Abstract
The small eye suggests an apparently robust anatomy with a more resistant sclera, good trabecular function, good uvea trophicity, a healthy retina, with a full papilla. The volume of these eyes is small. Usually, the volume of the eye is related to the sagittal diameter of the eye. However, the volume of a sphere varies with the third power of the radius of the sphere. These small eyes have a volume smaller than their sagittal diameter suggests. In this volume, highly decreased develop certain anatomical components without having to keep proportions (lens, choroid), and some have a continuous growing volume (lens).
On long term, there is a balance inside these eyes despite a disproportion between their components. This internal disproportion inside the small eye can erupt through pressure differences between its structures: pupillary block, angle closure or a disproportionate response in case of typically uncomplicated surgery, which alters the apparent internal balance of these eyes.
Seemingly simple surgeries, such as phacoemulsification or filtering surgery can trigger storm (storms occur by differences in atmospheric pressure) with the following characteristics:
- intraocular “precipitation” in the form of uveal effusions, massive choroidal hemorrhage exudative retinal detachment, CME
- breaks in anatomical barriers, lens posterior capsular tear
- deviations courses: aqueous misdirection
Surgical operations on these eyes are like a dangerous storm surfing, with risks, incidents, with unpredictable but great experience and courage request.
Keywords: small eye, hyperopia, glaucoma, cataract, relative anterior microphthalmia
Hyperopic eye has an apparent allure of a robust anatomical structure with thicker sclera, voluminous choroid, with a good trabecular filtration with retina without vulnerable areas and full papilla (often-physiological excavation absence).
From the early childhood, the hyperopic eye has intrinsic accommodative possibilities of compensating its refractive errors, providing accommodative resources in excess of its needs, however being able to reach ametropic amblyopia. Requiring excessive accommodative efforts, small eye trains excessive convergence and leads to esotropia or esophoria, affecting the binocularity.
When these are added to certain degrees of hyperopic anisometropia, the risk of unilateral amblyopia increases greatly.
This is the paradox of hyperopic eye. Behind such a robust anatomy, some ocular structures (choroid, lens) in the small eye do not meet certain normal harmony with their normal development in a small space, and there is an increased pressure gradient between the anterior and posterior segment.
This makes the depth of the AC lower in these eyes, together with any intervention that decompress the AC. Moreover, paracentesis and filtering operations can trigger a chain of events that may cause an unfavorable traveled surgical act. There are several types of small eyes.
Nanophthalmia
Nanophthalmic eye has its sagittal diameter of less or equal to 20mm, proportional and with normal functions. There is a nanophthalmia associated with systemic manifestations and one insolated. Isolated forms can be genetically transmitted autosomal dominant or recessive. Genetic determinism of nanophthalmia seems to be related to the synthesis of a protein called MFRP (Frizzled Related Protein Membrane - type), located in the apical portion of the retinal pigment epithelium cells [1,2]. It seems to be involved in the normal development of the eye at birth and in the subsequent emmetropization by increasing its axial length.
Nanophthalmic eye (Fig. 1) has high degrees of hyperopia (+ 25D to + 8D), small corneal diameter, shallow anterior chamber, narrow angle, lens with normal or increased volume, located in a low-volume eye. In these eyes, sclera and the choroid are thicker than normal and this predisposes to redoubtable intraoperative risks, large suprachoroidal hemorrhages, and prolonged athalamia.
Fig. 1.
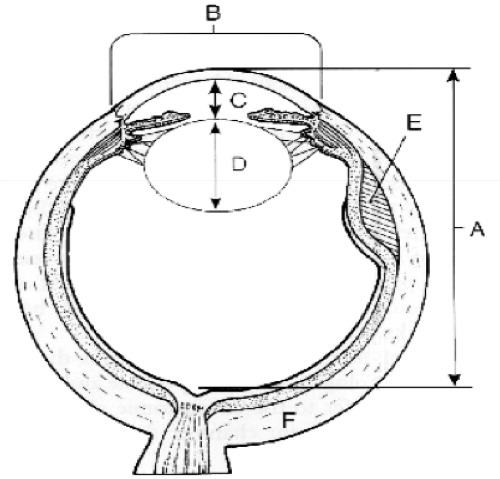
Nanophthalmic eye. A. Sagittal diameter <20mm, B. Small corneal diameter, C. Shallow, D. Normal/ increased lens volume, E. uveal effusion, F. thick sclera
In these first years of life, these eyes can have an affected visual acuity with monocular or binocular amblyopia, or strabismus [3]. Nanophthalmic eyes in childhood predisposes to the appearance of recurrent blepharitis, strabismus or amblyopia. An optimal optical correction and prevention of amblyopia retinoscopia with cycloplegic exam and paying attention to the accommodative amplitude of these eyes is generally recommended: nanophthalmic eyes may have incomplete accommodative amplitude requiring bifocal correction.
