Abstract
Goal 1 of the National Plan to Address Alzheimer’s Disease is to prevent and effectively treat Alzheimer disease and Alzheimer disease–related dementias by 2025. To help inform the research agenda toward achieving this goal, the NIH hosts periodic summits that set and refine relevant research priorities for the subsequent 5 to 10 years. This proceedings article summarizes the 2016 Alzheimer's Disease–Related Dementias Summit, including discussion of scientific progress, challenges, and opportunities in major areas of dementia research, including mixed-etiology dementias, Lewy body dementia, frontotemporal degeneration, vascular contributions to cognitive impairment and dementia, dementia disparities, and dementia nomenclature.
Dementia is a major public health problem with substantial personal, social, and financial burden, affecting more than 47 million people worldwide.1 Alzheimer disease (AD) contributes to about two-thirds of dementia cases and affects more than 5 million people in the United States alone. Although AD is the most prevalent dementia diagnosis, the majority of dementia cases among the elderly show histologic changes in addition to the classic AD pathology of β-amyloid (plaques) and tau-containing aggregates (neurofibrillary tangles).2–7 These additional non-AD pathologic changes, typically vascular, Lewy bodies, or TAR DNA-binding protein (TDP)–43 pathology, occur in individuals with clinical AD, as well as in other types of dementias, and conversely, classic AD pathology is frequently present when the dementia diagnosis is not AD, as well as in older persons without dementia.8–15 Because of such close clinical and pathologic relationships with AD, frontotemporal, Lewy body, vascular, and mixed dementias are considered AD-related dementias (ADRD) and are included in the National Plan to Address Alzheimer’s Disease. ADRD contribute to millions of dementia cases2,7,9,11,16 in the United States. Combined, the toll of AD and ADRD on individuals, caregivers, and society is enormous and will continue to increase as the United States population ages.17–22 An organized, comprehensive, and multisector approach is necessary to coordinate and more effectively use national resources to mitigate physical, emotional, and economic burden of these devastating diseases. Prioritized recommendations from the ADRD Summit 2016 are now formalized, with success criteria, in the National Plan to Address Alzheimer's Disease as milestones23 that will both drive critical new research and track progress toward the goal of preventing and effectively treating AD and ADRD by 2025.
A COORDINATED APPROACH TO ADVANCING AD/ADRD RESEARCH
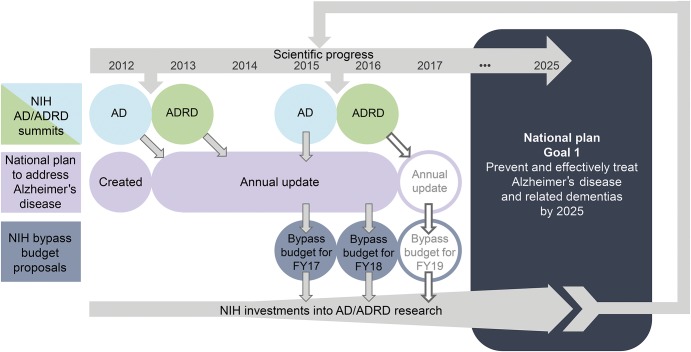
In 2011, the National Alzheimer's Project Act was signed into law, requiring the Secretary of the US Department of Health and Human Services to create and coordinate an integrated National Plan to address AD.24 First released in 2012, this Plan is updated annually and includes an ambitious research goal (Goal 1) of preventing and effectively treating AD/ADRD by 2025. To inform the national AD/ADRD research agenda toward this goal, the National Institute on Aging and the National Institute of Neurological Disorders and Stroke (NINDS) at the NIH host periodic AD and ADRD summits, inviting national stakeholders and international partners to identify and refine AD/ADRD research priorities for a 5- to 10-year time frame. Following approval by the NINDS Council, ADRD research recommendations are included in the National Plan as research milestones23 that shape development of the annual NIH AD/ADRD bypass budget proposals and guide progress toward the goal of preventing and effectively treating AD/ADRD by 2025 (figure 1).25,26
Figure 1. Role of Alzheimer disease (AD) and Alzheimer disease–related dementias (ADRD) summits in the National Plan to Address Alzheimer's Disease.
National Plan to Address Alzheimer's Disease hosted AD and ADRD summits review progress in AD/ADRD research and generate or refine recommendations based on recent scientific discoveries and input from a wide range of stakeholders, including the public. These recommendations inform research milestones that are included in the annually updated national plan. The milestones not only help guide and track progress, but also inform the annual bypass budget proposals developed each year by the NIH and delivered to congress that estimate additional funding needed to reach Goal 1 of the National Plan.
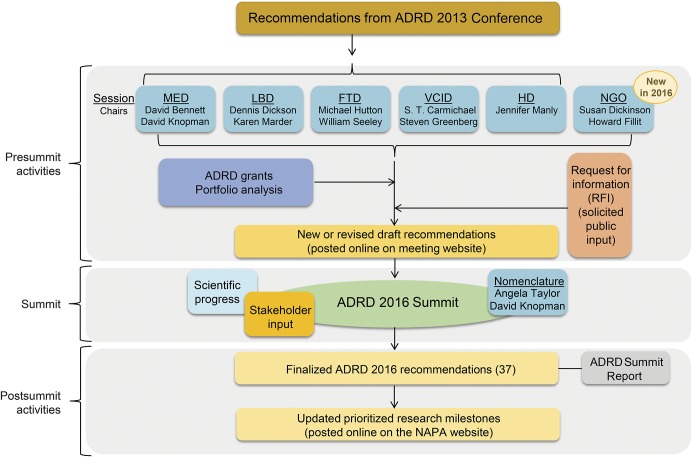
In 2013, the initial detailed ADRD-specific research priorities were established by the ADRD Conference 2013.27 Three years later, NIH hosted the second ADRD Summit on March 29–30, 2016, to visit progress and refine recommendations as needed (figure 2). The 2016 Summit also included a session led by nongovernmental organizations (NGOs) to broaden stakeholder input and increase public–private partnership. The remainder of these proceedings will focus on the ADRD Summit 2016 by presenting the updated research recommendations that reflect the most important opportunities in ADRD research based on scientific progress, broad stakeholder input, and research gaps, and will highlight ADRD-scientific advances since the 2013 conference.
