Abstract
Over the past 10 years, the Tumor Microenvironment Network (TMEN), supported by the NCI (Bethesda, MD), has promoted collaborative research with the explicit goal of fostering multi-institutional and transdisciplinary groups that are capable of addressing complex issues involving the tumor microenvironment. The main goal of the TMEN was to generate novel information about the dynamic complexity of tumor–host interactions in different organ systems with emphasis on using human tissues and supplemented by experimental models. As this initiative comes to a close, members of the TMEN took time to examine what has been accomplished by the Network and importantly to identify the challenges and opportunities ahead. This consensus document summarizes for the broader scientific community discussions that occurred at the two final meetings of the TMEN in 2015 and 2016.
Introduction
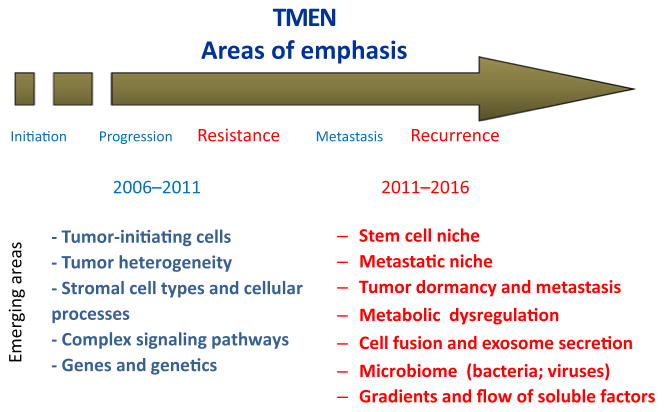
In 2006, the NCI (Bethesda, MD) launched the Tumor Microenvironment (TME) Initiative and reissued it in 2010 to fund collaborative centers across the country (see tmen.nci.nih.gov). The primary objective of the TME Initiative was to delineate mechanisms of interactions in human cancers between neoplastic cells and the various host cell types, as well as the extracellular matrix (ECM) that they recruit to form the tumor-associated stroma, the latter defined as the TME. The initiative intended to do so by generating a comprehensive understanding of stromal composition, understanding the role of stroma in normal tissues, uncovering the dynamic interactions between various subtypes of stromal cells, and discerning the role of stroma in tumor initiation, progression, and metastases as well as responses to treatment (Fig. 1). A second but equally important component of the initiative was the formation of a TME Network (TMEN), wherein each program grantee participated in collaborative efforts with other TMEN members to develop resources such as repositories of critical reagents that can be disseminated to the broader cancer research community (Table 1).
Figure 1.
TME Network 2006–2016. Areas of priority for the TMEN in the first 2006 RFA are indicated in blue; areas of priority for the 2011 RFA are indicated in red.
Table 1.
TME Network resources
| Resource | Description | Status | Contact/access |
|---|---|---|---|
| EHS (Engelbreth–Holm–Swarm) sarcoma-derived laminin-rich matrix | A pool of EHS ECM for distribution to the cancer research community who work on 3D models to study tumor–host interactions. | Available | http://tmen.nci.nih.gov/Pages/ResearchResources.aspx |
| Novel antibodies to detect cancer stem cells and stromal cells | This resource consists of validated commercially available antibody reagents for cancer stem cells and stromal cell–specific genes or proteins (biomarkers) that are critical for studying tumor–host interactions. | Available | http://tmen.nci.nih.gov/Pages/ResearchResources.aspx |
| Human xenograft tumor bank with characterized stem cell populations | A tumor bank of characterized human breast and colon solid tumors containing xenografts. Vials of frozen tumor cells from each tumor type have been stored for use. | Available | http://tmen.nci.nih.gov/Pages/ResearchResources.aspx |
| Bone marrow–derived cells from donor mice | The BMDC bank contains bone marrow cells from C57BL/6J and C57BL/6-Tg-(UBC-GFP) 30 Scha/J mouse lines in cryopreserved aliquots that can be reinfused as needed. | Available | http://tmen.nci.nih.gov/Pages/ResearchResources.aspx |
| RCAS constructs | RCAS(A)-GFP: This is an avian retroviral vector for GFP expression in TVa mice. The vector needs to be introduced in DF1 cells for virus generation. Normally, the DF1 cells themselves are introduced into mice directly. RCAS(B)-DsRed: This is an avian retroviral vector for DsRed expression in TVb mice. | Available | http://tmen.nci.nih.gov/Pages/ResearchResources.aspx |
| The Matrisome Project | Characterizes the in vivo matrisome, i.e., the ensemble of ECM and ECM-associated proteins, in normal tissues and in tumors and predicts bioinformatically the genes encoding the matrisome. Provides details of methods for analyses, an atlas of ECM proteins in diverse tissues and tumors, and incorporates an interactive database with links to external sources. | Available | http://matrisomeproject.mit.edu |
| Standard protocol for the characterization of the cellular populations in the TME in human breast cancer | The protocol uses IHC on three serial FFPE tissue sections to quantify up to 30 proteins expressed by stromal cells allowing the spatial quantification of subpopulations of stromal cells in a tumor. | Available | Lisa Coussens (lcoussens@ohsu.edu) |
| TMEM test | TMEM, consisting of direct contact between a macrophage, an endothelial cell, and a tumor cell, is associated with metastasis. TMEM score is predictive of distant metastasis in breast cancer. | Available | John Condeelis (john.condeelis@einstein.yu.edu) |
