Abstract
Introduction
Cancer is a disease with a global burden and is a major and increasing threat to public health. The demand for new modalities to treat and prevent cancer is high. Given the toxic side effects of standard treatments, such as chemotherapy, there is greater research interest in naturally derived compounds due to their selective toxicity to cancer cells. This study aimed to test the anticancer activity of a crude extract of Hopea odorata on hepatocellular carcinoma (HCC) HepG2 cell line.
Methods
Methanol extracts of H. odorata were prepared from the bark of H. odorata plants (H. odorata extract). The in vitro cytotoxicity of H. odorata extracts on human HCC cell line HepG2 compared to normal human fibroblasts (HFs) was assessed by Alamar Blue assay. Caspase-3/7 was detected using a reagent that consists of DEVD peptide conjugated to a nucleic acid-binding dye. Apoptosis induction by the H. odorata plant extract on HepG2 was evaluated by Annexin V/7-AAD using flow cytometry. Disintegrated nuclei of plant-treated cells were observed under a fluorescent microscope using Hoechst and propidium iodide (PI) staining. In addition, using the Hoechst/PI staining technique, the ratio of dead to total cells was determined by distinguishing Hoechst and PI fluorescent signals.
Results
We found that the IC50 value of H. odorata extract on HepG2 was 12.67±5 µg/mL and on HF was 44±3 µg/mL. The IC50 value of doxorubicin on HepG2 was 153.3±15 ng/mL and on HF was 6.3±0.6 ng/mL. The selectivity index (SI) of H. odorata extract for HepG2 cells was ~3.48, while the SI of doxorubicin for HepG2 cells was ~0.04. The ratio of dead to total cells increased in a dose-dependent manner for HepG2 cells when observed under a fluorescent microscope, while the ratio of dead to total cells barely changed for HF cells. The H. odorata extract inhibited HepG2 cells via the activation of caspase-3/7. At 250 µg/mL concentration of the H. odorata extract, 35% of HepG2 cells were induced into apoptosis, and the cells exhibited disintegrated nuclei under a fluorescent microscope.
Conclusion
These findings demonstrate that the methanolic bark extracts of H. odorata plant induce apoptosis and selective cytotoxicity toward HepG2 but not HF. Therefore, purification of compounds from H. odorata bark extracts may be useful as anticancer agents, and thus, more studies are warranted to investigate the anticancer properties of H. odorata.
Keywords: apoptosis, caspase-3, HepG2, Hopea odorata, human fibroblast, methanol extract, selectivity index
Introduction
There is a rising interest in natural product resources, particularly medicinal plants, for drug discovery and development of therapeutic agents. More than 35,000 plant species have been reported around the world for medicinal use.1 Plants contain many biologically active compounds that have potential to be developed as anticancer therapeutic agents. Some of these compounds include curcumin (from turmeric) that has shown anticancer effects against colon cancer2–4 and brain cancer,5,6 apigenic (from parsley) that has been demonstrated to induce apoptosis in breast cancer,7,8 and crocetin (from saffron) that has been reported to be cytotoxic against pancreatic cancer9,10 and skin carcinoma.11,12 By recent estimation, ~50%–60% of cancer patients in the US utilize agents from natural products exclusively or as a complement for traditional therapeutic regimens, such as chemotherapy or radiation therapy.13
Hepatocellular carcinoma (HCC) is one of the most common gastrointestinal diseases and generally carries a poor prognosis since it is highly malignant. According to the latest statistics, ~600,000 patients are diagnosed with HCC worldwide each year, and some of them die within 7–8 months after diagnosis.14 Although there are many advanced options available for the treatment of HCC, these methods have shown various adverse effects, such as immune system disorders and liver toxicity. Common treatments, including surgery and even liver transplantation, only provide limited results. Chemotherapy and radiotherapy cause severe side effects such as cytotoxicity on normal cells and drug resistance.15,16 Therefore, more effective alternative therapies or medicines, with fewer side effects, are critically needed for HCC. Herbal medicine has emerged as an alternative medicine with great potential for HCC therapy.
Hopea odorata belongs to the Dipterocarpaceae family of plants, which is widely distributed in lowland forests of Vietnam and is native to Southeast Asia.17 Its local name is “sao den”. Different parts of this plant have been used as traditional medicine. In Vietnam, the extract of the H. odorata bark has been used as a remedy for gingivitis and for diarrhea treatment. The resin of the trunk of H. odorata can be applied to sores and wounds for the relief of inflammation and to stop bleeding. A recent study showed that the methanol extract of H. odorata strongly inhibited gene expression of proinflammatory cytokines and chemokines, such as interferon-beta, interleukin-12, and monocyte chemotactic protein-1.18 To our knowledge, there is no information of H. odorata as a cancer treatment, from indigenous medicine until now. Owing to its long and traditional use in Southeast Asia to treat inflammation, we questioned if it might have an effect on cancer cells since the cancer milieu is proinflammatory.19–21 In this study, we aimed to determine whether methanol extracts of H. odorata bark showed antitumor efficacy against the HCC cell line HepG2 and human normal human fibroblast (HF) in vitro. The cytotoxic ability of the plant extract (PE) was investigated and compared to the reference anticancer drug doxorubicin.