Figure 2. Organization structure of the Alzheimer's Disease–Related Dementias (ADRD) Summit 2016 broken down into the pre-Summit activities, the Summit, and post-Summit activities.
FTD = frontotemporal dementia; LBD = Lewy body dementia; MED = multiple etiology dementia; NGO = nongovernmental organizations; VCID = vascular contributions to cognitive impairment and dementia.
MIXED-ETIOLOGY DEMENTIAS: THE COMPLEXITIES OF DIAGNOSING DEMENTIA WITH MULTIPLE ETIOLOGIES IN THE 21ST CENTURY
The first step in diagnosis of dementia disorders is detection of cognitive impairment. Although cognitive assessment is included in the annual wellness visit covered by Medicare,28 identifying cognitive impairment, including dementia, continues to be a substantial challenge in the current health care system even when a person or his or her relative or caregiver voices a concern to a health care provider. Important barriers are financial (e.g., reimbursement for neurologic and neuropsychological evaluation),29 technical (e.g., lack of simple, accurate, rapid, culturally appropriate, and standardized detection paradigms),30 and social (e.g., stigma for patients and families; reluctance of medical professions to whom the value of detection is unclear).31 As a result, cognitive impairment, including dementia, often goes undetected, and even when it is detected, follow-up resulting in a diagnosis occurs only about half of the time.32,33 When cognitive complaints and other warning signs are evident but there is no diagnosis it can delay or prevent treatment of reversible conditions, use of appropriate medical and support services, and care planning in a critical time window. Nowhere are these barriers more evident than in primary care, the main locus of care for older people in the United States. This need is addressed by mixed-etiology dementias (MEDs) Recommendation 1 (table 1).
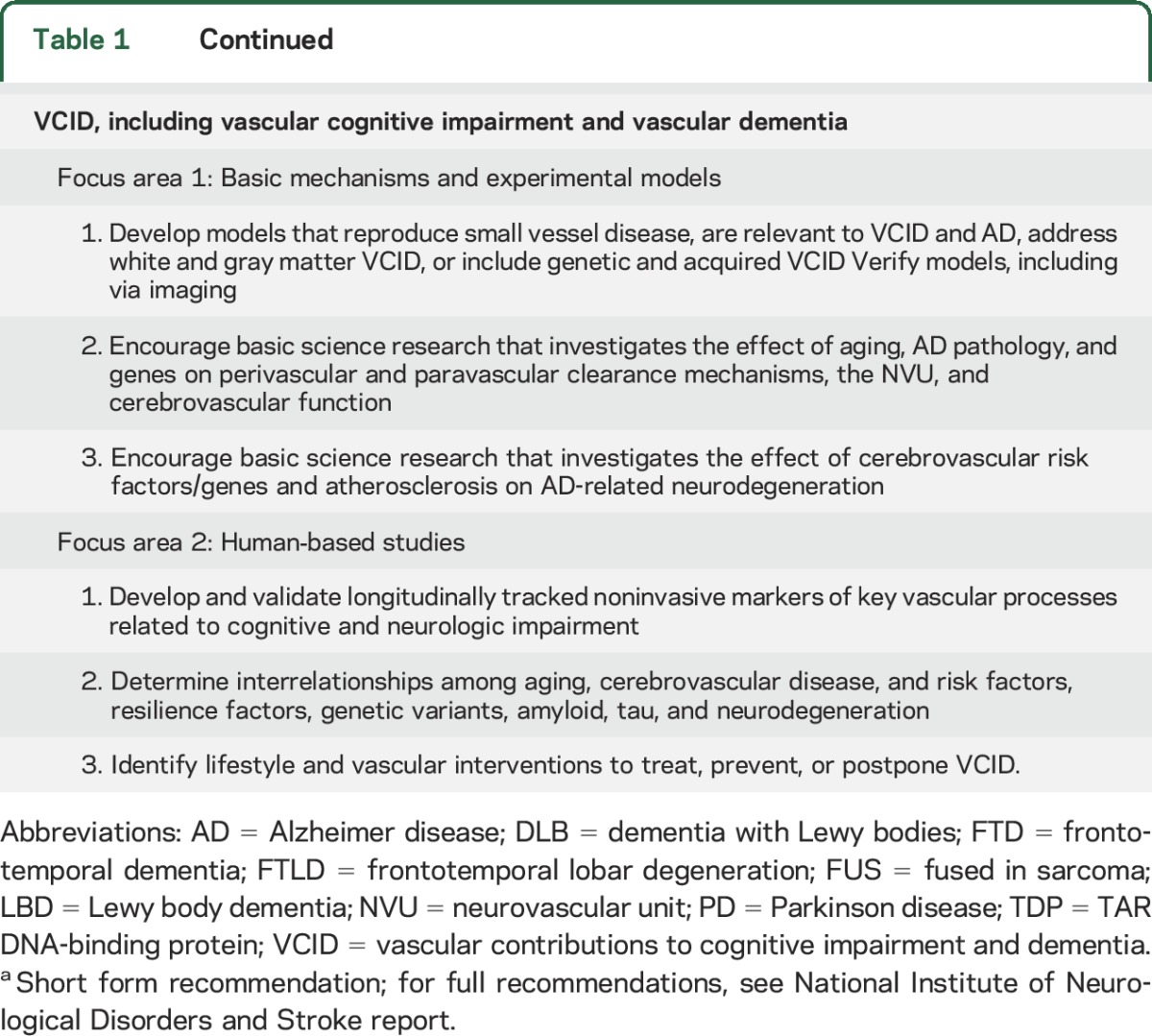
Table 1.