| NANIVID | The NANIVID is a multifunctional nanosystem composed of a chemoattractant source (hydrogel-EGF), capsule (cell trap), counter (transparent, interdigitated electrode arrays for sensing cell arrival), and remote reporter (readout electronics). After implanting in a tumor, the device can be retrieved and the cells harvested for subsequent assay. | Available | John Condeelis (john.condeelis@einstein.yu.edu), James Castracane (jcastracane@sunycnse.com) |
| HIS | A 75-gene panel validated in tumor cells to contain the essential molecules involved in tumor metastasis. The HIS panel has been shown to predict recurrence in breast cancer patients. | Available | John Condeelis (john.condeelis@einstein.yu.edu) |
| MenaCalc test | A quantitative method of metastasis assessment based on the MenaINV-high/Mena11a-low (MenaCalc) isoform splicing pattern. | Available | John Condeelis (john.condeelis@einstein.yu.edu) |
| TMEN-active | TMEM-active is a companion diagnostic derived from the known micropharmacology of TMEM function that can be used to assess the pharmacodynamics for drugs designed to inhibit TMEM function. TMEM-active looks at the vascular permeability at TMEM sites that is associated with tumor cell intravasation. | Available | John Condeelis (john.condeelis@einstein.yu.edu) |
| Cofilin activity test | The cofilin activity test is performed in FFPE tissues to determine the relative levels of cofilin and P-cofilin in breast tumor tissue. Elevated activity levels of the cofilin pathway can lead to tumor cell migration to blood vessels and dissemination via TMEM. | Available | John Condeelis (john.condeelis@einstein.yu.edu) |
| Standard protocol for the characterization of the cellular populations in the TME in human melanoma, pancreatic cancer, prostate cancer, and glioblastoma | Protocol for the characterization of stromal cells in the TME by flow cytometry using a specific set of well-characterized antibodies | Available, validation in progress | Melanoma-Jennifer Wargo (JWargo@mdanderson.org); prostate cancer-Kenneth Pienta (kpienta1@jhmi.edu), Raghu Kalluri (RKalluri@mdanderson.org; pancreatic cancer-Michael A. Hollingsworth (mahollin@unmc.edu); glioblastoma-Dolores Hambardzumyan (dolores.hambardzumyan@emory.edu) |
| RNA sequencing profiling of murine brain microglia and bone marrow–derived macrophages | Catalog of differentially expressed transcripts | Available | Gene Expression Omnibus (GEO) accession numbers GSE46686-GSE46690 |
| RNA sequencing profiling of murine normal resident microglia and low-grade glioma-associated microglia | Catalog of differentially expressed transcripts | Pending | Accession numbers pending, David Gutmann (gutmannd@neuro.wustl.edu) |
NOTE: The table lists and describes reagents, methodologies, and devices developed by the TME Network since its creation in 2006, which are available to the scientific community.
Abbreviations: FFPE, formalin-fixed paraffin-embedded; HIS, Human Invasion Signature; NANIVID, NANo IntraVItal Device.
At their annual meetings in 2015 and 2016, members of the NCI TMEN reviewed accomplishments of the Network and identified some of the challenges as well as future research opportunities. These are summarized in this article. This document does not claim to have identified all issues and unanswered questions and recognizes that some aspects may not have been highlighted. However, it reflects the opinion of a large number of investigators in the TMEN who have worked together over the past decade to expand our understanding of the critical role the TME plays in cancer progression.
Accomplishments
Dissemination of scientific knowledge
Over its 10 years, the scientific endeavors of NCI TMEN investigators have significantly advanced our understanding of the TME, as evidenced by more than 670 scientific publications that resulted from their collaborative work. Although it is beyond the scope of this article to comprehensively identify all of these findings, the areas of research focus and some key discoveries are highlighted here. Investigators in the first 5 years of the TMEN consortium (2006–2011) identified prominent roles for several host cell types in cancer. These include the recruitment of myeloid-derived suppressor cells, leading to enhanced invasion and metastasis (1), the recruitment of bone marrow–derived suppressor cells to the tumor site beginning at an early stage and increasing after therapy, with implications for therapeutic resistance (2), and the discovery by TMEN investigators that carcinoma-associated fibroblasts (CAF), known to promote an aggressive tumor phenotype, originate in the bone marrow and that their production and mobilization into the circulation represents a rate-limiting step in tumor growth (2). TMEN investigators also demonstrated that neurogenesis occurs in human prostate cancer and is correlated with tumor recurrence in patients (3).