In adulthood, a number of complications can seriously threaten the visual function by angle closure glaucoma, exudative retinal detachment, CMA, or surgical complications [4].
The CT scan shows the thickening of the macular region of these eyes, the absence of the physiological excavation or the retinal folds. Any maneuver that suddenly lowers the eye pressure, even a simple peripheral iridotomy, can trigger a uveal effusion and could evolve into retina take off, choroidal hemorrhage or malignant glaucoma.
Lens surgery on the nanophthalmic eyes
In the pre phacoemulsification era, lens extraction in nanophthalmic eyes has had catastrophic results [5].
In these eyes, phacoemulsification continues to be a great risk surgery that requires a special preoperative, intraoperative, and postoperative approach.
Preoperative problems
IOL power value assessment has difficulties in these eyes, in which specific formulas such as Hoffer Q and Holladay 2 , have to be used.
In addition to the difficulties regarding the calculation of IOL power in these eyes, ax errors have big consequences on refractive errors [5,6].
If a normal eye has a sagittal diameter of 22,5-24mm, an error of 1mmin measuring the sagittal axis is expressed through a 3D refractive error, in one eye less than 22mm, errors of 1mm could be expressed by an error refractive 4-5D.
In addition, the estimation of the effective lens position is difficult in these cases, when a lens thickness of 4mm is replaced with a 1mm one. Often, these are amblyopic eye and the patient should be aware of both the surgery risks and the preoperative realistic visual outcomes.
IOL values can reach 40-50D. These values cannot be provided by a single IOL, therefore will use a second IOL. The most powerful IOL available will be placed in the bag, and the residual dioptric power will require IOL in sulcus in accordance with piggyback formulas lens calculation [7].
The situation changes because complications (tear of capsulorhexis or posterior capsule) cannot place the IOL in the bag. Placing the lens in the sulcus can change the dates of the final IOL power. One solution would be the placing of the second lens in a later stage after evaluating the results of the first refraction surgery. The preoperative reducing of the ocular pressure to reduce back pressure and the risk of shallow AC or iris, prolapses after the first incision (acetazolamide or mannitol infusion of 15-30 minutes before surgery) [8,9].
Topical anesthesia is preferable but a good akinesia is useful to relieve eyelid pressure.
Retrobulbar anesthesia should be avoided to rule out the risk of retrobulbar extra pressure. It is recommended to apply the Honan balloon a few minutes after the anesthetic.
Anterior chamber evaluation
If the AC is shallow, surgery can start with a pars plana anterior vitrectomy through the trocar placement, 3,25-3,3mm from limb [10]. These eyes have a very short pars plana, but the lens has a normal size or even larger than normal so, vitreo tip will be directed toward the papilla.
Vitrectomy reduces vitreous volume, deepens CA, and allows a capsulorhexis without a risk of endothelial damage and capsulorhexis tear.
Intraoperative difficulties and precautions
If lens surgery is not started with vitrectomy, corneal incisions will be made more attentively according to wound construction and small incision length, so as to provide a stable AC during surgery. AC is filled with viscoelastic or can use a maintainer of AC connected to the BSS bottle, adjusting a bottle of ascension in relation with the AC deep, allowing a sufficient space to perform a rhexis without any risk. Anterior chamber depths must be monitored all the time. They should be restored immediately when they are shallow or a possible uveal effusion should be discovered. Inserting or taking the tools from the anterior chamber will be done with great caution, each time making sure that the anterior chamber depths are restored.
If during the operation, the anterior chamber is shallow, a uveal effusion can occur.
Any drop in pressure in the anterior chamber may favor the serous or hemorrhagic choroid detachment. A guided positioning of the scleral incisions to depress the eye becomes necessary and useful in the imminent uveal effusion. Some authors generally recommended a prophylactic four scleral incision performance in the four areas when phacoemulsification was present in nanophthalmos eyes.
With these safeguards in these cases, phacoemulsification favorable results declined in recent years. The favorable results are cited in literature after phacoemulsification in nanophthalmic eyes [10,11].