Research recommendations for Alzheimer disease–related dementias (ADRD) named in the national plana

After incident cognitive impairment is detected, differential diagnosis among dementia syndromes and differentiation of these from medical conditions that may be reversible is a high priority (table 1; MED Recommendation 2). Even though accurate diagnosis is possible to a significant (although imperfect) extent, only a subset of highly specialized clinicians has the requisite tools and training. Many in the United States do not have access to such specialists. Some patients would also benefit from improved diagnostic means to identify reversible causes of cognitive impairment (including, but not limited to, medication side effects, sleep disorders, normal-pressure hydrocephalus, substance abuse, anxiety, and depression). Relatively accurate diagnostic criteria for Lewy body dementia (LBD) are available, but underused, resulting in initial misdiagnosis, potentially harmful treatment with bradykinesia-causing antipsychotics, and delaying benefit from effective pharmacologic management.34 Revised diagnostic criteria are also available for the behavioral variant of frontotemporal dementia (FTD), which may also benefit from specific treatments.30
Accurate clinical diagnosis of the specific type of dementia is challenging because multiple pathologies can give rise to similar clinical syndromes.9,15 Further complicating matters, multiple pathologies frequently occur in an individual with a single dementia diagnosis.9,15 Classic AD pathology (plaques and tangles) in the elderly, for example, is often accompanied by additional disease processes that may contribute independently to cognitive decline and dementia. Contributing further to diagnostic complexity is a lack of tools to gauge the degree to which different underlying brain pathologies (e.g., AD pathology, cerebrovascular disease, Lewy bodies, TDP-43-opathy) contribute to observed cognitive decline and dementia.15 The current AD trials targeting amyloid attempt to include patients with pure Alzheimer pathology. Should these trials prove successful, attention will shift to understand how, or if, the treatment benefit generalizes to the elderly population with a mix of etiologies.
Two clinical-pathologic studies of aging and AD provide some insight into the relationship of common pathologies to dementias and offer implications for study and clinical management of MEDs. The Religious Orders Study, begun in 1993, follows 1,350 older nuns, priests, and religious brothers, without known dementia at enrollment, from across the United States. The Memory and Aging Project, begun in 1997, involves 1,850 lay people from northeastern Illinois. Participants in each study have consented to annual clinical evaluation and brain donation. These studies indicate that the effects of cerebrovascular disease, Lewy body pathology, TDP-43, and hippocampal sclerosis on cognition are independent of AD pathology. Moreover, clinically diagnosed probable AD and mild cognitive impairment are pathologically mixed and heterogeneous disorders that typically exhibit other pathologies in addition to β-amyloid and tau pathology.35,36 Recognition that the common clinical diagnosis of AD is actually heterogeneous in its pathologic etiology points to the need for biomarkers that reflect the underlying biology.
Learning from a series of unsuccessful clinical trials for AD, researchers are now using genetic and imaging-based biomarkers to define, recruit, and stratify participants eligible for clinical trials, and have shifted their focus to primary prevention or very early stages of dementia. Examples include the A4 trial37 using amyloid imaging as a biomarker as well as the Alzheimer's Prevention Initiative38 and the Dominantly Inherited Alzheimer's Network39 using genetic markers. Increased and earlier detection of impending cognitive impairment, including in primary care, followed by more accurate differential diagnosis that leverages genetic, imaging, and fluid-based biomarkers will be essential to successfully treat the different disease processes within and across individuals with neurodegenerative disorders. This emerging consensus is reflected both in the ADRD Summit 2016 recommendations reported here (table 1; see LBD Recommendation 4, FTD Clinical Science Recommendation 4, VCID Human-Based Studies Recommendation 1; table 2; see HD Recommendation 1) and in reviews regarding unsuccessful clinical trials in dementia.40,41
Table 2.
Health disparities recommendations for Alzheimer disease (AD)/Alzheimer disease–related dementias (ADRD)a

LBD: CLINICAL TRIALS REMAIN TOP RESEARCH PRIORITY
LBD, including both dementia with Lewy bodies (DLB) and Parkinson disease dementia (PDD), features include dementia with some combination of visual hallucinations, fluctuation in level of alertness or consciousness, parkinsonism, and sleep abnormalities such as REM sleep behavior disorder.34 Cognitive dysfunction and signs of dementia occur either before or close to the onset of first motor symptoms. Diagnostic criteria for DLB were recently updated and continue to emphasize these features.42 In contrast, in PDD, disabling cognitive dysfunction and dementia appear at least a year after the onset of typical Parkinson disease indications including motor symptoms.37,38 Pathologically, these disorders are characterized by aggregates of α-synuclein that are indicative of neurodegeneration in specific but widespread brain regions with affected dopaminergic neurons and their terminals. Individuals with LBD commonly have a MED that also includes components of both Alzheimer and vascular pathology.43
Recent research has led to a better understanding of the role of genetics, brain network changes, and cell biology in LBD.44,45 Cell to cell trans-synaptic spread of aggregates of α-synuclein potentially explains the widespread Lewy body pathology in PDD and DLB.46,47 Substantial progress is also reflected by publication of a large-scale genetic association study with evidence that genetic risk for LBD shares similar degrees of overlap with genetic risk factors for both AD and PD. This includes a strong association of both AD and LBD at the APOE locus.44,48 Additional large whole-genome association, whole-exome sequencing, and targeted resequencing studies are ongoing.