TMEN investigators in the 2006–2011 consortium firmly established that tumor–stroma interactions play an important role in tumor progression. ECM molecules in the tumor stroma were shown to have the capacity to impose stem cell fate decisions (4). Importantly, TMEN investigators established tumor stroma as a predictive factor, showing that the rate of progression of human prostate cancer to biochemical recurrence is associated with reactive stroma volume (5). This was one of the first reports demonstrating that a signature not directly related to the cancer cell is useful in predicting outcome (5). Another informative signature involving direct contact of invasive carcinoma cells, macrophages, and endothelial cells, the TME of metastasis signature, was shown to be associated with the development of distant breast cancer metastasis (6). TMEN investigators also found significant age-associated changes in the prostatic microenvironment, termed senescence-associated secretory phenotype, that promote tumor growth, metastasis, and chemoresistance (7). Another important contribution of the TMEN consortium was the demonstration that the gene expression differences in stroma adjacent to prostate cancer compared with normal are not due to genetic alterations in the cancer stroma, suggesting that they are likely due to tumor-derived factors, inflammatory signals, or epigenetic changes (8).
On the basis of these findings and other developments in the TME field, the second phase of the TMEN consortium (2011–2016) research efforts focused on many emerging themes, including understanding the role of immune cells in cancer initiation, progression, and metastasis, characterization of the stem cell niche, the role of epigenetic changes in the TME, targeting stromal cells, and the emerging role of the microbiome in tumor progression. TMEN investigators made important contributions to our understanding of the role of inflammation in cancer progression and therapeutic resistance, in particular on the central regulatory function of B cells (9, 10). Also, TMEN investigators comprehensively characterized the human matrisome (the ensemble of ECM and ECM-associated proteins) from primary and metastatic cancers of colon and breast carcinoma (11). Findings indicate that both tumor cells and stromal cells contribute to the tumor matrix and that tumors of varying metastatic ability differ in both tumor- and stromal-derived components. TMEN researchers also identified receptors and ligands on disseminated/dormant tumor cells as they reside in the hematopoietic stem cell niche in the bone marrow (12) and delineated the relevant signaling pathways (13). In addition, TMEN investigators made advances in the understanding of the role of the gastrointestinal microbiome in tumor development and progression in non-intestinal tissue and the role of specific cell types of the TME in this process (14).
TMEN investigators made important contributions to the understanding of the role of the TME in therapeutic resistance. It was found that genotoxic therapies in prostate cancer patients induced production of secreted cytokines and growth factors by the stroma that effect cancer cells and promote progression (15). A seminal observation from another TMEN group showed that deletion of proinflammatory, proangiogenic, and prometastatic CAFs in genetically engineered mouse models of pancreatic ductal adenocarcinoma resulted in aggressive pancreatic lesions and early lethality, suggesting that caution needs to be exercised in using TME-targeting approaches to treat patients, and that a more in-depth understanding of tumor–stromal interactions is needed (16). Mechanisms of resistance to antiangiogenic therapies have also been identified by TMEN investigators (17). Another major area of research in the second TMEN consortium was the role of tumor exosomes. TMEN investigators showed that tumor-derived exosomes can initiate premetastatic niche formation and determine organ-specific metastasis (18). Furthermore, cancer cell exosomes were identified on the basis of the expression of the cell surface proteoglycan, glypican-1. Cancer-derived exosomes could be detected specifically in pancreatic cancer patient serum at both early and late stages (19).
Currently, the field of TME is an area with enormous excitement, as new possibilities to target the TME are emerging. Advances in this sphere of research have stimulated many cancer biologists to incorporate TME studies into their study of cancer biology and therapeutics.
Progress in the TMEN Program outlined here was the result not only of the efforts of the 20 U54 centers funded over the 10-year span (9 centers funded during 2006–2011 and 11 centers funded during 2011–2016) but also through the outreach U01 program that funded investigators not supported by TMEN to collaborate with TMEN investigators. This program extended participation to a population of investigators that brought additional expertise to the network. A total of 10 TMEN collaborative U01s were supported. In addition, a TMEN collaborative supplement program provided for 28 collaborative projects between U54 centers or between U54 centers and U01 projects. Semiannual steering committee meetings of the Network were key to the development and exchange of reagents/technologies and for scientific discussions among this highly interactive group. Over the course of the TMEN program, 20 TMEN steering committee meetings were held with 70 to 90 attendees per meeting.
Resources for the scientific community
Among the specific objectives of the TME Network was the development of resources in the form of standardized reagents and methodologies made available to the broader community of TME investigators outside of the Network. The list of these 16 resources with contact information is included in Table 1. Whereas some are readily available, others are presently under development and should be available soon. These resources were used by investigators both within and outside the TMEN community, and we anticipate a greater use as information about these resources is further disseminated.
