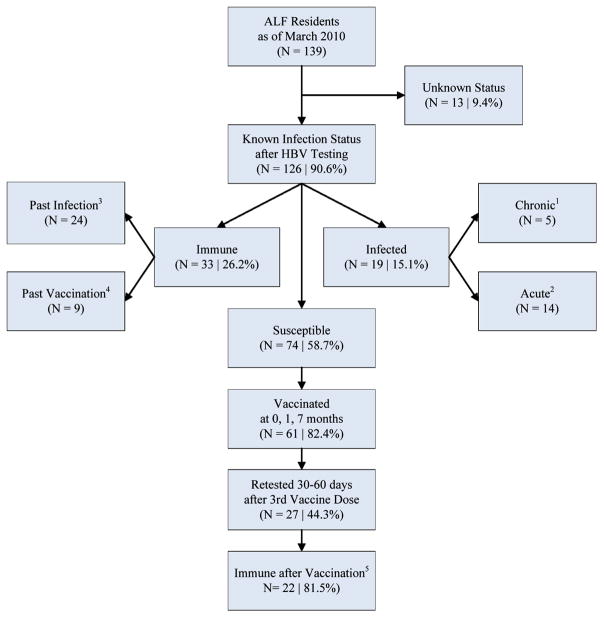
We collected an initial blood sample from all ALF residents who consented to initial screening or, for those residents unable to consent, whose conservators provided consent. Specimens were tested at CDC Division of Viral Hepatitis Laboratory for hepatitis B surface antigen (HBsAg), total core antibody (anti-HBc), IgM core antibody (IgM-anti-HBc), and surface antibody (anti-HBs) by using VITROS® ECi Immunodiagnostic System (Ortho-Clinical Diagnostics, Inc., Rochester, NY). Based upon results from serologic testing of these pre-vaccination blood samples, residents were classified as acutely or chronically infected, immune, or susceptible to HBV infection according to standard criteria (Fig. 1) [5].
Fig. 1.
Participant flow chart for hepatitis B screening and vaccination—Virginia, 2010. ALF, assisted living facility; HBV, hepatitis B virus; anti-HBs, antibody to hepatitis B surface antigen; anti-HBc, antibody to hepatitis B core antigen; HBsAg, hepatitis B surface antigen; immune, anti-HBs ≥10 mIU/mL. 1 Serologic markers indicating chronic infection: positive hepatitis B surface antigen (HBsAg) and total hepatitis B core antibody (anti-HBc), negative hepatitis B IgM core antibody (IgM anti-HBc). 2 Serologic markers indicating acute infection: positive HBsAg and IgM anti-HBc. One resident was determined to have acute infection based on a well-documented HBsAg seroconversion even though IgM-anti-HBc remained negative. 3 Serologic markers indicating immunity to hepatitis B due to past infection: positive hepatitis B antibody to surface antigen (anti-HBs) and total hepatitis B core antibody (anti-HBc). 4 Serologic markers indicating immunity to hepatitis B due to past vaccination: positive anti-HBs (level ≥10 mIU/mL) and anti-HBc negative. 5 Seroprotective response defined as anti-HBs ≥10 mIU/mL.
Patients who were susceptible to HBV infection who consented to vaccination or, for those residents unable to consent whose conservators provided consent, were vaccinated using a 23-gauge one-inch needle in the right or left deltoid muscles with one 1-mL dose of TWINRIX™ provided by VDH (GlaxoSmithKline Biologicals, lot numbers: AHBVB640BA, AHABB174AA, AHABB167BA, AHABB162CA, AHABB189AB, AHABB185AA) on a 0, 1, 7 month schedule [17]. (The third dose, usually given at 6 months, was delayed by 1 month due to logistical challenges.)
Because of logistical constraints only those vaccinated patients who were able to give informed consent without the assistance of a conservator were approached for consent to post-vaccination testing. Blood samples were collected at the standard time period for assessing serologic response to hepatitis B vaccine, 1–2 months after receipt of the third dose [17]. Anti-HBs concentrations ≥10 mIU/mL were considered indicative of seroprotection [17].