Effective clinical trials based on a strong foundation of research remains the top priority in LBD research (table 1; LBD Recommendation 1), as it was in 2013, following the first ADRD conference.27 Since 2013, 4 DLB trials have been completed. Three have been open-label or open-label extensions, with only one randomized, double-blind, placebo-controlled trial.49–51 Most of these studies focused on Food and Drug Administration–approved drugs for AD, and at least one trial contributed to the approval of donepezil for DLB in Japan in September 2014. In LBD clinical trials, there is growing interest in new types of primary outcome measures for cognition, as reflected in results reported for a memantine intervention.52 Three PDD trials have been completed since 2013, featuring drug formulation (rivastigmine patch vs oral pill),53 caregiver burden/secondary analysis of a memantine trial,54 and psychosis in PD (randomized, double-blind trial with pimavanserin).55
LBD OUTCOMES ARE EMBLEMATIC OF THE CROSS-CUTTING CHALLENGE, IMPORTANCE, AND BENEFIT OF ACCURATE DIAGNOSIS EVEN IN THE ABSENCE OF DISEASE-MODIFYING THERAPY
The committees of all 6 sessions of the Summit believed that accurate differential diagnosis of the many forms of dementia is of the utmost importance. LBD, which represents a significant proportion of dementia diagnoses,12,56,57 can clinically mimic not only AD but also Parkinson disease, commonly leading to underdiagnosis, misdiagnosis, or a delayed diagnosis. The challenges of differential diagnosis are clearly illustrated by the fact that about three-quarters of people with LBD received a different initial diagnosis before ultimately learning they had LBD, and the process required, on average, visits to 3 different physicians.58–60 Misdiagnosis or no diagnosis is problematic in terms of planning and medical management across the AD/ADRD spectrum. In LBD, misdiagnosis or no diagnosis is especially dangerous because of potential adverse reactions, such as heavy sedation, increased hallucinations, and parkinsonism in response to medications (e.g., typical neuroleptics) used in care settings for behavioral management of disabled elderly. Moreover, improving differential diagnosis among dementias will facilitate better clinical research and trials. To improve diagnostic capabilities as well as useful measures to test target engagement in clinical trials, NINDS issued a funding opportunity announcement for studies of LBD biomarkers.e1,e2
FRONTOTEMPORAL LOBAR DEGENERATION: GENETIC DISCOVERIES INCREASE UNDERSTANDING OF MECHANISMS
Among rare early age at onset dementias, FTD is a prevalent clinical diagnostic category that encompasses diverse clinical syndromes.e3 The average age at onset is in the middle to late 50s,e4 making FTD a condition with much greater midlife burden compared to typical late age at onset AD. In most patients with FTD, the pathologic correlate is frontotemporal lobar degeneration, a heterogeneous category in which neurons and glia form inclusions containing tau, TDP-43, or fused in sarcoma (FUS). FTD has an autosomal dominant genetic cause in 10%–20% of patients. Despite this complexity, considerable progress has been made since 2013, including new insights into the pathogenic mechanisms and clinical symptomatology of FTD and the FTD/amyotrophic lateral sclerosis (ALS) disease spectrum.e5
Recent research has provided new insights into the disease mechanisms of FTD/ALS caused by a hexanucleotide repeat motif expansion in the chromosome 9 open reading frame 72 genee6 (C9ORF72; see below). For example, recent studies led to the discovery of disruptions in ribonucleoprotein granule function due to liquid-to-solid phase transitions of TDP-43 and FUS.e7 Scientists now also have a better understanding of the molecular mechanisms underlying FTD due to tau pathology.e8–e10 Aiding this investigation are new techniques to study the propagation of synthetic tau strains in vitro and in vivo. Such methods have been shown to recapitulate features of human disease pathology in animal models. Ongoing research efforts responsive to the recommendations from the 2013 ADRD Conference include 3 NIH-funded longitudinal cohort studies of Mendelian, genetically influenced, and sporadic FTD to better understand disease progression, to identify new biomarkers for diagnosis, progression, and prognosis, and to establish a clinical research consortium to support FTD therapy development. In 2016, NIH issued a funding opportunity announcement to stimulate research on tau pathogenesis as it relates to FTD.e1,e2
ROLE OF C9ORF72 IN FTD/ALS
The so far most common mutation associated with familial or sporadic FTD/ALS is an expanded hexanucleotide repeat motif (GGGGCC) located in intron 1 of the C9ORF72 gene. Three main hypotheses are currently being tested to explain how this repeat expansion causes disease. One possibility is that decreased expression of the C9ORF72 mRNAs leads to a loss-of-function phenotype, as a result of the expanded repeats interfering with transcription or translation. Another possibility is that foci of RNAs formed by the repeat-expanded sense or antisense transcripts sequester essential RNAs, leading to neurotoxicity. Finally, toxic dipeptide repeat proteins produced by repeat-associated non-ATG-initiated translation of the expanded sense and antisense transcripts could be pathogenic. These putative disease mechanisms are not mutually exclusive and require further exploration.e6 This mechanistic uncertainty led the FTD committee to include research on the molecular basis of C9ORF72 repeat expansion–related neurodegeneration in Basic Science (table 1; FTD Recommendation 2).
VASCULAR CONTRIBUTIONS TO COGNITIVE IMPAIRMENT AND DEMENTIA: UNDERSTANDING MECHANISMS AND DEVELOPING BIOMARKERS FOR BETTER DEMENTIA OUTCOMES
Because diagnoses of pure vascular dementia are comparatively infrequent in the United States, vascular contributions to cognitive impairment and dementia (VCID) is frequently underestimated both in terms of disease burden and potential for understanding and preventing dementia. The most common picture of brain pathology in older persons with dementia includes vascular pathology together with varying amounts of classic AD plaques and tangles. Numerous studies report that cardiovascular and cerebrovascular risk factors, cerebral arteriosclerosis, diffuse white matter disease, and infarcts increase risk for cognitive impairment and dementia in humans as well as in animal models.e11–e16 In addition, recent epidemiologic studies report downward trends in the prevalence of dementia in high-income countries that parallel downward trends in the incidence of stroke that occurred due to improved control of vascular risk factors.e17,e18 These findings are consistent with the hypothesis that addressing cardiovascular and cerebrovascular risk factors in midlife will prevent cerebrovascular disease and stroke in later life with consequent decreased risk of dementia. Therefore, health care providers should be aware of cardiovascular and cerebrovascular risk factors and the potential importance of their management to prevent cognitive impairment and dementia.
Recognizing this scientific nexus between vascular disease and dementia, and the potential for a positive influence on public health through research in this area, in 2014 the NIH started tracking VCID in its Research, Condition, and Disease Categorization, a classification system that NIH uses to report funding. Recent scientific progress in VCID features new animal models exhibiting different types of ischemia, cerebrovascular disease, and white matter pathology as well as comorbidity with relevant human conditions. For example, AD-transgenic mice with diet-induced hyperhomocysteinemiae19 exhibit increased amyloid deposition in arterioles.e20 In hypertensive stroke-prone rats, researchers were able to study the relationship among age, small-vessel disease, parenchymal β-amyloid, and tau pathology. In 2015, the NIH established the M2OVE AD Consortium, a project that funds interdisciplinary research to understand the vascular etiology of AD.e21
Updates to the VCID recommendations included identifying emerging areas of research such as the study of perivascular and peravascular clearance mechanisms,e22,e23 translational brain imaging, and the role of aging, resilience factors, and genetic factors as well as the relationship of cerebrovascular disease to tau-related neurodegeneration.e20 The research community also emphasized the need for a new generation of human-based VCID studies that interrogate not only the known vascular risk factors contributing to dementia, but also the complex and diverse roles of the different aspects of late-life changes in cerebral blood vessels (table 1). This can only be accomplished if new clinical research recruits and stratifies research participants from high-risk, high-burden populations with racial, ethnic, geographic, socioeconomic, and other real-life diversity that reflects the spectrum of vascular pathology in United States populations. One potential strategy to achieve this is by adding relevant VCID components to existing population cohort studies.