Junior investigators network
Over the 10 years of its existence, the TMEN has supported annual meetings organized by and for senior trainees working in the laboratories of TMEN investigators. These meetings, which were attended each year by 30 to 65 junior investigators, were excellent opportunities for networking, for sharing research experiences and career development issues, and for informal meetings with TMEN senior investigators. Many of the participants at these meetings are now independent investigators who continue to focus on the TME.
Suggestions for the future
There is little question that the TMEN greatly contributed to stimulating research in the area of the TME, to encouraging young investigators to perform research in the field, and to providing to the scientific community standardized reagents and methodologies. It stimulated research efforts among the members of each center, supported collaborations between TMEN member and non-TMEN investigators via collaborative U01s, and stimulated collaborations among TMEN groups via collaborative supplements. Collaborative projects and resource development were undertaken by TMEN groups and supported by TMEN opportunity funds. Despite its successes, some areas could have been strengthened. Although joint meetings were held with the NCI’s Integrative Cancer Biology Program, the TMEN would have benefited from including additional investigators with a non-TME background or even a noncancer background, such as engineers, immunologists, geneticists, or population scientists. Thus, future efforts should be developed in a way that strongly encourages such individuals to participate and contribute to the convergence of minds and disciplines in the TME field.
Challenges and Opportunities: What Needs to be Better Understood and What to do About It
A better understanding of the TME of metastasis
The emphasis of studies on the TME over the last 10 years has been on better understanding the TME of the primary tumor and how it promotes metastasis. Conversely, with the exception of the bone marrow, relatively little emphasis has been placed on studying the TME of metastatic lesions, as these lesions are rarely the subject of biopsies and tumor resections. This has left a number of fundamental questions about metastases unaddressed. For example, what is the relationship of the target organ TME and solitary cancer cells and how does the TME change as these cells grow into micro- and macrometastasis? Are changes in the TME during micro- and macrometastatic stages different or the same as those seen in the primary tumor, and do these changes occur before and/or after tumor cells extravasate into the parenchyma of distant organs? Are these changes driven by genetic or epigenetic events? Does therapy promote some of these changes and influence the formation of distant metastases? How different are the cancer cells in a distant metastasis from the bulk of cells in a primary tumor?
Current research also places much emphasis on organ-specific metastasis studies at the macro level, and this is certainly a problem that has intrigued oncologists for over a century. However, the reality is that many patients suffer from, or are at risk of, concomitant metastasis to multiple organs. This raises the question whether differences measured in the TME composition of a primary tumor and a metastatic lesion in a specific organ also apply to a diverse array of metastasis in various organs. More knowledge of common mediators of metastatic colonization and regrowth after therapy would provide clues for the improved elimination of residual disease in sites distant from excised primary tumors.
Among the various organ sites in which metastases form, the brain represents a particular challenge. Progress in the area of brain metastasis has been slow. One of the reasons is that there is a paucity of experimental models to better delineate the molecular mechanisms of brain metastasis formation and its peculiar intrinsic resistance to therapy. A better understanding of its underlying mechanisms is urgently needed. Specific unanswered questions in this area include the basis for the notorious drug resistance of metastatic cells in the brain microenvironment, the need to understand intratumoral heterogeneity and the relative contributions of individual clones during migration, invasion, and metastatic colonization, and whether the brain and the blood–brain barrier represent a unique TME for tumor cells.
A clinical dilemma that is poorly understood is the mechanism of differential host response in various target organs to the presence of metastatic colonies. For example, in humans, hepatic metastasis (even with heavy tumor burden) can still result in normal liver function, while small tumor burden in pulmonary metastasis can have dire consequences in terms of aberrant respiratory function. There is thus a need to define the normal ecosystem to better understand tumor-induced changes in the adjacent host tissues.
To be able to answer some of these questions will require elimination of several barriers that in the past have prevented easy access to metastatic lesions of good quality in patients. This is not a simple issue to resolve, as metastatic sites are seldom biopsied in terminally ill patients. There are thus ethical as well as financial constraints. With the recruitment of more patients into molecularly driven clinical protocols, there will be more attempts to obtain pre- and posttherapy tumor samples.
Suggestions
All cancer research institutions and cancer centers should be encouraged to establish rapid autopsy programs for patients in their clinical protocols and to develop the needed infrastructure (standardized informed consent, assistance from pathologists, and tissue banking). Such programs have been successfully developed at many institutions (e.g., for breast, prostate, pancreatic cancers; ref.20). Extending them would have the potential to enhance the pace of new discoveries as well as to generate novel tools and reagents.
In patients with recurrent and metastatic disease, not only the primary recurrent tumor should be biopsied to obtain the needed molecular information but also metastatic lesions. Whereas this is relatively simple in cases of skin or bone marrow metastasis, it is more challenging in the case of bone or brain metastasis, for example, and clinical care as well as ethical factors needs to be taken into consideration. Minimally invasive techniques play an important role, and in this regard, the NANIVID device developed by TMEN investigators at Albert Einstein College of Medicine (21) and the human invasion signature (HIS) developed by the same group could provide a minimally invasive method to collect tumor cells and accompanying stromal cells from metastatic lesions.