Cataract surgery is very stressful in these situations, which sometimes can finish dramatically by expulsive choroid hemorrhage or malignant glaucoma.
The discovery of intraoperative uveal effusions or intraoperative choroid hemorrhage needs a prompt and quick attitude of the surgeon, who will have to locate the maximal choroid detachment place.
Then, the suprachoroidal liquid will be drained by scleral incisions placed 4-5mm from limb in a quadrant where the choroid is maximally bulging.
Postoperative complications
Even in cases in which surgery occurred successfully, a postoperative EMC may occur. The scleral thickness, which is more pronounced in these eyes, may frequently predispose to uveal effusion.
Sometimes, the uveal effusion may be accompanied by a retinal exudative detachment [12].
The exudative retinal detachment can occur in these eyes after cataract surgery, after filtering surgery or after laser peripheral iridotomy. Anti-inflammatory treatment, or, in case of failure, scleral incisions with suprachoroidal fluid drainage, can resolve these cases [12].
In these eyes, cataract surgery is a challenge and supports extensions from the anterior vitrectomy that may precede surgery to the necessary scleral incisions.
Filtering surgery nanophthalmic eye
Angle closure may be expressed clinically as acute angle closure or chronic angle closure glaucoma. In chronic forms, there can be large IOP fluctuations [13,14].
Nanophthalmic eye responds poorly to topical anti glaucoma treatment. Miotics may worsen the pupillary block by relaxing the zonula. LPI can remove some of the pupillary block and AL iridoplasty is recommended when LPI does not open the closed appositional angle.
There is an angle closure in these eyes by pushing the root of the iris by the ciliary body. These eyes can have a circular ciliary body detachment and choroidal effusion that can push forward and rotate the ciliary body.
After the appearance of PAS, LPI has no effect. In this stage, even transparent lens extraction may be used.
Nanophthalmic eye decompression shows an increased risk of serous or hemorrhagic choroidal detachment with a shallow anterior chamber and a postoperative ocular hypotonia, which can lead to the flattening of the filter bubble and finally to the filtration failure.
The shallow anterior chamber accompanied by postoperative ocular hypertonia can be an expression of malignant glaucoma. In these situations, the nanophthalmic eye conformation allows a cilio-lenticular block with aqueous misdirection. The treatment consists of drugs that lower the aqueous production, cycloplegics, and multiple LPI.
In case of failure of medical treatment, a surgical peripheral iridectomy guided by an UBM exam can be used. It will be placed near the place in which the aqueous humor collection is present in vitreous. By making an anterior vitrectomy, thereby this surgical iridectomy, the collection of aqueous humor misdirected in vitreous can be reached.
When this procedure fails, transparent lens extraction can be practiced. In this case, phacoemulsification is performed on one eye with a shallow AC or athalamia, an ocular hypertension, in which there is a great risk of corneal endothelium damage, and a great risk of expulsive hemorrhage.
In these cases, it is recommended that the surgical treatment of glaucoma generally starts even with a clear lens extraction, not having to resort to lens extraction under more dangerous conditions (Fig. 2).
Fig. 2.
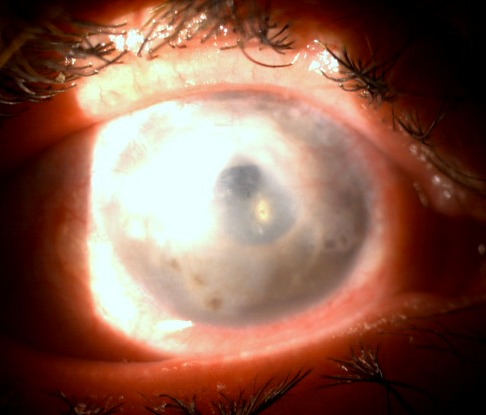
Prolonged athalamia after a filtering surgery for angle closure glaucoma in the hyperopic eye. The corneal damage persists after lens extraction
Clear lens extraction is not performed as a first option, only if laser peripheral iridotomy plus maximum tolerated topical treatment is not controlled by IOP. The role of the lens in angle closure for these eyes has to be documented by UBM examination, anterior segment OCT and biometry [15-17].
Although the lens has major implications in the etiology and mechanism of angle closure, clear lens extraction surgery is risky and often avoided. However, in these cases, lens extraction releases pupillary block, open the angle and, if the trabeculae did not suffer too much phacoemulsification by itself, can provide a good control of IOP [18,19]. The condition is to be done before extended PAS has occurred or the trabeculae were severely impaired and it will not be accompanied by intra and postoperative events, such as athalamia, malignant glaucoma, and expulsive hemorrhage.