REDEFINING THE NEUROVASCULAR UNIT FOR VCID AND BEYOND
An area of increasing focus and a critical part of VCID research is the neurovascular unit (NVU). The concept of the NVU highlights the close interaction between brain and vascular cells in development, normal function, and disease. Scientific interest and cognate publications have increased dramatically since the NVU was first defined at the 2002 NINDS Stroke Progress Research Group. The concept has continued to evolve and grow in importance, including following a 2010 call for reevaluation of the NVU's role in health and disease, and in particular, in VCID.e12 Increased study of the NVU has shed light on the blood–brain barrier, highlighting roles of paracellular movement, including transcytosis. These insights have pointed to new roles for astrocytes, microglia, and extracellular matrix proteins after injury, including brain clearance of potentially toxic agents.e24
From a conceptual standpoint, the NVU was initially static, but now it is very clear that the exact makeup of the NVU differs in different brain regions. It has become important to revisit the NVU with our increased understanding of its complex interactions with multiple neuronal and vascular cell types such as astrocytes, neurons, myocytes, pericytes, and endothelial cells. At each level of the cerebrovascular tree, the architecture of the NVU changes to meet the functional requirement of delivering blood to the brain. Thus, understanding the NVU at the extraparenchymal arteriole is different from understanding the NVU at the capillary. Understanding how the function of the NVU changes with age, hypertension, atherosclerosis, and concomitant proteinopathies, including AD, is critical to understanding and preventing VCID.
HEALTH DISPARITIES: RECOGNIZING DEMENTIA DISPARITIES, ADVANCING UNDERSTANDING AND SOLUTIONS
There is evidence that the prevalence of cognitive impairment is higher in nonwhite populations in the United States, including African American, Hispanic, and Latino populations.e25–e32 At the same time, people from certain racial and ethnic groups, along with socioeconomically disadvantaged and rural populations, are less likely to have dementia diagnosed, and diagnosis is typically at later stages of disease, with more neuropsychiatric symptoms present than among whites.e33,e34 There are also geographic disparities in the burden of dementia. For example, African Americans and whites born in the United States stroke belt are at higher risk of dementia mortality than those born in other states, even for people that at some point move out of this southern region of the country.e35 Finally, more women are affected than men.e36–e39 Due to disparities in access to specialty diagnostic care, current AD/ADRD datasets are lacking in research data from diverse populations. This is because of insufficient diversity in most cohort studies, and data capture methods that are typically composed of registries with limited catchment areas. As a result, there are important gaps in the AD/ADRD research and evidence base, which is built largely from non-Latino white participants.
Nonetheless, since the 2013 ADRD conference, research progress has been made. Existing studies of diverse cohorts have been leveraged to include neuropsychological and biomarker assessments. Researchers are engaging local expertise to evaluate AD/ADRD in diverse communities, developing assessment tools for use in disparate populations, and studying molecular mechanisms for health disparities. To help further close the disparity gap, NIH also released 2 targeted funding initiatives that address health disparities in AD and AD-related dementias.e1,e2 Because culturally appropriate assessment, approaches, validation, and community partnerships are critical to address dementia disparities, these figure prominently in the recommendations (table 2).
RECRUITMENT IS CRITICAL TO ANY SUCCESSFUL APPROACH FOR ADDRESSING DEMENTIA HEALTH DISPARITIES
There are substantial barriers to recruitment of minorities as well as persons affected by health disparities to clinical trials and clinical research. These include inadequate connection with health systems, lack of community-based participatory research, lack of engagement and partnership with the community, and cultural differences in beliefs such as about the body after death.e40 Mistrust of scientific research in disparate populations has historical roots (e.g., atrocities such as the Tuskegee Syphilis Study) as well as current realities (e.g., minorities continue to have experiences of discrimination in medical settings).e41,e42 Despite these and other challenges, including stigma associated with cognitive impairment and dementia, there is potential to address heath disparities in AD/ADRD, as indicated by the many Latino and African American people, as well as those living in rural areas and those with lower socioeconomic status, who report interest in participation in research and clinical trials but are not routinely eligible or asked to participate.e43 The Heath Disparities Session embraced the task of solving these critical challenges (table 2; Focus area 4) by recommending that a Health Disparities Task Force be established to focus exclusively on recruitment issues in AD/ADRD research.
NGO: CATALYZING RESEARCH THROUGH UNIQUE PARTNERSHIPS
The 2016 ADRD Summit included a session led by representatives from NGOs in the AD/ADRD field that fund biomedical research among other activities. The NGO committee highlighted the value of partnerships and collaborations in AD/ADRD research. Collaborations among NGOs, government, industry, and academia can foster unique research opportunities across diseases, national borders, and stakeholders. Members of the committee discussed shared principles of NGO research funding, including flexibility, swift turnaround, focus, and an emphasis on collaboration. These allow NGOs to provide research funding that is both complementary to and synergistic with federal support (table 3).
Table 3.