Liquid biopsies, based on circulating tumor cells, should be studied not only for genetic and epigenetic alterations but also for their ability to interact with stromal cells in in vitro 2D and 3D models.
Future research should pursue the identification of target organ TME commonalities and differences to pinpoint and target the mediators of metastasis to multiple organ microenvironments and tumor types. For these cases, identifying common mediators of metastatic colonization as therapeutic targets would be of value. For example, current checkpoint immunotherapy and its encouraging clinical success are based on the premise that immune evasion is a shared feature of metastatic disease irrespective of organ site.
A better understanding of how therapy affects the TME
Much is known about the adhesion-dependent and adhesion-independent mechanisms by which the TME promotes resistance to therapy. However, there is a paucity of information on the effect of therapies on the TME, including its cellular composition and biological functions. Do therapies polarize the TME in a direction that is more favorable to the establishment of dormant and/or resistant tumor cells? What is the effect of radiation or chemotherapy on the composition of the TME in primary tumors and metastatic lesions or disseminated disease in patients? Considering the toxicity of most chemotherapeutic agents to the bone marrow, the effect of these agents on the TME of the bone marrow has been poorly understood. Do such injuries promote or inhibit the recruitment of bone marrow precursor cells to the site of a primary tumor or to premetastatic sites? Are these recruited cells pro- or antitumorigenic?
Suggestions
As patients with recurrent disease will increasingly be treated with molecularly informed protocols, the material from these biopsies should not only be used to study the presence of genetic or epigenetic alterations that could be targetable but also to examine the composition of the TME through a combination of transcriptomics and cell composition analysis using standardized protocols. In this regard, the standardized panel of antibodies developed by TME investigators has been one mechanism to provide such protocols to the scientific community (Table 1).
In the development of clinical trials for recurrent/refractory cancers, TME scientists and clinical investigators should join forces in the design of protocols using combinations of agents targeting the TME and the tumor cell.
Encourage longitudinal studies: The performance of longitudinal tumor biopsies is often not feasible in patients unless the tumor is easily accessible to multiple minimally invasive biopsies, as is the case in metastatic melanoma. Such efforts have been led by TMEN investigators at MD Anderson Cancer Center (Houston, TX; ref.22). Liquid biopsies (biomarkers present in the peripheral blood that reflect tumor behavior) could provide an alternate approach if these biopsies reflect changes related to the TME, such as changes in the production of chemokines and cytokines or activation of pathways operating within tumor cells that are the results of changes in the TME.
A better understanding of the heterogeneity of the stromal and inflammatory cells in the TME
The TME is heterogeneous at multiple levels, in its composition and its function as well as dynamically and spatially. The tumor stroma is complex in the diversity of its cellular components: fibroblasts, endothelial cells, neurons, adipocytes, adaptive, and innate immune cells, as well as its noncellular components, including the ECM and soluble products like chemokines, cytokines, growth factors, and extracellular vesicles. It is also complex in its physical properties, such as metabolic content, and oxygen and pH gradients. Although research has progressed in understanding the role of individual stromal cells in the primary tumor, we have yet to derive from this understanding paradigms that can predict whether the primary tumor will be less or more aggressive. Also, there is limited understanding of whether the composition of the TME within a given tumor sample differs substantially between one and another area of the tumor. The complexity of the TME also extends to its function. For example, tumor-associated fibroblasts and tumor-associated macrophages can be both pro-and antitumorigenic. Finally, the interaction between tumor cells and the TME is dynamic and reciprocal and needs to be explored within the context of the systemic biology of host and tumor over time.
This heterogeneous nature of the TME has an impact on tumor progression, as both tumor cells and stromal cells coevolve from local disease to become migratory, invasive, and angiogenic. Some stromal cells like myeloid cells and endothelial precursor cells can also disseminate to metastatic organs. This aspect raises several questions such as: Do different subtypes of a given type of carcinoma (e.g., breast cancer) recruit distinct or different subtypes of stromal cells to their respective stromata? How do carcinomas recruit stromal cells from local and distant sources? How does heterogeneity differ in different sites, for example, in the circulation or in the lymph nodes? How “tissue specific” is the inflammatory phenotype identified in various types of tumors? Is it “hardwired” during tissue development or a systemic response to stress, or alternatively, driven by evolution of the tumor cells? How much heterogeneity is there in immune checkpoints across tumor types and stages? Are there tumor-permissive and -suppressive subdomains within the tumor and are they the same in micro-and/or macrometastasis? Do these subdomains exist to the same extent in target organs when only solitary disseminated tumor cells (DTC) are present and patients still bear primary tumors?