2.3. Demographic data
We examined facility health records of all current residents to obtain basic demographic (age, sex, and race) data. Information on clinical characteristics that might affect vaccine response (e.g., smoking, body mass index, alcohol use, renal disease, liver disease, HIV status, use of immunosuppressive drugs or chemotherapy, and other comorbid conditions) was often missing from facility records because ALFs in Virginia are not required to maintain complete medical records.
2.4. Statistical analysis
Differences in the proportion of residents with adequate serologic response to hepatitis B vaccine (anti-HBs concentration ≥10 mIU/mL) based upon demographic characteristics were assessed and compared using Fisher’s exact test, with a p-value <0.05 considered statistically significant. For the purpose of summarizing the stimulated immunity for this population, quantitative anti-HBs concentrations were calculated as geometric mean concentrations (GMCs) with 95% confidence intervals (95% CIs). Each anti-HBs concentration value was log transformed and values below 1 mIU/mL were assigned a value of 1 mIU/mL before log transformation. SAS v9.2 (SAS Institute, Inc., Cary, NC) was used for statistical analysis.
3. Results
Among 139 residents present in the ALF in March 2010, HBV serologic status was determined for 126 (91%) (Fig. 1). Of these, 19 (15%) were HBV infected (5 chronic; 14 acute), 33 (26%) were immune (24 had evidence of past infection and 9 of previous vaccination), and 74 (59%) appeared susceptible to infection as indicated by an anti-HBs level <10 mIU/mL. Prevalence of diabetes necessitating receipt of AMBG was 13% among these residents, and 16/17 (94%) diabetic residents had experienced HBV infection by the time of investigation. Among the 61 susceptible residents (82%) who consented to vaccination and completed the 3-dose vaccination series, 27 (44%) consented to measurement of vaccine response. The anti-HBs GMC among the 27 residents was 91.7 mIU/mL (95% confidence interval, 35.17–239.2). Among these patients, 22 (81%) achieved anti-HBs concentrations ≥10 mIU/mL. Non-response status was shared with the five remaining patients’ physicians for consideration of re-vaccination, but re-vaccination and assessment of further response was not included in the protocol.
The median age of the 27 vaccinated residents was 60 years (range: 46–86 years). More men than women, but similar numbers of whites and blacks, were vaccinated (Table 1). None of the vaccinated residents had diabetes. The demographic characteristics (age, sex, and race) of the 47 (64%) susceptible residents who did not complete vaccination or did not have post-vaccination serological testing were similar to those 27 (36%) who completed vaccination and post-vaccination serological testing; all p-values were >0.05. Susceptible patients who did not have post-vaccination testing were likely to have a more severe neuropsychiatric disorder (e.g., history of traumatic brain injury) because only patients able to provide consent without healthcare conservators were approached for post-vaccination testing. Common comorbid conditions among the vaccinated and unvaccinated patients included schizophrenia, depression, and hypertension. The one HBV-susceptible patient with diabetes declined vaccination.
Table 1.
Concentration of antibody to Hepatitis B surface antigen (Anti-HBs) after 3 doses of hepatitis B vaccine among assisted living facility residents by demographic characteristics—Virginia, 2010.
| Characteristic | Total sample N (%) | Anti-HBs ≥10 mIU/mL n (%) | p-Valuea | Anti-HBs GMC | |
|---|---|---|---|---|---|
|
| |||||
| Mean GMC | 95% CI | ||||
| Total | 27 | 22 (81.5%) | – | 91.7 | 35.2–239.2 |
| Age | |||||
| 46–59 | 12 (44.4%) | 10 (83.3%) | Ref | 105.3 | 21.5–516.4 |
| 60–74 | 12 (44.4%) | 9 (75.0%) | 0.6 | 74.3 | 13.4–411.4 |
| ≥75 | 3 (11.1%) | 3 (100.0%) | 1.0 | 122.5 | b |
| Sex | |||||
| Male | 17 (63.0%) | 13 (76.5%) | Ref | 55.3 | 16.5–185.5 |
| Female | 10 (37.0%) | 9 (90.0%) | 0.4 | 217.1 | 38.9–1213.0 |
| Race | |||||
| White | 14 (51.9%) | 12 (85.7%) | Ref | 150.7 | 35.9–632.5 |
| Black | 13 (48.1%) | 10 (76.9%) | 0.6 | 53.7 | 13.1–219.8 |
Fisher’s exact.
Insufficient sample size.