Nongovernmental organization (NGO) session recommendationsa

WHAT'S IN A NAME? NGO AND THE MED SESSION FOCUS ON NOMENCLATURE
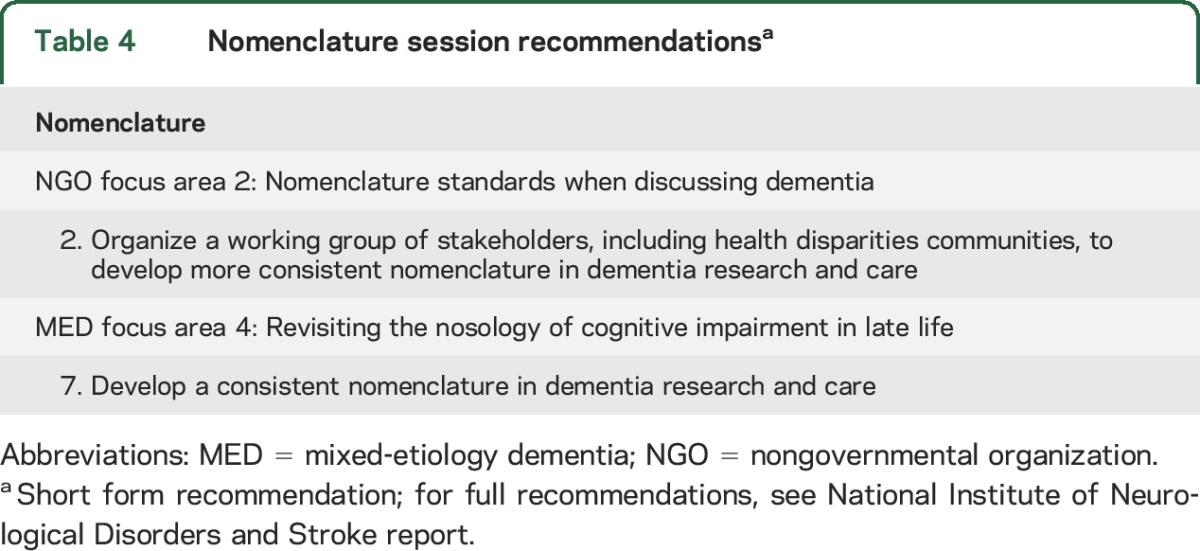
A joint session of the NGO and MED committees highlighted the need for developing AD/ADRD nomenclature standards for use among researchers and other stakeholders, including individuals living with dementia and their families, caregivers, health care providers, and government agencies (table 4). There are many factors that contribute to unclear and inconsistent nomenclature: undirected evolution of terminology, reductionist tendencies, confounding of clinical syndromes and etiologies, and lack of consensus regarding how to refer to the mildest symptomatic phases of cognitive impairment and dementia. In addition, AD is often used synonymously with or instead of the term dementia. The result is that some patients and families have not heard of the ADRD diagnoses, and thus lack context for making a connection between AD care and service and their own needs. A generalized lack of clarity in dementia terminology is counterproductive to the goals of all stakeholders; better clarity in terminology should increase clinicians' ability to engage in meaningful discussions. This is critical because misdiagnosis, changing diagnoses over disease progression, and lack of clarity in communicating a diagnosis can create tremendous confusion for patients and their family members. In the community at large, lack of both a basic understanding of dementia and an accurate vocabulary to address this contributes to stigma and confounds efforts to develop effective systems for appropriate, empathic care. It is time for an open national dialogue toward developing a universal lexicon that meets the needs of all. Several efforts are underway to achieve this goal: Dementia Friendly America,e44 the dementia language guidelines,e45 and the Dementia Engagement & Empowerment Programmee46 are 3 examples.
Table 4.
Nomenclature session recommendationsa
DISCUSSION
Rapid progress has been made in AD/ADRD research in the last few years and dedicated funding has accelerated its pace. However, continued attention to cross-cutting areas will be necessary to uncover poorly understood relationships among different diagnoses and pathophysiologic mechanisms. Notable areas of interdisciplinary scientific interest include the relationship among proteinopathies, genetics, metabolism including diabetes, immune signaling, neural circuits, circadian rhythms and sleep, and the NVU and blood–brain barrier.
Discussion at the ADRD Summit 2016 also called for a more effective cross-sector dialogue about activities and progress toward achieving ADRD research goals, especially in the years between the NIH-hosted ADRD Summits. Plans are in process to develop consensus and harmonization in AD/ADRD nomenclature that is effective for the broad range of stakeholders. Continued collaborative efforts from government, industry, and nonprofit organizations will be essential to meet the goal of preventing and effectively treating AD/ADRD by 2025.
Here we have characterized in some detail recent progress and research recommendations in the main ADRD focal areas: LBD, FTD, VCID, and multiple etiology dementias. Frequent assessment and recalibration of research directions is being accomplished through periodic and complementary AD and ADRD summits. A full listing of the ADRD Summit 2016 recommendations, with further comments and rationale not detailed here, is available online.e47
ACKNOWLEDGMENT
The membership of the ADRD Summit 2016 Organizing Committee and Summit Sessions appears as supplemental information (tables e-1 and e-2 at Neurology.org). The authors recognize the editorial contributions of Dr. Alison F. Davis.
GLOSSARY
- AD
Alzheimer disease
- ADRD
Alzheimer disease–related dementias
- ALS
amyotrophic lateral sclerosis
- DLB
dementia with Lewy bodies
- FTD
frontotemporal dementia
- FUS
fused in sarcoma
- LBD
Lewy body dementia
- MED
mixed-etiology dementia
- NGO
nongovernmental organization
- NINDS
National Institute of Neurological Disorders and Stroke
- NVU
neurovascular unit
- PDD
Parkinson disease dementia
- TDP
TAR DNA-binding protein
- VCID
vascular contributions to cognitive impairment and dementia
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
R.A.C., J.T.G., A.K.G., S.J., W.J.K., and D.M.H. organized pre-Summit activities, participated in the ADRD Summit 2016, and contributed to post-Summit activities and drafting a substantial portion of the manuscript. D.B., D.A.B., T.C., S.L.-J.D., D.W.D., M.E., H.F., S.M.G., M.L.H., D.S.K., J.J.M., K.S.M., C.S.M., C.H.P., C.L.T., P.A.S., W.W.S., B.-A.S., N.B.S., M.L.S., A.T., and S.P.W. organized pre-Summit activities, participated in the ADRD Summit 2016, and contributed to post-Summit activities.
STUDY FUNDING
The conference was supported by NINDS, organized in collaboration with the National Institute on Aging, with assistance from the Foundation for the NIH and additional support from the NIH Office of Disease Prevention, the Alzheimer's Association, Accelerate Cure/Treatments for Alzheimer's Disease (ACT-AD), the American Heart Association/American Stroke Association, the Association for Frontotemporal Degeneration, Axovant Sciences, the BrightFocus Foundation, and the LEAD Coalition (Leaders Engaged on Alzheimer's Disease Coalition).