Materials and methods
Collection of plant material
The plant was collected at Ma Da forest in Dong Nai Culture and Nature Reserve, Dong Nai Province, Vietnam, in April 2014. The plant was identified by Associate PhD. Hop Tran, Institute of Tropical Biology, Ho Chi Minh City, Vietnam. The voucher sample (MCE018) is preserved at the Division of Medicinal Chemistry, Faculty of Chemistry, University of Science, Vietnam National University Ho Chi Minh City, Vietnam.
Extraction of plant material
The dried bark of H. odorata plant (200 g) was cut into small pieces and extracted with MeOH (300 mL, reflux, 3 hours, ×3). The MeOH solution was evaporated under reduced pressure to give an MeOH extract (15 g).
Cell lines
The HepG2 cell line was obtained from American Type Culture Collection (Manassas, VA, USA). Cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% penicillin–streptomycin in a humidified incubator with 5% CO2 at 37°C; all regents were obtained from Thermo Fisher Scientific (Waltham, MA, USA).
HFs were isolated from foreskin, according to a previously published protocol. All patients provided written informed consent for the use of human skin tissue in this research. HF cells were isolated from human foreskin collected at Gia Dinh Hospital (Ho Chi Minh City, Vietnam). The cell isolation from human tissue was approved by the scientific ethics committee of the Stem Cell Institute, University of Science, Vietnam National University. Foreskin was stored in the PBS solution at 4°C and transferred to the laboratory for isolation and culture of fibroblasts. HFs from passage 4 to passage 5 were used for experiments.
Cell viability assessment of PE treatment and doxorubicin treatment
Briefly, 2,500 cells of HepG2 were seeded into each well of a 96-well tissue culture plate 1 day before treatment with PE. HF cells were used as controls and were assayed in parallel with HepG2. Cells were treated with PE in the culture medium for 48 hours at these serial extract concentrations: 1,000, 500, 250, 125, 62.5, 31, 15, and 0 µg (untreated). Methanol solvent in the culture medium was adjusted to 0.5% in all the concentration points. Subsequently, Alamar Blue (R-7071; Sigma-Aldrich Co., St Louis, MO, USA) was added to the medium to a final concentration of 10 µg/mL and incubated for 1 hour. The fluorescence at 560 nm was recorded using DTX 880 (Beckman Coulter, Brea, CA, USA). Each concentration was tested in triplicate. Experiments were independently repeated three times.
For doxorubicin treatment, doxorubicin (Sigma-Aldrich Co.) was evaluated at the following serial concentrations: 125, 62, 30, 15, 7, 3, and 0 µg (untreated). Each concentration was tested in triplicate. Experiments were independently repeated three times.
Caspase-3/7 activity
Cells were treated with H. odorata extract at a concentration of 250 µg/mL. Concurrently, CellEvent Caspase-3/7 Green Detection Reagent (C10423; Thermo Fisher Scientific) was added into the culture medium at a final concentration of 5 µM and incubated for 30 minutes. Cells were visualized by fluorescent microscopy.
Annexin V/propidium iodide (PI) apoptosis assay
Treated and untreated cells were harvested after trypsinization and pelleted by centrifugation at 500× g for 5 minutes at 4°C. Pellets were suspended, and cells were then stained with Annexin V and PI using a FITC Annexin V Apoptosis Detection Kit I (556547; BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. Stained cells were analyzed by a BD FACSCalibur flow cytometer (BD Biosciences). Data were analyzed by CellQuest software (BD Biosciences).
Hoechst 33342 and PI staining assay
Cell viability of HepG2 (following treatment with 250 µg/mL of extract) was evaluated by Hoechst 33342/PI staining. Skin-derived HF was used as the control cell line. Briefly, HepG2 and HF cells were treated with H. odorata extract at a concentration of 250 µg/mL; untreated cells were cultured in the normal medium complemented with a solvent. At day 2 of treatment, cells were stained with Hoechst 33342 and PI. The final concentration of Hoechst 33342, as well as PI, was 1 µg/mL in the culture medium. Then, cells were incubated in an incubator (37°C, 5% CO2) for 5 minutes. Stained cells were observed by a fluorescent microscope.
Statistical analysis
Data were analyzed by GraphPad Prism software (Graphpad Software, Inc., La Jolla, CA, USA) for the determination of IC50 values. All data were presented as the mean of triplicate experiments. Statistical significance was set at P<0.05.
Results
Fibroblast isolation from skin tissue
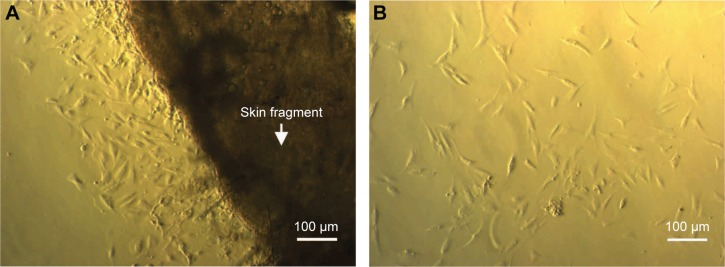
Fat was eliminated from skin tissue and cut into small pieces with sharp scissors. Skin fragments were then cultured for 10 days. At days 9–10, cells had spread out from the edge of the skin fragment (Figure 1). At this time, cells grew slowly. At day 15, cells proliferated faster and spread out from the skin fragment and exhibited the elongated morphology of HFs. Cells from passage 4 or 5 were used for experiments.