Hyperopia
The eye has a proportional development of anterior and posterior segments and an axial length of 20-22mm. These eyes keep the nanophthalmic eye characters more or less accentuated, depending on how close the dimensions from those of the nanophthalmic eye are.
Fig. 4.
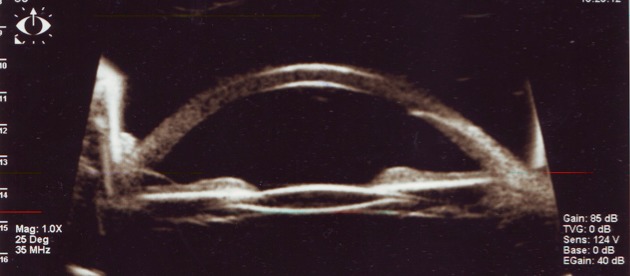
The eye in Fig. 3 - aspect of the angle after phacoemulsification
Relative Anterior Microphthalmia (RAM)
In these cases, the anterior segment is smaller than normal and the posterior segment has normal dimensions (Fig. 2).
Fig. 5.
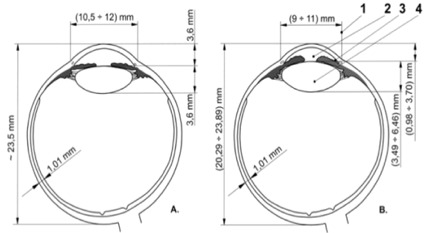
Relative anterior microphthalmia (B) compared with normal eye (A) shows: 1-corneal diameter < 11,5mm, 2 - shallow AC superficial, 3 - normal sagittal axis of the lens. Eye sagittal axis of 20,5mm-23mm. Posterior segment has normal dimensions
These eyes have a smaller corneal diameter but with a relatively normal posterior segment, so, they may have a low degree hyperopia or even a small myopia. Even the lens has normal dimensions; it is large relatively to the size anterior segment. These eyes can develop the most insidious forms of angle closure glaucoma, especially when you have a small myopia [20]. Narrow-angle glaucoma often progresses rapidly to advanced stages, and conventional treatment (iridotomy) does not open the angle.
Fig. 6.
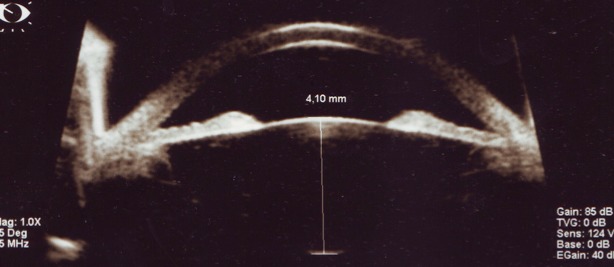
Relative anterior microphthalmia – lens sagittal diameter of 4,10mm
The lens may have a normal volume but its relative size is much higher compared to the anterior segment crowded space. This phenomenon makes the volume of the lens play a role in angle closure.
Being aware of the continuous increase in the lens volume by adding new fibrils, this report may increase with age and so does the role of the lens angle closure mechanisms.
The UBM exam and anterior chamber OCT can quantify the size of the lens and its relations with the anterior chamber structures.
Relative Posterior Microphthalmia (RPM)
These eyes have a normal development of the anterior segment but with an abnormal development of the posterior segment [21] (Fig. 3).
Fig. 3.
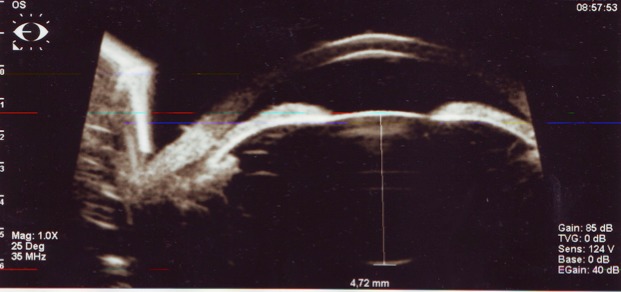
Hyperopic eye. Sagittal lens diameter of 4,72mm, sagittal ax of the eye of 20,58mm. Angle closure glaucoma
There is a discrepancy between the sclera development at this level, whose growth is slow, it is thicker and the retina develops normally but in a small space. This leads to the development of pa pillomacular retinal folds [22] at high-grade hyperopia and the difficulty in IOL power evaluation in cataract surgery [23,24].