Age was not a significant determinant of seroprotection. The proportion of residents who developed seroprotection was lower among residents aged 60–74 years (10/12 [75%]) than among residents aged 46–59 years (9/12 [83%]), but all three residents aged ≥75 years developed seroprotection. Likewise, the GMC was lower among residents aged 60–74 years (74.3 mIU/mL) than among residents aged 46–59 years (105.3 mIU/mL), but highest among the three residents aged ≥75 (122.5 mIU/mL).
4. Discussion
During an outbreak of HBV infection at an ALF where most residents had neuropsychiatric disorders [13], bivalent hepatitis B/hepatitis A vaccine was administered to residents who were identified as susceptible to HBV infection and willing to be vaccinated. Seroprotection (anti-HBs concentration ≥10 mIU/mL) was achieved by 81% of residents who were vaccinated and willing to have their anti-HBs concentration measured after receipt of the third vaccine dose.
We did not detect any new infections after re-testing for hepatitis B markers at re-screening approximately 1 month after the second vaccine dose or at post-vaccination testing 1–2 months past completion of the third dose of vaccine. Receipt of AMBG was the principal risk factor for acquiring HBV infection during this outbreak [13]. Inappropriate reuse of fingerstick devices on multiple residents was so efficient at transmitting HBV from resident to resident in this setting that only one resident with diabetes who received AMBG remained susceptible to infection. That resident declined to be vaccinated. Thus we cannot provide data on response to vaccination among persons with diabetes in this population.
In our study of ALF residents aged 46–86 years, age was not a significant determinant of seroprotection 1–2 months following a 3-dose vaccine series. In the few published studies that have assessed the response to hepatitis B vaccine among older adults (aged ≥60 years) reported seroprotection rates vary from 30% to 80% and depend on characteristics of the study population, vaccination history, vaccination schedule, and type of vaccine [23–31]. Some studies have shown poor response to bivalent hepatitis B/hepatitis A vaccine among elderly populations [23,29]. However, another study found higher response rates [24], with an approximately 65–70% response rate overall to simultaneous or combination hepatitis B and hepatitis A vaccine among persons 61–81 years of age in an unpublished sub-analysis [31].
Response to bivalent hepatitis A/B vaccine administered at standard dosing and schedule was also assessed during a 2010 HBV outbreak in two skilled nursing homes in another state; of these 86 residents aged 45–101, only 29 (34%) demonstrated seroprotective anti-HBs levels [29]. Surprisingly in this study the response rate among the younger individuals under age 60 (33%) was no better than among those older; this may be due to the multiple comorbid conditions present among these persons whose care required residence in a skilled nursing facility. Response to monovalent hepatitis B vaccine administered at standard dosing and schedule was also assessed during a 2011 ALF HBV outbreak in which only 6/22 (27%) diabetic and/or memory-impaired residents aged 58–92 demonstrated seroprotective anti-HBs levels, although response testing was delayed by 1–2 months (unpublished data, Watson J, Myrick-West A, Virginia Department of Health, Richmond, Virginia). In another recent study of response to monovalent hepatitis B vaccine among a population of ALF residents in Houston, TX, seroprotection was achieved by only 30% (7 of 23) of tested vaccine recipients aged 70–89 [30]. In contrast, among the small number (n = 4) of residents aged 70 or greater in our study, all responded to the bivalent vaccine.
Several factors might have contributed to the high proportion of seroprotection achieved among residents in this study. First, unlike other studies [30] in which an accelerated (0, 1, 4 months) vaccination schedule was implemented as part of the outbreak response effort, we used a 0, 1, 7 months vaccination schedule. Delaying the third dose beyond 4 months might have achieved a better booster effect. Second, data on most clinical characteristics that might affect vaccine response (e.g., smoking, body mass index, etc.) were missing from facility records. Third, age has been found to be a significant predictor of seroprotection in other study populations [30], and the majority of adults resident at this ALF were not significantly older than 60 years (median age 60) which likely favored the high proportion of seroprotection. Our sample size, particularly of residents aged ≥75 years, was small and limited to persons who could consent for themselves, thus estimates may not be widely generalizable. However, the data available from other studies for residents over 75 years old, while sparse, indicate that the development of seroprotection is achievable at least for some older adults [23–31].