Figure 1.
Isolation of fibroblasts from skin tissue.
Notes: Skin tissue after collection from hospital was cut into small pieces and laid on the surface of culture roux. After 10 days, cells were grown from the edge of the skin fragment (A). After 15 days, cells proliferated and spread out on the surface of culture roux (B).
Selectivity cytotoxicity of H. odorata extract on HepG2 but not HF
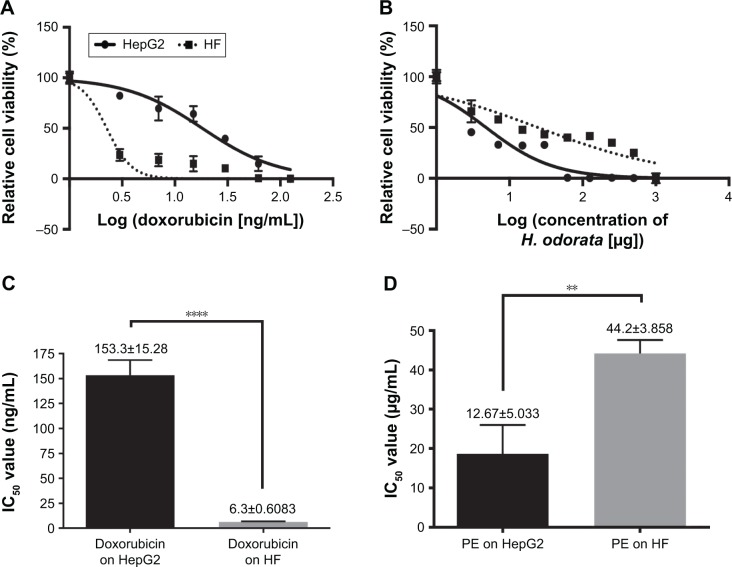
The mean IC50 values of doxorubicin on HepG2 and HF were 153.3±15.28 and 6.3±0.6 ng/mL, respectively. The IC50 values of H. odorata extract on HepG2 and HF were 12.67±5.03 and 44.2±3.85 µg/mL, respectively (Figure 2). We calculated the selectivity index (SI) of cytotoxicity of the agent by the ratio of IC50 value on HF (control cells) to that on HepG2 (cancer cells). The results showed that SI of H. odorata is 87-fold greater than SI of doxorubicin. H. odorata extract is less cytotoxic to HF than to HepG2 cells. Conversely, doxorubicin is more cytotoxic against HF than to HepG2.
Figure 2.
Cytotoxicity of doxorubicin and H. odorata PE on HepG2 and HF.
Notes: HepG2 and HF cells were treated with doxorubicin, from 0 ng/mL (untreated) to 125,000 ng/mL. Similarly, both of these cell lines were treated with H. odorata at concentrations of 0 µg/mL (untreated) to 2,000 µg/mL. After 48 hours of treatment, Alamar Blue was added. The fluorescence of cell samples was measured after 4 hours of incubation with dye. The experiment was conducted three independent times. (A) Inhibition curve of HepG2 and HF treated with doxorubicin for 48 hours. (B) Inhibition curve of HepG2 and HF treated with H. odorata extract for 48 hours. (C) IC50 value of doxorubicin on HepG2 and HF after 24 hours or 48 hours of treatment. (D) IC50 value of H. odorata extract on HepG2 and HF after 24 hours or 48 hours of treatment. Data shown as mean ± standard deviation. ****P<0.0001 for C and **P=0.0053 for D using t-test analysis of Graphpad Prism. Mean ± SEM, n=1 independent experiment for (A and B) and n=3 independent experiments for (C and D).
Abbreviations: HF, human fibroblast; H. odorata, Hopea odorata; PE, plant extract; SEM, standard error of the mean.