Suggestions
We need to integrate all of the different systems of analysis (genomics, transcriptomics, proteomics, epigenomics, and cytometry) within a comprehensive set of data that should provide a classification of the TME that reflects its complexity and at the same time provides clear markers that can be used by the preclinical and clinical research communities. Such effort based on FACS analysis of myeloid cells in dissociated tumor samples was pioneered by TMEN investigators (23), and new solutions will be provided by data analysis software, such as the CIBERSORT platform that characterizes cell composition of complex tissues on the basis of a comprehensive analysis of their gene expression profiles (24). IHC protocols that on three serial tissue sections allow the identification of 30 protein markers have been developed by TMEN investigators at Oregon Health Sciences University (Portland, OR). Mass spectrometry–based cytometry, such as cytometry by time of flight (CyTOF), could provide another answer to the problem. The standardization of these protocols will be critical to ensure a common language is used to identify the variety of stromal cells in the TME.
We need to continue to encourage research on exosomes in cancer, as this field is still in its infancy as the characterization and isolation of exosomes from other extracellular vesicles remains a challenge. Tumor-derived exosomes however have the attractive potential to reflect in one isolate, the heterogeneous nature of the cancer they derive from.
We need to share reagents and methods. Although an important long-term objective, the establishment of markers that are commonly accepted by all laboratories will remain a challenge, as many markers initially found to be specific to one subtype of cells or one phenotype of the same cells are less specific than initially envisioned. There is presently no consensus as to what is the best method to approach the important issue of heterogeneity within the TME. Methods based on IHC, flow cytometry, CyTOF, and transcriptomics have provided helpful information. However, it has not always been easy to compare and integrate information obtained from the various techniques used. Other tools and technologies needed include new tools with small analytic input that allow broad conclusions, and real-time imaging in vivo techniques that can track cell–cell interactions and allow determination of the dynamic spatial characterization of tumors.
A better understanding of the TME of minimal residual disease and dormancy
The systemic nature of metastatic disease, the heterogeneity of metastatic tumors, the multitude of genes and pathways involved in different organs, and the many mechanisms of drug resistance generate a sobering picture of the highly complex problem and the future prospects of addressing overt metastatic disease. Prevention of relapse ostensibly is the goal of systemic therapy delivered after the removal of a primary tumor but in the continued presumed presence of dormant micrometastatic disease in distant organ sites. However, most agents used in the adjuvant therapy setting target growing cancer cells, not dormant cells that appear to form much of the residual disease during periods of metastatic latency. TMEN investigators were among the first to address tumor dormancy after diagnosis and before therapy, to establish preclinical models and to demonstrate the homing of DTCs to the bone marrow niche and to delineate the mechanisms of dormant versus invasive cells (13). There is a critical need to further characterize circulating tumor cells (CTC) in blood samples and DTC in bone marrow samples, changes in the TME during single-cell dormancy, and early changes in the TME prior to the development of clinical metastases. A better understanding of the basis for metastatic colonization, in particular of its latent phase, is therefore needed to develop better treatments.
To these ends, we would suggest directing attention to the following questions: What gives cancer cells the ability to enter a dormant state for up to several years while retaining tumor-initiating capacity? Do metastatic cells utilize the same or different niches for their initial survival, for dormancy and for aggressive outgrowth in host organs? What is the role of immunity in the latent state? Are latent metastatic cells slow-cycling cancer stem cells? What are the signals that allow cancer cells to exit dormancy and reactivate their proliferative programs? Are organs that serve as sanctuary sites for dormant metastatic cells the same organs as those in which overt metastases eventually emerge? Would therapeutic targeting of the mechanisms that specifically support the survival of dormant metastatic cells prove an efficient strategy to prevent metastasis? What factors modulate residual disease (e.g., age of host, cancer subtype, as well as effects of therapies)? How do different target organs shape DTC evolution and affect dormancy and reactivation? What is the role of adult stem cell niches in driving dormancy and reactivation?
Suggestions
We need to further study DTCs and CTCs: The challenge here is to isolate sufficient numbers of these cells to be able to study them and the availability of models that closely mimic the interaction of these cells with their TME. In this respect, recently, there have been several methods to successfully isolate DTCs and CTCs (liquid biopsies) combined with in vitro models that mimic the TME of the bone marrow niche, and it will be possible to address some of the critical questions listed above (25). It will be important to correlate DTC and CTC phenotype (dormant vs. activated) and gene expression profiles in DTC and CTC with cues present in the microenvironment of specific stromal cells and their state of activation, that is, can the TME provide a surrogate marker for monitoring DTC and CTC phenotype?
A better understanding of how the macroenvironment influences the TME
The contribution of the host systemic environment to cancer initiation and progression is well known, but its effect on the TME is less well characterized. How much does the interindividual variability influence the TME during cancer development? Thus, animal studies have shown that different mouse strains exhibit different susceptibilities to cancer development and that strains with enhanced susceptibility to carcinogens have a higher Th2 native environment. With the advent of CRISPR technologies, it will now be feasible to determine the effects that specific genetic loci have on the TME.