In addition to characteristics of our study population that may have resulted in higher seroprotection, this study has some other potential limitations. It is possible some persons vaccinated in this study might have been previously vaccinated in the past without documentation in the currently available medical chart. In such vaccine recipients the response to vaccination would actually have been a response to revaccination with preserved immune memory in the absence of detectable antibody, which could have falsely elevated our estimate of primary vaccine response. Self-reported data on previous hepatitis B vaccination was not collected from the vaccinated residents because of logistical constraints and concerns about the reliability of patient recall for specific vaccinations received in the possibly quite distant past.
In the absence of a more immunogenic hepatitis B vaccine, adults anticipated to be at risk for bloodborne pathogen exposure are more likely to benefit from vaccination at earlier ages when immune response is most robust [16]. Adults with diabetes who do not currently require daily glucose monitoring might eventually require such monitoring, with or without assistance; recipients of AMBG in ALFs are at increased risk of bloodborne pathogen exposure [3,5,7,29] when infection control practices in such facilities are compromised [14]. In an effort to address this problem, ACIP recently recommended that all previously unvaccinated adults with diabetes aged 19 through 59 years be vaccinated against hepatitis B as soon as possible after a diagnosis of diabetes is made [16]. Persons with mental illness, if housed in a congregate setting for protracted periods, may also be at increased risk for HBV infection [18–22]. However, outbreaks have occurred among neuropsychiatric ALF populations linked with AMBG as well as other infection control breaches and patient risk behavior [11,14,32,33]. Protection of a growing and vulnerable ALF population should include interventions such as improved infection control oversight at ALFs, appropriate training of staff members performing AMBG, and prompt investigation of acute HBV infections [11].
At this ALF, vaccine recipients without diabetes aged 60–86 years of age demonstrated a robust capacity for achieving sero-protection in response to the hepatitis B vaccine by standard measures [17]. The role for vaccination in interrupting HBV transmission during an outbreak remains unclear because of the timeline required for vaccine series completion and immunogenicity, but concerns about age-related response to hepatitis B vaccine may be insufficient to justify foregoing vaccination of susceptible residents.
Acknowledgments
Funding
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Use of trade names and commercial sources is for identification only and does not imply endorsement.
We would like to thank the following persons for their generous assistance with this project: Margaret Tipple, M.D., Kaye Carrithers, B.S.N., M.P.H., Barbara Hummell, B.S.N., Donald R. Stern, M.D., M.P.H., and Danny Avula, M.D., M.P.H., Virginia Department of Health, Richmond, VA; Cathy Wyatt, B.S.M.T., Division of Consolidated Laboratory Services, Virginia Department of General Services, Richmond, VA; Deborah A. Lloyd, B.S.N., Lynne A. Williams, Lisa Abraham, B.S.N., Vashti L. Colson, and DeNyce B. Bonaparte, M.S.W., Virginia Department of Social Services, Richmond, VA; Sarah Schillie, M.D., M.P.H., M.B.A.; Rania A. Tohme, M.D., M.P.H., Yury Khudyakov, Ph.D., and Natasha Khudyakov, M.S., Division of Viral Hepatitis, CDC, Atlanta, GA; Matthew E. Wise, Ph.D., M.P.H., Nicola D. Thompson, Ph.D., M.S., Priti R. Patel, M.D., M.P.H., Division of Healthcare Quality Promotion, CDC, Atlanta, GA; Sheryl B. Lyss, M.D., M.P.H., Office of Surveillance, Epidemiology, and Laboratory Services, CDC, Atlanta, GA.
Footnotes
Ethical considerations
The investigation followed the guidelines of the U.S. Department of Health and Human Services regarding protection of human subjects.