By that, SI of doxorubicin (SIdoxorubicin) is
SI of H. odorata (SIH. odorata) is
The ratio of (SIH. odorata) to (SIdoxorubicin) is
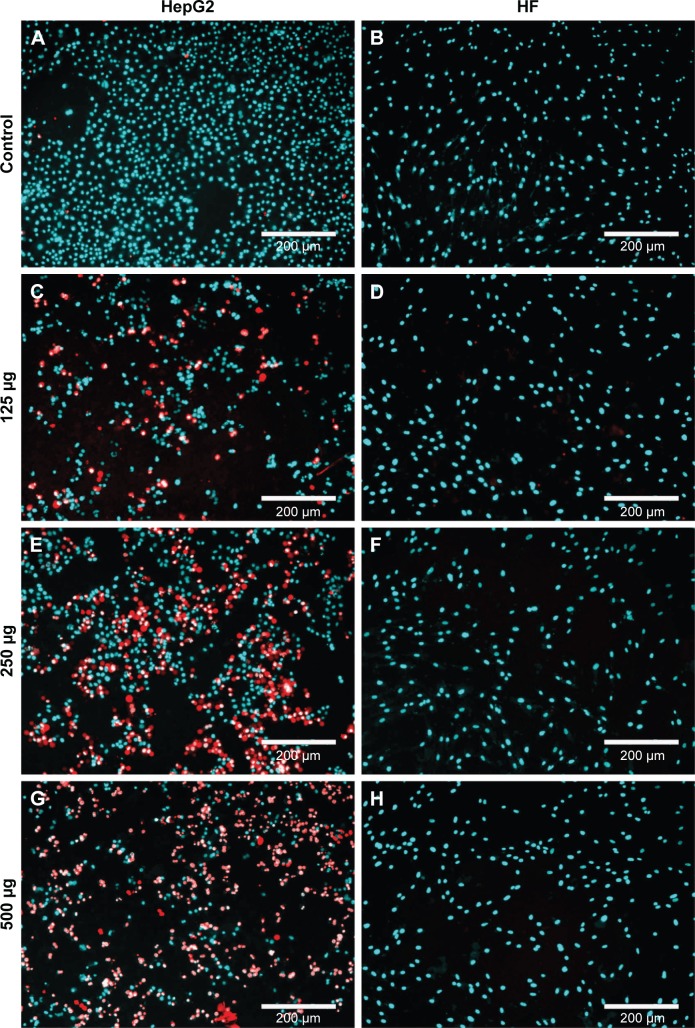
The SI of H. odorata extract was supported by the observation of dead cells by fluorescent microscopy. Dead cells were determined by PI-positive cells. Nuclei of total cells were visualized by Hoechst 33342 staining. The staining assays showed that H. odorata extract killed HepG2 cells in a dose-dependent manner (Figure 3). At concentrations of 60 µg/mL, 250 µg/mL, and 500 µg/mL of H. odorata extract, the percentage of cell death induced was approximately 20%, 30%, and 80%, respectively. However, no dead cells were observed for HFs even when the concentration of H. odorata extract was raised up to 500 µg/mL. According to our observations, while the H. odorata extract at a high concentration (eg, 500 µg/mL) did not kill HF cells, it appeared to reduce the proliferative capacity of HFs.
Figure 3.
Fluorescence of HepG2 and HF cells after 48 hours of H. odorata treatment.
Notes: HepG2 (A, C, E, G) and HF (B, D, F, H) cells were treated with H. odorata PE at concentrations of 500 µg/mL (G and H), 250 µg/mL (E and F), and 125 µg/mL (C and D). After 48 hours of treatment, cells were stained with fluorescent dye Hoechst 33342 and PI at final concentrations of 1 µg/mL for each dye and then incubated further for 10 minutes at 37°C, 5% CO2. Cells were then observed under a fluorescent microscope at magnification 5×. Hoechst 33342 was detected by DAPI filter, and PI was detected by PI filter.
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; HF, human fibroblast; H. odorata, Hopea odorata; PE, plant extract; PI, propidium iodide.
Using ImageJ, we calculated the ratio of red signal to blue signal to determine the ratio of dead cells to live cells (data not shown). Cell death of HepG2 increased in a dose-dependent manner after treatment with H. odorata extract. The percentage of dead HepG2 cells following treatment with H. odorata extract at 60 µg/mL, 250 µg/mL, and 500 µg/mL concentrations were approximately 20%, 25%, and 80%, respectively. Conversely, there were rarely any cell deaths of HF cells after treatment with H. odorata extract; even the dose of H. odorata extract was increased.
H. odorata extract-induced apoptosis of HepG2 cells
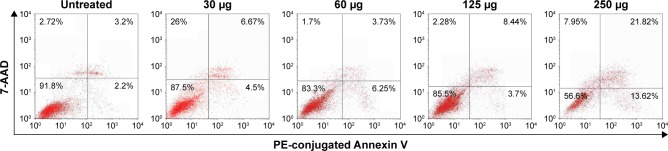
Following treatment of HepG2 cells with H. odorata at concentrations of 30 µg/mL, 60 µg/mL, 125 µg/mL, and 250 µg/mL, the percentage of apoptotic cells increased dose dependently (Figure 4). For instance, at 250 µg/mL concentration of extract, the percentage of dead cells significantly increased to 35.3%. Approximately 56% of cells remained alive, 13.6% of cells went on to early apoptosis, 21.8% of cells went into late apoptosis, and 7.9% of cells underwent necrosis.
Figure 4.
Flow cytometry analysis of treated HepG2 cells.
Notes: HepG2 were treated with H. odorata extract at concentrations of 30 µg/mL, 60 µg/mL, 125 µg/mL, and 250 µg/mL. After 48 hours, cells were stained with Annexin V/PI and incubated for 15 minutes. Cells were then analyzed by CellQuest Pro using a FACSCalibur system.
Abbreviations: H. odorata, Hopea odorata; PE, plant extract; PI, propidium iodide.