Paradoxically, these small eyes with high degree hyperopia do not develop angle closure.
Fig. 7.
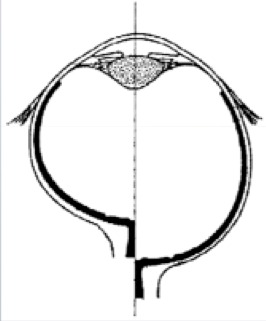
Relative posterior microphthalmia
Conclusion
Small eyes have a volume smaller than their sagittal diameter suggests. In this volume, highly decreased develop certain anatomical components without having to keep proportions (lens, choroid), and some have a continuum growing volume (lens).
There is a balance inside these eyes on long term, despite a disproportion between theirs components. This internal disproportion inside the small eye can erupt through pressure differences between its structures: pupillary block, angle closure or a disproportionate response in case of typically uncomplicated surgery, which alters the apparent internal balance of these eyes.
Seemingly simple surgeries, such as phacoemulsification or filtering surgery can trigger storm (storms occur by differences in atmospheric pressure) with the following outcomes:
- intraocular “precipitation” in the form of uveal effusions, massive choroidal hemorrhage exudative retinal detachment, CME
- breaks in anatomical barriers, lens posterior capsular tear
- deviations courses: aqueous misdirection.
Surgical operations on these eyes are like a dangerous storm surfing, with risks, incidents, unpredictable but with great experience and courage request.
Small eyes, such as simple nanophthalmia or hyperopia, or those with a relative anterior microphthalmia, may require lens surgery due to its opacity. Sometimes, lens surgery is performed for reasons of angle closure, or angle closure glaucoma. There may be situations when it is compulsory to make a transparent lens extraction as a last resort treatment for narrow-angle glaucoma in small eyes.
Lens extraction in these eyes is a stressful operation that requires a good experience surgery and surgery itself can be one extended beyond the initial intentions: from the possible commencement of the operation with an anterior vitrectomy, until the end of the surgery, by scleral incisions to remove the suprachoroidal fluid.
Complications can be extended during the postoperative period with prolonged choroidal detachment or exudative retinal detachment.
Filtering surgery on small eyes is more risky due to the emphasizing of the pressure gradient between the posterior segment and the previous one, with sometimes-dramatic developments to a hypotensive athalamia (by uveal effusion) or a hypertension athalamia (by aqueous misdirection).
Sometimes transparent lens extraction may be used in small eyes as a last resort surgical opening angle. This can make it a more effective topical treatment, and in some cases, it can even control its own PIO.
The lower the eye, the more the filtering cataract surgery or more complex intra and postoperative complications can be riskier.
Small eye is a small stump, which can challenge and tilt a great surgery.
References
- 1.Sundin OH, Leppert GS, Silva ED, et al. Extreme hyperopia is the result of null mutations in MFRP, which encodes a frizzled-related protein. Proc Natl Acad Sci. 2005 doi: 10.1073/pnas.0501451102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sundin OH, Dharmaraj S, Bhutto IA, et al. Developmental basis of nanophthalmos: MFRP is required for both postnatal ocular growth and postnatal emmetropization. Ophthalmic Genet. 2008;19(1):1-9.02:9553–9558. doi: 10.1080/13816810701651241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sener EC, Mocan MC, Sarac OI, Gedik S, Sanac AS. Management of strabismus in nanophthalmic patients. A long-term follow-up. Ophthalmology. 2003;110:1230–1236. doi: 10.1016/S0161-6420(03)00267-7. [DOI] [PubMed] [Google Scholar]
- 4.Singh OS, Simmons RJ, Brockhurst RJ, Trempe CL. Nanophthalmos: a perspective on identification and therapy. Ophthalmology. 1982;89(9):1006–1012. [PubMed] [Google Scholar]