References
- 1.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 2.Louie RF, Lau MJ, Lee JH, Tang Z, Kost GJ. Multicenter study of the prevalence of blood contamination on point-of-care glucose meters and recommendations for controlling contamination. Point Care. 2005;4:158–63. [Google Scholar]
- 3.Thompson ND, Perz JF, Moorman AC, Holmberg SD. Nonhospital health care-associated hepatitis B and C virus transmission: United States, 1998–2008. Ann Intern Med. 2009;150:33–9. doi: 10.7326/0003-4819-150-1-200901060-00007. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Transmission of hepatitis B virus among persons undergoing blood glucose monitoring in long-term-care facilities—Mississippi, North Carolina, and Los Angeles County, California, 2003–2004. MMWR Morb Mortal Wkly Rep. 2005;54:220–3. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Notes from the field: deaths from acute hepatitis B virus infection associated with assisted blood glucose monitoring in an assisted-living facility—North Carolina, August–October 2010. MMWR Morb Mortal Wkly Rep. 2011;60:182. [PubMed] [Google Scholar]
- 6.Quale JM, Landman D, Wallace B, Atwood E, Ditore V, Fruchter G. Deja vu: nosocomial hepatitis B virus transmission and fingerstick monitoring. Am J Med. 1998;105:296–301. doi: 10.1016/s0002-9343(98)00256-3. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Nosocomial hepatitis B virus infection associated with reusable fingerstick blood sampling devices—Ohio and New York City, 1996. MMWR Morb Mortal Wkly Rep. 1997;46:217–21. [PubMed] [Google Scholar]
- 8.Polish LB, Shapiro CN, Bauer F, Klotz P, Ginier P, Roberto RR, et al. Nosocomial transmission of hepatitis B virus associated with the use of a spring-loaded finger-stick device. N Engl J Med. 1992;326:721–5. doi: 10.1056/NEJM199203123261101. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Nosocomial transmission of hepatitis B virus associated with a spring-loaded fingerstick device—California. MMWR Morb Mortal Wkly Rep. 1990;39:610–3. [PubMed] [Google Scholar]
- 10.Food and Drug Administration. Safety alert: hepatitis B transmission via spring-loaded lancet devices. Rockville, MD: Department of Health and Human Services, US Public Health Service, Food and Drug Administration; 1990. [Google Scholar]
- 11.Centers for Disease Control and Prevention. Multiple outbreaks of hepatitis B virus infection related to assisted monitoring of blood glucose among residents of assisted living facilities—Virginia, 2009–2011. MMWR Morb Mortal Wkly Rep. 2012;61(19):339–43. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Notes from the field—ongoing transmission of HBV among ALF residents despite ongoing public health efforts, Virginia 2012. Morbid Mortal Weekly Rep. 2013;62(19):389. [PMC free article] [PubMed] [Google Scholar]
- 13.Bender TJ, Wise ME, Utah O, Xing J, Moorman AC, Sharapov UM, et al. Outbreak of hepatitis B virus infections associated with assisted monitoring of blood glucose in an assisted living facility—Virginia, 2010. PLoS One. 2012;7(12):e50012. doi: 10.1371/journal.pone.0050012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. [accessed 08.08.13];Healthcare-associated hepatitis B and C outbreaks reported to the Centers for Disease Control and Prevention (CDC) in 2008–2012. http://www.cdc.gov/hepatitis/Statistics/HealthcareOutbreakTable.htm.
- 15.Counard CA, Perz JF, Linchangco PC, Christiansen D, Ganova-Raeva L, Xia G, et al. Acute hepatitis B outbreaks related to fingerstick blood glucose monitoring in two assisted living facilities. J Am Geriatr Soc. 2010;58:306–11. doi: 10.1111/j.1532-5415.2009.02669.x. [DOI] [PubMed] [Google Scholar]
- 16.Sawyer MH, Hoerger TA, Murphy TV, Schillie SF, Hu D, Spradling PR, et al. Use of hepatitis B vaccination for adults with diabetes mellitus: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2011;60:1709–11. [PubMed] [Google Scholar]
- 17.Mast EE, Weinbaum CM, Fiore AE, Alter MJ, Bell BP, Finelli L, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep. 2006;55:1–33. quiz CE1-4. [PubMed] [Google Scholar]