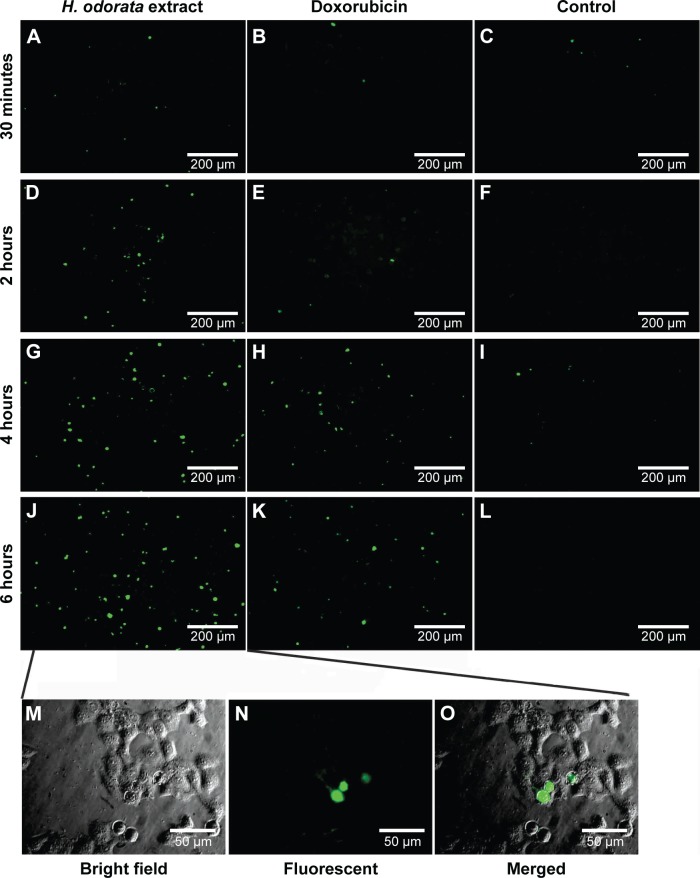
With caspase-3/7 green reagent staining, cells that underwent apoptosis appeared as green fluorescent signals under a fluorescent microscope. The results showed that HepG2 cells treated with the H. odorata extract at 250 µg/mL concentration induced apoptosis after 30 minutes of treatment (Figure 5A). The apoptotic signals increased as the time of incubation with H. odorata extract increased to 2 hours, 4 hours, and 6 hours (Figure 5D, F, G, and J). In the case of treatment with doxorubicin, there were no apoptotic signals after 30 minutes of treatment (Figure 5B). Apoptosis of doxorubicin-treated cells appeared after 4 hours and 6 hours of treatment (Figure 5E, H, and K). In those cells treated with 0.5% methanol, apoptosis was not observed at any of the time points (Figure 5C, F, I, and L). Apoptosis of HepG2 cells was discerned by shrinkage of cells into round shapes with unsmooth surfaces (Figure 5M–O). These observations indicate that H. odorata extract has the ability to induce apoptosis of HepG2 in a time-dependent manner through caspase-3/7 activation.
Figure 5.
The activation of caspase-3/7 in HepG2.
Notes: HepG2 cells were loaded with CellEvent™ caspase-3/7 green reagent at a concentration of 5 µM and then treated with either 250 µg/mL of H. odorata extract (A, D, G, and J), 100 mM of doxorubicin (B, E, H, and K), or 0.5% methanol solvent control (C, F, I, and L). HepG2 cells were treated for 30 minutes, 2 hours, 4 hours, or 6 hours. Cells were observed under fluorescent microscope for each time point. Untreated cells have minimal fluorescence, while treated cells show a significant increase in fluorescence (shown in green), indicating apoptosis (M, N, and O). H. odorata-treated cells were observed under a microscope after 6 hours of treatment at magnification 40×. Apoptotic HepG2 cells after treatment with H. odorata extract shrunk into a round shape. Unlike neighboring normal cells with smooth shape, the surface of apoptotic HepG2 cells was rough and shrunken.
Abbreviation: H. odorata, Hopea odorata.