The effect of aging on the TME is also not well understood. It is well known that aging affects our immune system and thus the ability of the TME to sustain an antitumorigenic reaction against malignant tumor cells, but beyond this area, little is known. Many immune cell phenotypes differ in young and old individuals, for example, T- and B-cell infiltration, IgG levels, and macrophages. Thus, age should be considered when building models and/or designing experiments and clinical trials, and there is a paucity of models using older mice. Late relapse can span 10 to 20 years, which suggests that metastatic relapse may also be influenced by an aging population and mechanisms linked to aging. Are dormancy-inducing mechanisms lost or attenuated in aging niches? The sympathetic nervous system could also contribute to the TME and affect its influence on cancer initiation and progression, but its role has been left almost entirely unexplored.
Another important question related to the macroenvironment is whether we will be able to define a macroenvironment that leads to a premalignant TME. Studies on obesity and cancer suggest that obesity, by increasing the number and mass of white adipose tissue or the levels of circulating insulin-like growth factor, could lead to a TME that induces therapeutic resistance. For example, does liver steatosis promote the establishment of liver cancer or the presence of liver metastasis?
Suggestions
These broad questions will be better addressed by a team of investigators with scientific background and interests in endocrinology, epidemiology and population-based science, aging, immunology, nutrition, and even global environment. The formation of interdisciplinary teams of scientists, appropriately supported, should be encouraged.
We need better tools and models to study the TME
Better analytic and experimental tools to study the TME are needed, at least in the three major areas: for analysis of the target organs and metastasis as well as primary tumor TME components, for imaging tumor cell–stromal cell interactions, and for the development of truly appropriate genetically defined mouse models.
One of the major limitations of many current modeling studies is the reliance on cell sorting to isolate populations of stromal cells for sequencing. A potential investment in mouse lines engineered with bacterial artificial chromosome and translating ribosome affinity purification (26) or other similar approaches into specific stromal cell type loci (endothelial cells, macrophages, etc.) might allow for simultaneous and rapid isolation of these populations without long processing times. The use of CyTOF on tumor tissues is another promising approach that allows identification of specific proteins at the cellular and subcellular level through antibodies tagged with rare earth metals. Single-cell methods for genomic, epigenomic, and transcriptomic analyses are a rapidly growing area of investigation that will lead to a better understanding of the heterogeneity in the TME.
Imaging tools to monitor the TME spatially and temporally are also needed for multimodal and multiscale imaging (e.g., microscopy, MRI, PET). Such tools can use smaller analytic inputs but broad outputs (to address heterogeneity issues), for example, availability of appropriate probes, such as measurements of metabolic signatures. Tumor cells and stromal cells have different metabolic signatures, and tools to image live animals to superimpose data on tumor imaging and physical parameters are needed. There is also a critical need for imaging micrometastases, especially the ability to improve live imaging for TME that are efficient, cost effective, and can analyze big data more efficiently. Here, also single-cell methods will be powerful tools.
The issue of relevant preclinical models to study the TME is a critical one not only in preclinical therapeutic studies but also in biological studies. Although the use of immunodeficient mice grafted with patient-derived tumors [patient-derived xenograft (PDX) models] has gained increased acceptance in preclinical therapeutic studies in cancer, the need for immunodeficient mice in these models is a major limitation when they are employed to study the TME. Humanized mouse models may be more appropriate, but they remain expensive and do not necessarily recruit the types of stromal cells needed to model the stroma in human patient tumors. For example, mesenchymal stromal cells do not engraft in mice transplanted with human cord blood cells or human bone marrow cells. The use of murine cancer stem cells might be instructive for elucidating the stromal determinants required for engraftment, with future applications to human PDX modeling.
Preclinical models that mimic human cancer with cells that have driver and passenger mutations may also be important in understanding immune responses to neoantigens derived from passenger mutations. Similarly, there is an urgent need to develop models of stochastic tumor initiation yielding tissues in which not all cells are transformed, in contrast to many current, commonly employed models that are highly penetrant, making the study of metastasis extremely difficult as primary tumor burden is rate limiting. There is also a paucity of models of tumor dormancy that mimic clinical stable disease and are amenable to addressing issues of therapeutic resistance and the interactions between the DTC and the local TME where the dormant cells reside. Models based on tissue engineering (both in vitro and in vivo) could be helpful in studying aberrant wound healing in tumor cells, architectural differences between benign (indolent) versus malignant tumor cells, and tissue regeneration.
Suggestions
In this area also, a multidisciplinary effort is needed, and engineers, imaging scientists, and clinical investigators will provide important input in joining forces with TME investigators.
Conclusion: A More Integrated Scientific Community to Study the TME
Our knowledge of the TME has grown significantly over the past 10 years, and the networking of investigators in this field has substantially contributed to the expansion of such knowledge. As we better appreciate the tremendous heterogeneity in the TME and better understand the role of the immune system, it is clear that the study of the TME is at the convergence of two fundamental disciplines, one traditionally focused on the study of the tumor vasculature, the ECM, and the pathophysiology of metastasis, and one focused on the role of the immune system and on immunotherapy. Traditionally, these two disciplines have been distinct, with separate organizations, meetings, and journals, and with few opportunities to closely interact with one another. It is also clear that to meet the challenges ahead, the field needs to go beyond the two disciplines mentioned above and reach out to other scientific disciplines, such as engineering, imaging, nutrition and metabolism, and even environmental science. Further progress in the field will require the convergence of many different minds that will learn to share a common language, apply complementary approaches and methodologies to address common themes, and establish a new synthetic conceptual framework for studying the TME.