- 18.Pirl WF, Greer JA, Weissgarber C, Liverant G, Safren SA. Screening for infectious diseases among patients in a state psychiatric hospital. Psychiatr Serv. 2005;56:1614–6. doi: 10.1176/appi.ps.56.12.1614. [DOI] [PubMed] [Google Scholar]
- 19.Brunette MF, Drake RE, Marsh BJ, Torrey WC, Rosenberg SD. Responding to blood-borne infections among persons with severe mental illness. Psychiatr Serv. 2003;54:860–5. doi: 10.1176/appi.ps.54.6.860. [DOI] [PubMed] [Google Scholar]
- 20.Essock SM, Dowden S, Constantine NT, Katz L, Swartz MS, Meador KG, et al. Risk factors for HIV, hepatitis B, and hepatitis C among persons with severe mental illness. Psychiatr Serv. 2003;54:836–41. doi: 10.1176/appi.ps.54.6.836. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg SD, Swanson JW, Wolford GL, Osher FC, Swartz MS, Essock SM, et al. The five-site health and risk study of blood-borne infections among persons with severe mental illness. Psychiatr Serv. 2003;54:827–35. doi: 10.1176/appi.ps.54.6.827. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg SD, Goodman LA, Osher FC, Swartz MS, Essock SM, Butterfield MI, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91:31–7. doi: 10.2105/ajph.91.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolters B, Junge U, Dziuba S, Roggendorf M. Immunogenicity of combined hepatitis A and B vaccine in elderly persons. Vaccine. 2003;21:3623–8. doi: 10.1016/s0264-410x(03)00399-2. [DOI] [PubMed] [Google Scholar]
- 24.Van der Wielen M, Van Damme P, Chlibek R, Smetana J, von Sonnenburg F. Hepatitis A/B vaccination of adults over 40 years old: comparison of three vaccine regimens and effect of influencing factors. Vaccine. 2006;24:5509–15. doi: 10.1016/j.vaccine.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Stoffel M, Lievens M, Dieussaert I, Martin I, Andre F. Immunogenicity of Twinrix in older adults: a critical analysis. Expert Rev Vaccines. 2003;2:9–14. doi: 10.1586/14760584.2.1.9. [DOI] [PubMed] [Google Scholar]
- 26.Rendi-Wagner P, Kundi M, Stemberger H, Wiedermann G, Holzmann H, Hofer M, et al. Antibody-response to three recombinant hepatitis B vaccines: comparative evaluation of multicenter travel-clinic based experience. Vaccine. 2001;19:2055–60. doi: 10.1016/s0264-410x(00)00410-2. [DOI] [PubMed] [Google Scholar]
- 27.Denis F, Mounier M, Hessel L, Michel JP, Gualde N, Dubois F, et al. Hepatitis-B vaccination in the elderly. J Infect Dis. 1984;149:1019. doi: 10.1093/infdis/149.6.1019. [DOI] [PubMed] [Google Scholar]
- 28.Averhoff F, Mahoney F, Coleman P, Schatz G, Hurwitz E, Margolis H. Immunogenicity of hepatitis B Vaccines Implications for persons at occupational risk of hepatitis B virus infection. Am J Prev Med. 1998;15:1–8. doi: 10.1016/s0749-3797(98)00003-8. [DOI] [PubMed] [Google Scholar]
- 29.Williams RE, Sena AC, Moorman AC, Moore ZS, Sharapov UM, Drobenuic J, et al. Hepatitis B vaccination of susceptible elderly residents of long term care facilities during a hepatitis B outbreak. Vaccine. 2012;30:3147–50. doi: 10.1016/j.vaccine.2012.02.078. [DOI] [PubMed] [Google Scholar]
- 30.Tohme RA, Awosika-Olumo D, Nielsen C, Khuwaja S, Scott J, Xing J, et al. Evaluation of hepatitis B vaccine immunogenicity among older adults during an outbreak response in assisted living facilities. Vaccine. 2011;29:9316–20. doi: 10.1016/j.vaccine.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van der Wielen M Department of Health and Human Services, Centers for Disease Control and Prevention, Advisory Committee on Immunization Practices (ACIP) [accessed 05.09.13];Summary Report. 2010 Jun 23–24;:89. [unpublished data]. http://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-jun10.pdf.
- 32.Jasjua S, Thompson ND, Peters PJ, Khudyakov YE, Patel MT, Linchangco P, et al. Investigation of hepatitis B virus and human immunodeficiency virus transmission among severely mentally ill residents at a long term care facility. PLoS One. 2012;7(8):e43252. doi: 10.1371/journal.pone.0043252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wise ME, Marquez P, Sharapov U, Hathaway S, Katz K, Tolan S, et al. Outbreak of acute hepatitis B virus infections associated with podiatric care at a psychiatric long-term care facility. Am J Infect Control. 2012;40(1):16–21. doi: 10.1016/j.ajic.2011.04.331. [DOI] [PubMed] [Google Scholar]