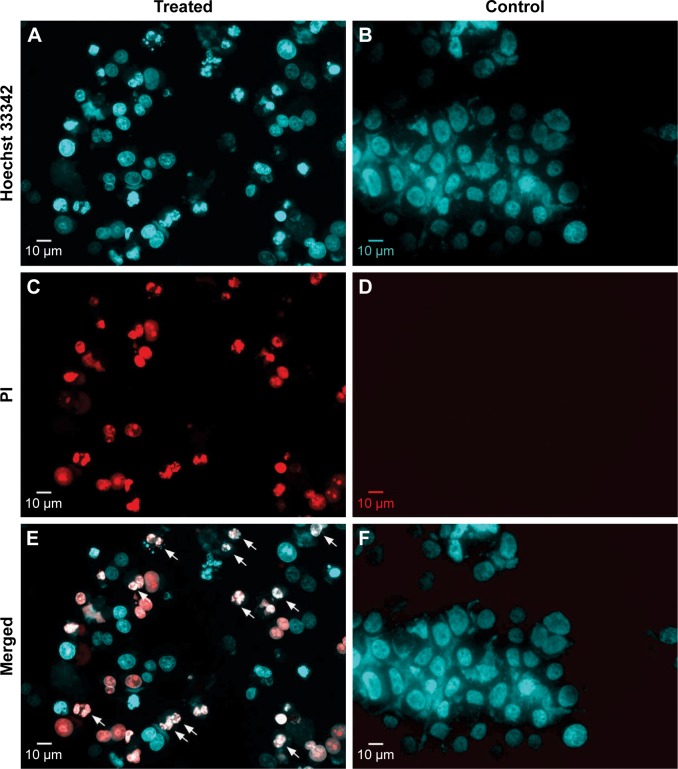
H. odorata extract (250 µg/mL) was used to treat HepG2 cells for 48 hours, and then, treated cells were stained with Hoechst 33342 to assess nuclei of all cells and with PI to assess nuclei of dead cells only. Control cells were treated with 0.5% methanol solvent, which is the same concentration of methanol solvent in H. odorata extract-treated samples. H. odorata-treated cells appear like dead cells with disintegration of nuclei (Figure 6A, C, and E). The cells also exhibited nuclei condensation and appeared to have brighter staining than the surrounding live cells. Conversely, control cells stained with Hoechst 33342 but not with PI; this means that there was no death cell in the control cells (HFs) when compared to treated HepG2 cells (Figure 6B, D, and F).
Figure 6.
Disintegrated nuclei of H. odorata extract-treated cells.
Notes: After treatment with H. odorata extract (250 µg/mL) for 48 hours, HepG2 cells were harvested and stained with Hoechst 33342 (A and B) and PI (C and D) at a final concentration of 1 µg/mL for each dye. Nuclei of treated cells and control cells were then observed under a fluorescent microscope. Disintegrated nuclei are indicated with white arrows (E and F).
Abbreviations: H. odorata, Hopea odorata; PI, propidium iodide.
Discussion
Chemotherapy, radiation therapy, and surgery have been used for cancer treatment as a standard. However, these therapies have not been fully effective and the side effects are of concern. Most antitumor agents currently used in chemotherapy are toxic to normal cells and cause weakening of the immune system. Therefore, there is an increased demand to seek out new anticancer agents with lower side effects than those of current agents.
Herbal medicines have been used in many cultures for thousands of years. In fact, many plant-derived compounds can cure health problems, such as inflammation, virus-related disease, or even cancer. Natural resources have become increasingly popular to study. Many current studies seek the identification of new anticancer drugs from natural plants. In this study, we aimed to investigate the in vitro anticancer activity of methanolic extract of H. odorata against HepG2 cancer cell line.
The cytotoxicity effect of anticancer agents was measured from the activity of the reducing environment within the cytosol of the live cells. Resazurin, the active compound of Alamar Blue reagent, is blue in color and virtually non-fluorescent. Upon entering cells, resazurin is reduced to resofurin, which has a red color and is highly fluorescent. Inhibition effects can be assessed by change in the fluorescent intensity. The results showed that the methanolic extract of H. odorata showed selective inhibition toward HepG2 but not to HF as compared to doxorubicin. The selective index (SI) of H. odorata extract (SIH. Odorata) was 87-fold that of doxorubicin (SIDoxorubicin). This means that H. odorata inhibited HepG2 but not HF. Moreover, doxorubicin inhibited HF but not HepG2.
It is known that chemotherapy for cancer treatment causes side effects, such as hair loss, vomit, and nausea.22,23 The high and selective cytotoxicity of H. odorata extract against HepG2 but not HF makes the extract a potential agent for cancer therapy. Previous studies have demonstrated the selectivity effect of butanolic extract of heartwood of H. odorata against HepG2 cells but not against normal liver cells.24 The IC50 of H. odorata extract on HepG2 was 12.67±5 µg/mL, which is nearly similar to the IC50 of 20.14 µg/mL on HepG2 found in a previous report.24 Interestingly, our study evaluates methanolic extract (not butanolic extract) from stem bark (not heartwood) of H. odorata. This demonstrates that different parts of H. odorata plant possess potential anticancer activity.