Acknowledgments
The authors thank all the investigators of the TMEN who contributed to the discussions held during the TMEN meetings that were used for the writing of the white paper. The authors also thank in particular Drs. Robert Weinberg and Richard Hynes for critically reviewing the document and providing excellent input.
Footnotes
Disclosure of Potential Conflicts of Interest
S. Mohla is a consultant/advisory board member. No potential conflicts of interest were disclosed by the other authors.
Authors’ Contributions
Conception and design: Y.A. DeClerck, K.J. Pienta, D.S. Singer
Writing, review, and/or revision of the manuscript: Y.A. DeClerck, K.J. Pienta, E.C. Woodhouse, D.S. Singer, S. Mohla
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): S. Mohla
Study supervision: Y.A. DeClerck, S. Mohla
References
- 1.Bierie B, Moses HL. Transforming growth factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth Factor Rev. 2010;21:49–59. doi: 10.1016/j.cytogfr.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–72. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayala GE, Dai H, Powell M, Li R, Ding Y, Wheeler TM, et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res. 2008;14:7593–603. doi: 10.1158/1078-0432.CCR-08-1164. [DOI] [PubMed] [Google Scholar]
- 4.LaBarge MA, Nelson CM, Villadsen R, Fridriksdottir A, Ruth JR, Stampfer MR, et al. Human mammary progenitor cell fate decisions are products of interactions with combinatorial microenvironments. Integr Biol. 2009;1:70–9. doi: 10.1039/b816472j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yanagisawa N, Li R, Rowley D, Liu H, Kadmon D, Miles BJ, et al. Stromogenic prostatic carcinoma pattern (carcinomas with reactive stromal grade 3) in needle biopsies predicts biochemical recurrence-free survival in patients after radical prostatectomy. Hum Pathol. 2007;38:1611–20. doi: 10.1016/j.humpath.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Rohan TE, Xue X, Lin HM, D’Alfonso TM, Ginter PS, Oktay MH, et al. Tumor microenvironment of metastasis and risk of distant metastasis of breast cancer. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju136. pii:dju136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean JP, Nelson PS. Profiling influences of senescent and aged fibroblasts on prostate carcinogenesis. Br J Cancer. 2008;98:245–9. doi: 10.1038/sj.bjc.6604087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianchi-Frias D, Basom R, Delrow JJ, Coleman IM, Dakhova O, Qu X, et al. Cells comprising the prostate cancer microenvironment lack recurrent clonal somatic genomic aberrations. Mol Cancer Res. 2016;14:374–84. doi: 10.1158/1541-7786.MCR-15-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, et al. Bruton tyrosine kinase-dependent immune cell cross-talk drives pancreas cancer. Cancer Discov. 2016;6:270–85. doi: 10.1158/2159-8290.CD-15-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25:809–21. doi: 10.1016/j.ccr.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. The matrisome: insilico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteomics. 2012;11:M111.014647. doi: 10.1074/mcp.M111.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121:1298–312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14:611–22. doi: 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lakritz JR, Poutahidis T, Mirabal S, Varian BJ, Levkovich T, Ibrahim YM, et al. Gut bacteria require neutrophils to promote mammary tumorigenesis. Oncotarget. 2015;6:9387–96. doi: 10.18632/oncotarget.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med. 2012;18:1359–68. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–34. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivera LB, Bergers G. Angiogenesis. Targeting vascular sprouts Science. 2014;344:1449–50. doi: 10.1126/science.1257071. [DOI] [PubMed] [Google Scholar]
- 18.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–35. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–82. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubin MA, Putzi M, Mucci N, Smith DC, Wojno K, Korenchuk S, et al. Rapid (“warm”) autopsy study for procurement of metastatic prostate cancer. Clin Cancer Res. 2000;6:1038–45. [PubMed] [Google Scholar]
- 21.Raja WK, Padgen MR, Williams JK, Gertler FB, Wyckoff JB, Condeelis JS, et al. Development path and current status of the NANIVID: a new device for cancer cell studies. J Micro Nanolithogr MEMS MOEMS. 2012;11:pii:013013. doi: 10.1117/1.JMM.11.1.013013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen PL, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov. 2016;6:827–37. doi: 10.1158/2159-8290.CD-15-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A. 2012;109:2796–801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–7. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Toom EE, Verdone JE, Gorin MA, Pienta KJ. Technical challenges in the isolation and analysis of circulating tumor cells. Oncotarget. 2016 doi: 10.18632/oncotarget.11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–48. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]