DISCLOSURE
R.A. Corriveau, W.J. Koroshetz, J.T. Gladman, S. Jeon, D. Babcock, D.A. Bennett, S.T. Carmichael, S.L.-J. Dickinson, D.W. Dickson, M. Emr, H. Fillit, and S.M. Greenberg report no disclosures relevant to the manuscript. M.L. Hutton is a full-time employee of Eli Lilly and company. D.S. Knopman, J.J. Manly, K.S. Marder, C.S. Moy, C.H. Phelps, P.A. Scott, W.W. Seeley, B.-A. Sieber, N.B. Silverberg, M.L. Sutherland reports, A. Taylor, C.L. Torborg, S.P. Waddy, and A.K. Gubitz report no disclosures relevant to the manuscript. D.M. Holtzman cofounded and is on the scientific advisory board of C2N Diagnostics. DMH is an inventor on a submitted patent “Antibodies to Tau” that is licensed by Washington University to C2N Diagnostics. This patent was subsequently licensed to AbbVie. D.M. Holtzman consults for Genentech, Denali, AbbVie, GlaxoSmithKline, and Proclara Biosciences. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Dementia: Fact Sheet N°362 [online]. Available at: who.int/mediacentre/factsheets/fs362/en/. Accessed August 2, 2017. [Google Scholar]
- 2.Bachman DL, Wolf PA, Linn RT, et al. Incidence of dementia and probable Alzheimer's disease in a general population: the Framingham Study. Neurology 1993;43:515–519. [DOI] [PubMed] [Google Scholar]
- 3.Hebert LE, Scherr PA, Beckett LA, et al. Age-specific incidence of Alzheimer's disease in a community population. JAMA 1995;273:1354–1359. [PubMed] [Google Scholar]
- 4.Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology 2000;54:2072–2077. [DOI] [PubMed] [Google Scholar]
- 5.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol 2002;59:1737–1746. [DOI] [PubMed] [Google Scholar]
- 6.Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol 2003;60:185–189. [DOI] [PubMed] [Google Scholar]
- 7.James BD, Bennett DA, Boyle PA, Leurgans S, Schneider JA. Dementia from Alzheimer disease and mixed pathologies in the oldest old. JAMA 2012;307:1798–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalaria RN. The role of cerebral ischemia in Alzheimer's disease. Neurobiol Aging 2000;21:321–330. [DOI] [PubMed] [Google Scholar]
- 9.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69:2197–2204. [DOI] [PubMed] [Google Scholar]
- 10.Jellinger KA, Attems J. Neuropathological evaluation of mixed dementia. J Neurol Sci 2007;257:80–87. [DOI] [PubMed] [Google Scholar]
- 11.Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol 2010;119:421–433. [DOI] [PubMed] [Google Scholar]
- 12.Jellinger KA, Attems J. Prevalence and pathology of dementia with Lewy bodies in the oldest old: a comparison with other dementing disorders. Demen Geriatr Cogn Disord 2011;31:309–316. [DOI] [PubMed] [Google Scholar]
- 13.Gardner RC, Valcour V, Yaffe K. Dementia in the oldest old: a multi-factorial and growing public health issue. Alzheimers Res Ther 2013;5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer's disease: lessons from pathology. BMC Med 2014;12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jellinger KA, Attems J. Challenges of multimorbidity of the aging brain: a critical update. J Neural Transm 2015;122:505–521. [DOI] [PubMed] [Google Scholar]
- 16.Barker WW, Luis CA, Kashuba A, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord 2002;16:203–212. [DOI] [PubMed] [Google Scholar]
- 17.Fillit H, Hill J. Economics of dementia and pharmacoeconomics of dementia therapy. Am J Geriatr Pharmacother 2005;3:39–49. [DOI] [PubMed] [Google Scholar]
- 18.Chiao CY, Wu HS, Hsiao CY. Caregiver burden for informal caregivers of patients with dementia: a systematic review. Int Nurs Rev 2015;62:340–350. [DOI] [PubMed] [Google Scholar]
- 19.Vandepitte S, Van Den Noortgate N, Putman K, Verhaeghe S, Verdonck C, Annemans L. Effectiveness of respite care in supporting informal caregivers of persons with dementia: a systematic review. Int J Geriatr Psychiatry 2016;31:1277–1288. [DOI] [PubMed] [Google Scholar]
- 20.Vandepitte S, Van Den Noortgate N, Putman K, Verhaeghe S, Faes K, Annemans L. Effectiveness of supporting informal caregivers of people with dementia: a systematic review of randomized and non-randomized controlled trials. J Alzheimers Dis 2016;52:929–965. [DOI] [PubMed] [Google Scholar]
- 21.Allen AP, Curran EA, Duggan A, et al. A systematic review of the psychobiological burden of informal caregiving for patients with dementia: focus on cognitive and biological markers of chronic stress. Neurosci Biobehav Rev 2017;73:123–164. [DOI] [PubMed] [Google Scholar]
- 22.Deb A, Thornton JD, Sambamoorthi U, Innes K. Direct and indirect cost of managing Alzheimer's disease and related dementias in the United States. Expert Rev Pharmacoecon Outcomes Res 2017;17:189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Institute of Neurological Disorders and Stroke. Alzheimer's Disease-Related Dementias (ADRD) Summit 2016 Prioritized Research Milestones [online]. Available at: aspe.hhs.gov/alzheimers-disease-related-dementias-adrd-summit-2016-prioritized-research-milestones. Accessed October 23, 2017. [Google Scholar]
- 24.US Department of Health & Human Services. National Alzheimer's Project Act [online]. Available at: aspe.hhs.gov/national-alzheimers-project-act. Accessed October 29, 2015. [Google Scholar]
- 25.An act making consolidated appropriations for the fiscal year ending September 30, 2015, and for other purposes. Public and Private Laws. 113th Congress ed.2014.
- 26.Collins FS on behalf of the NIH. Stopping Alzheimer's Disease and Related Dementias: Advancing Our Nation's Research Agenda: NIH Bypass Budget Proposal for Fiscal year 2018. Washington, DC: US Department of Health and Human Services; 2016.