We found that HepG2 cell death induced by H. odorata was due to the activation of apoptosis. One of the hallmarks of apoptosis is the exposure of phosphatidylserine (PS). Since PS has an affinity for Annexin V,25 we detected the apoptotic cells in our study by Annexin-positive labeling. By this principle, we found that HepG2 cells underwent apoptosis in a dose-dependent manner with increasing concentrations of H. odorata extract. At the 250 µg/mL concentration, apoptosis of cells (including early and late stages) could be observed in ~35% of cells. The ability of H. odorata extract to induce apoptosis was strongly established in this study. However, we did not distinguish which pathway of apoptosis was activated. Methanolic extracts of H. odorata bark have been shown to suppress the kinase activities of enzymes Src and Syk, resulting in reduced signaling of NF-kB activation in Raw264.7 cells.18 Other studies showed that the inhibition of NF-kB in HepG2 led to enhancement of caspase-3-dependent apoptosis.26,27
There are two known pathways of apoptosis. In the extrinsic apoptosis pathway, the binding of a ligand to a dead receptor initiates the activation of caspase-8. Active caspase-8 then activates executioner caspase-3/7. In the intrinsic apoptosis pathway, cellular stresses lead to cytochrome c release from mitochondria to form the apoptosome, resulting in the activation of caspase-9. Active caspase-9 then activates caspase-3/7. Therefore, caspase-3/7 is considered to be executioner caspase of both extrinsic and intrinsic apoptosis pathways. In this study, the activation of caspase-3/7 in HepG2 cells appeared in a time-dependent manner in the presence of H. odorata extract at 250 µg/mL concentration. The activation of executioner caspase-3/7 triggers a cascade of proteolytic events leading to apoptotic destruction of the cell, including nuclear degradation, which is regarded as one of the hallmarks of apoptosis.28
In the study herein, HepG2 cells showed disintegrated nuclei due to the presence of H. odorata extract for 48 hours. Apoptotic HepG2 cells exhibited a different cell shape (and mostly shrunken) and were seen floating/detached. Morphological changes indicate the cleavage of cytoskeleton downstream of caspase activation.29 Caspase-dependent cleavage of the components of the focal adhesion complex leads to detachment of apoptotic cells.30–32 Taken together, the study results demonstrate that the methanol extract of H. odorata possesses the ability to induce apoptosis of HepG2 cells. In this study, H. odorata methanol extract showed high cytotoxicity against HepG2 but not against control HFs, either alone or in comparison to doxorubicin. Therefore, compounds from H. odorata extracts may be useful as anticancer agents.
Conclusion
Traditional medicine has now become more important in the modern world. We found that methanolic bark extract of H. odorata, a plant commonly used for anti-inflammation purposes in Southeast Asia, has selective in vitro cytotoxicity against HCC (HepG2 cells) but not against HF cells. H. odorata extract has the ability to induce HepG2 cells into caspase-dependent apoptosis. Thus, H. odorata bark extract may have great potential as a therapeutic agent for HCC. Further studies are warranted to evaluate H. odorata bark-derived compounds as anticancer agents.
Acknowledgments
This work was supported by the Vietnam National University, Ho Chi Minh City, Vietnam, under grant A2015-18-01.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lewington A. Medicinal Plants and Plant Extracts: A Review of Their Importation into Europe. Cambridge, UK: Traffic International; 1993. [Google Scholar]
- 2.Tong W, Wang Q, Sun D, Suo J. Curcumin suppresses colon cancer cell invasion via AMPK-induced inhibition of NF-kappaB, uPA activator and MMP9. Oncol Lett. 2016;12(5):4139–4146. doi: 10.3892/ol.2016.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantara C, O’Connell M, Sarkar S, Moya S, Ullrich R, Singh P. Curcumin promotes autophagic survival of a subset of colon cancer stem cells, which are ablated by DCLK1-siRNA. Cancer Res. 2014;74(9):2487–2498. doi: 10.1158/0008-5472.CAN-13-3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buhrmann C, Kraehe P, Lueders C, Shayan P, Goel A, Shakibaei M. Curcumin suppresses crosstalk between colon cancer stem cells and stromal fibroblasts in the tumor microenvironment: potential role of EMT. PLoS One. 2014;9(9):e107514. doi: 10.1371/journal.pone.0107514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das SS, Nanda GG, Alone DP. Artemisinin and curcumin inhibit Drosophila brain tumor, prolong life span, and restore locomotor activity. IUBMB Life. 2014;66(7):496–506. doi: 10.1002/iub.1284. [DOI] [PubMed] [Google Scholar]
- 6.Purkayastha S, Berliner A, Fernando SS, et al. Curcumin blocks brain tumor formation. Brain Res. 2009;1266:130–138. doi: 10.1016/j.brainres.2009.01.066. [DOI] [PubMed] [Google Scholar]
- 7.Sung B, Chung HY, Kim ND. Role of apigenin in cancer prevention via the induction of apoptosis and autophagy. J Cancer Prev. 2016;21(4):216–226. doi: 10.15430/JCP.2016.21.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nabavi SM, Habtemariam S, Daglia M, Nabavi SF. Apigenin and breast cancers: from chemistry to medicine. Anticancer Agents Med Chem. 2015;15(6):728–735. doi: 10.2174/1871520615666150304120643. [DOI] [PubMed] [Google Scholar]