- 27.Montine TJ, Koroshetz WJ, Babcock D, et al. Recommendations of the Alzheimer's disease-related dementias conference. Neurology 2014;83:851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Centers for Medicare & Medicaid Services. Medicare Annual Wellness Visit: Your Medicare Coverage [online]. Available at: medicare.gov/coverage/preventive-visit-and-yearly-wellness-exams.html. Accessed March 24, 2017. [Google Scholar]
- 29.Fillit H, Geldmacher DS, Welter RT, Maslow K, Fraser M. Optimizing coding and reimbursement to improve management of Alzheimer's disease and related dementias. J Am Geriatr Soc 2002;50:1871–1878. [DOI] [PubMed] [Google Scholar]
- 30.Tsoi KK, Chan JY, Hirai HW, Wong SY, Kwok TC. Cognitive tests to detect dementia: a systematic review and meta-analysis. JAMA Intern Med 2015;175:1450–1458. [DOI] [PubMed] [Google Scholar]
- 31.Bunn F, Goodman C, Sworn K, et al. Psychosocial factors that shape patient and carer experiences of dementia diagnosis and treatment: a systematic review of qualitative studies. PLoS Med 2012;9:e1001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eichler T, Thyrian JR, Hertel J, et al. Rates of formal diagnosis in people screened positive for dementia in primary care: results of the DelpHi-Trial. J Alzheimers Dis 2014;42:451–458. [DOI] [PubMed] [Google Scholar]
- 33.Eichler T, Thyrian JR, Hertel J, et al. Rates of formal diagnosis of dementia in primary care: the effect of screening. Alzheimers Demen 2015;1:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mueller C, Ballard C, Corbett A, Aarsland D. The prognosis of dementia with Lewy bodies. Lancet Neurol 2017;16:390–398. [DOI] [PubMed] [Google Scholar]
- 35.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 2009;66:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider JA, Arvanitakis Z, Yu L, Boyle PA, Leurgans SE, Bennett DA. Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain 2012;135:3005–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sperling RA, Rentz DM, Johnson KA, et al. The A4 study: stopping AD before symptoms begin? Sci Transl Med 2014;6:228fs213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reiman EM, Langbaum JB, Fleisher AS, et al. Alzheimer's prevention initiative: a plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis 2011;26(suppl 3):321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bateman RJ, Benzinger TL, Berry S, et al. The DIAN-TU Next Generation Alzheimer's Prevention Trial: adaptive design and disease progression model. Alzheimers Dement 2017;13:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karran E, Hardy J. A critique of the drug discovery and phase 3 clinical programs targeting the amyloid hypothesis for Alzheimer disease. Ann Neurol 2014;76:185–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karran E, Hardy J. Antiamyloid therapy for Alzheimer's disease: are we on the right road? N Engl J Med 2014;370:377–378. [DOI] [PubMed] [Google Scholar]
- 42.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 2017;89:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kovacs GG, Alafuzoff I, Al-Sarraj S, et al. Mixed brain pathologies in dementia: the BrainNet Europe consortium experience. Demen Geriatr Cogn Disord 2008;26:343–350. [DOI] [PubMed] [Google Scholar]
- 44.Guerreiro R, Escott-Price V, Darwent L, et al. Genome-wide analysis of genetic correlation in dementia with Lewy bodies, Parkinson's and Alzheimer's diseases. Neurobiol Aging 2016;38:214.e7–214.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Surmeier DJ, Obeso JA, Halliday GM. Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci 2017;18:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luk KC, Kehm VM, Zhang B, O'Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med 2012;209:975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Longhena F, Faustini G, Missale C, Pizzi M, Spano P, Bellucci A. The contribution of alpha-synuclein spreading to Parkinson's disease synaptopathy. Neural plasticity 2017;2017:5012129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bras J, Guerreiro R, Darwent L, et al. Genetic analysis implicates APOE, SNCA and suggests lysosomal dysfunction in the etiology of dementia with Lewy bodies. Hum Mol Genet 2014;23:6139–6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ikeda M, Mori E, Matsuo K, Nakagawa M, Kosaka K. Donepezil for dementia with Lewy bodies: a randomized, placebo-controlled, confirmatory phase III trial. Alzheimers Res Ther 2015;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ikeda M, Mori E, Kosaka K, et al. Long-term safety and efficacy of donepezil in patients with dementia with Lewy bodies: results from a 52-week, open-label, multicenter extension study. Demen Geriatr Cogn Disord 2013;36:229–241. [DOI] [PubMed] [Google Scholar]
- 51.Mori E, Ikeda M, Nakagawa M, Miyagishi H, Yamaguchi H, Kosaka K. Effects of donepezil on extrapyramidal symptoms in patients with dementia with Lewy bodies: a secondary pooled analysis of two randomized-controlled and two open-label long-term extension studies. Demen Geriatr Cogn Disord 2015;40:186–198. [DOI] [PubMed] [Google Scholar]
- 52.Wesnes KA, Aarsland D, Ballard C, Londos E. Memantine improves attention and episodic memory in Parkinson's disease dementia and dementia with Lewy bodies. Int J Geriatr Psychiatry 2015;30:46–54. [DOI] [PubMed] [Google Scholar]
- 53.Emre M, Ford PJ, Bilgic B, Uc EY. Cognitive impairment and dementia in Parkinson's disease: practical issues and management. Mov Disord 2014;29:663–672. [DOI] [PubMed] [Google Scholar]
- 54.Leroi I, Atkinson R, Overshott R. Memantine improves goal attainment and reduces caregiver burden in Parkinson's disease with dementia. Int J Geriatr Psychiatry 2014;29:899–905. [DOI] [PubMed] [Google Scholar]
- 55.Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson's disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet 2014;383:533–540. [DOI] [PubMed] [Google Scholar]
- 56.Hogan DB, Fiest KM, Roberts JI, et al. The prevalence and incidence of dementia with Lewy bodies: a systematic review. Can J Neurol Sci 2016;43(suppl 1):S83–S95. [DOI] [PubMed] [Google Scholar]
- 57.Vann Jones SA, O'Brien JT. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol Med 2014;44:673–683. [DOI] [PubMed] [Google Scholar]
- 58.Lewy Body Dementia Association Investigators. Caregiver Burden in Lewy Body Dementias Challenges in Obtaining Diagnosis and Providing Daily Care [online]. Available at: http://lbda.org/go/caregiverburden. Accessed October 23, 2017. [Google Scholar]
- 59.Galvin JE, Duda JE, Kaufer DI, Lippa CF, Taylor A, Zarit SH. Lewy body dementia: caregiver burden and unmet needs. Alzheimer Dis Assoc Disord 2010;24:177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galvin JE, Duda JE, Kaufer DI, Lippa CF, Taylor A, Zarit SH. Lewy body dementia: the caregiver experience of clinical care. Parkinsonism Relat Disord 2010;16:388–392. [DOI] [PMC free article] [PubMed] [Google Scholar]