- 9.Rangarajan P, Subramaniam D, Paul S, et al. Crocetinic acid inhibits hedgehog signaling to inhibit pancreatic cancer stem cells. Oncotarget. 2015;6(29):27661–27673. doi: 10.18632/oncotarget.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhar A, Mehta S, Dhar G, et al. Crocetin inhibits pancreatic cancer cell proliferation and tumor progression in a xenograft mouse model. Mol Cancer Ther. 2009;8(2):315–323. doi: 10.1158/1535-7163.MCT-08-0762. [DOI] [PubMed] [Google Scholar]
- 11.Mathews-Roth MM. Effect of crocetin on experimental skin tumors in hairless mice. Oncology. 1982;39(6):362–364. doi: 10.1159/000225672. [DOI] [PubMed] [Google Scholar]
- 12.Gainer JL, Wallis DA, Jones JR. The effect of crocetin on skin papillomas and Rous sarcoma. Oncology. 1976;33(5–6):222–224. doi: 10.1159/000225150. [DOI] [PubMed] [Google Scholar]
- 13.Gutheil WG, Reed G, Ray A, Anant S, Dhar A. Crocetin: an agent derived from saffron for prevention and therapy for cancer. Curr Pharm Biotechnol. 2012;13(1):173–179. doi: 10.2174/138920112798868566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhanasekaran R, Limaye A, Cabrera R. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis, and therapeutics. Hepat Med. 2012;4:19–37. doi: 10.2147/HMER.S16316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen ML, Fang CH, Liang LS, Dai LH, Wang XK. A meta-analysis of chemotherapy regimen fluorouracil/leucovorin/oxaliplatin compared with fluorouracil/leucovorin in treating advanced colorectal cancer. Surg Oncol. 2010;19(1):38–45. doi: 10.1016/j.suronc.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Maor Y, Malnick S. Liver injury induced by anticancer chemotherapy and radiation therapy. Int J Hepatol. 2013;2013:815105. doi: 10.1155/2013/815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen T, Tran T, Nguyen M, Sierens T, Triest L. Genetic population of threatened Hopea odorata Roxb. in the protected areas of Vietnam. Journal of Vietnamese Environment. 2015;6(1):69–76. [Google Scholar]
- 18.Yang Y, Yu T, Lee YG, et al. Methanol extract of Hopea odorata suppresses inflammatory responses via the direct inhibition of multiple kinases. J Ethnopharmacol. 2013;145(2):598–607. doi: 10.1016/j.jep.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 19.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang XY, Zhang PY, Aboul-Soud MA. From inflammation to gastric cancer: role of Helicobacter pylori. Oncol Lett. 2017;13(2):543–548. doi: 10.3892/ol.2016.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korniluk A, Koper O, Kemona H, Dymicka-Piekarska V. From inflammation to cancer. Ir J Med Sci. 2017;186(1):57–62. doi: 10.1007/s11845-016-1464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warr DG. Chemotherapy- and cancer-related nausea and vomiting. Curr Oncol. 2008;15(suppl 1):S4–S9. doi: 10.3747/co.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poon KS, Un MK, Low XH, Cheung YT, Yap KY, Chan A. Impact of cancer-related fatigue on chemotherapy-induced nausea and vomiting in Asian cancer patients. Pharmacoepidemiol Drug Saf. 2013;22(12):1345–1351. doi: 10.1002/pds.3516. [DOI] [PubMed] [Google Scholar]
- 24.Norizah A, Zakiah I, Zurinah WN. An evaluation of the anticancer activity in Hopea odorata extracts. J Med Plants Res. 2012;6(8):1382–1388. [Google Scholar]
- 25.Boersma HH, Kietselaer BL, Stolk LM, et al. Past, present, and future of annexin A5: from protein discovery to clinical applications. J Nucl Med. 2005;46(12):2035–2050. [PubMed] [Google Scholar]
- 26.Wu LF, Li GP, Su JD, et al. Involvement of NF-kappaB activation in the apoptosis induced by extracellular adenosine in human hepatocellular carcinoma HepG2 cells. Biochem Cell Biol. 2010;88(4):705–714. doi: 10.1139/O10-008. [DOI] [PubMed] [Google Scholar]
- 27.Kim YS, Schwabe RF, Qian T, Lemasters JJ, Brenner DA. TRAIL-mediated apoptosis requires NF-kappaB inhibition and the mitochondrial permeability transition in human hepatoma cells. Hepatology. 2002;36(6):1498–1508. doi: 10.1053/jhep.2002.36942. [DOI] [PubMed] [Google Scholar]
- 28.Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res. 2000;45(3):528–537. doi: 10.1016/s0008-6363(99)00384-3. [DOI] [PubMed] [Google Scholar]
- 29.Mashima T, Naito M, Tsuruo T. Caspase-mediated cleavage of cytoskeletal actin plays a positive role in the process of morphological apoptosis. Oncogene. 1999;18(15):2423–2430. doi: 10.1038/sj.onc.1202558. [DOI] [PubMed] [Google Scholar]
- 30.Mian MF, Kang C, Lee S, et al. Cleavage of focal adhesion kinase is an early marker and modulator of oxidative stress-induced apoptosis. Chem Biol Interact. 2008;171(1):57–66. doi: 10.1016/j.cbi.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki H, Kotsuji F, Tsang BK. Caspase 3-mediated focal adhesion kinase processing in human ovarian cancer cells: possible regulation by X-linked inhibitor of apoptosis protein. Gynecol Oncol. 2002;85(2):339–350. doi: 10.1006/gyno.2002.6632. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Fernandez L, Cerezo-Guisado MI, Langmesser S, Bragado MJ, Lorenzo MJ, Garcia-Marin LJ. Cleavage of focal adhesion proteins and PKCdelta during lovastatin-induced apoptosis in spontaneously immortalized rat brain neuroblasts. FEBS J. 2006;273(1):1–13. doi: 10.1111/j.1742-4658.2005.05023.x. [DOI] [PubMed] [Google Scholar]