- 5.Steijns D, Bijlsma W, Van der Lelij A. Cataract Surgery in Patients with Nanophthalmos. Ophthalmology. 2012:1–5. doi: 10.1016/j.ophtha.2012.07.082. [DOI] [PubMed] [Google Scholar]
- 6.Wu W, Dawson D, Sugar A, et al. Cataract surgery in patients with nanophthalmos: results and complications. J Cataract Refract Surg. 2004;30(3):584–590. doi: 10.1016/j.jcrs.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Cao KY, Sit M, Braga-Mele R. Primary piggyback implantation of 3 intraocular lenses in nanophthalmos. J Cataract Refract Surg. 2007 Apr;33(4):727–730 . doi: 10.1016/j.jcrs.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 8.Day AC, MacLaren RE, Bunce C, et al. Outcomes of phacoemulsification and intraocular lens implantation in microphthalmos and nanophthalmos. J Cataract Refract Surg. 2013;39:87–96. doi: 10.1016/j.jcrs.2012.08.057. [DOI] [PubMed] [Google Scholar]
- 9.Olson RJ, Jin GJ, Ahmed IK, Crandall AS, Cionni RJ, Jones JJ. Cataract surgery from routine to complex: A practical guide. Chapter 11: Nanophthalmos. SLACK Incorporated, Thorofare NJ; 2011. [Google Scholar]
- 10.Steijns D, Bijlsma WR, Van der Lelij A. Cataract surgery in patients with nanophthalmos. Ophthalmology. 2013;122:266–270. doi: 10.1016/j.ophtha.2012.07.082. [DOI] [PubMed] [Google Scholar]
- 11.Faucher A, Hasanee K, et al. Phacoemulsification and introcular lens implantation in nanophthalmic eye: report of medium size series. J Cataract Refractive Surgery. 2002;28 doi: 10.1016/s0886-3350(01)01161-0. [DOI] [PubMed] [Google Scholar]
- 12.Krohn J, Seland JH. Exudative retinal detachment in nanophthalmos. Acta Ophthalmol Scand. 1998;76:499–502. doi: 10.1034/j.1600-0420.1998.760421.x. [DOI] [PubMed] [Google Scholar]
- 13.Yalvac IS, Satana B, Ozkan G, Eksioglu U, Duman S. Management of glaucoma in patients with nanophthalmos. Eye. 2008;22:838–843. doi: 10.1038/sj.eye.6702742. [DOI] [PubMed] [Google Scholar]
- 14.Burgoyne C, Tello C, Katz LJ. Nanophthalmia and chronic angle closure glaucoma. J Glaucoma. 2002;11:525–528. doi: 10.1097/00061198-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Yip LW, Aquino MC, Chew PT. Measurement of anterior lens growth after acute primary angle-closure glaucoma. Can J Ophthalmol. 2007 Apr;42(2):321, 322. [PubMed] [Google Scholar]
- 16.Wang BS, Narayanaswamy A, Amerasinghe N, Zheng C, He M, Chan YH, et al. Increased iris thickness and association with primary angle closure glaucoma. Br J Ophthalmol. 2010 Jun 7; doi: 10.1136/bjo.2009.178129. [DOI] [PubMed] [Google Scholar]
- 17.Tham CC, Kwong YY, Leung DY, et al. Phacoemulsification vs. phacotrabeculectomy in chronic angle-closure glaucoma with cataract: complications. Arch Ophthalmol. 2010;128:303, 311. doi: 10.1001/archophthalmol.2010.12. [DOI] [PubMed] [Google Scholar]
- 18.Tham CC, Leung DY, Kwong YY, et al. Effects of phacoemulsification versus combined phaco-trabeculectomy on drainage angle status in primary angle closure glaucoma (PACG) Glaucoma. 2010;19:119–123. doi: 10.1097/IJG.0b013e31819d5d0c. [DOI] [PubMed] [Google Scholar]
- 19.Tham CC, Kwong YY, Baig N, et al. Phacoemulsification versus trabeculectomy in medically uncontrolled chronic angle-closure glaucoma without cataract. Ophthalmology. 2013;120:62–67. doi: 10.1016/j.ophtha.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Buckley SA, Reeves B, Burdon M, et al. Acute angle closure glaucoma: relative failure of YAG iridotomy in affected eyes and factors influencing outcome. Br J Ophthalmol. 1994;78(7):529–533. doi: 10.1136/bjo.78.7.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spitznas M, Gerke E, Bateman VB. Hereditary posterior microphthalmos with papillomacular fold and high hyperopia. Arch Ophthalmol. 1983;101:413–417. doi: 10.1001/archopht.1983.01040010413014. [DOI] [PubMed] [Google Scholar]
- 22.Feledelius H, Rosenberg T. Extreme hypermetropia and posterior microphthalmos in three siblings. An oculometric study. in: Ossoinig KC. Ophthalmic Echography. Nijhoff M, Junk W. Doc Ophthalmol Proc Ser. 1987:89–91,48. [Google Scholar]
- 23.Meire F, Leys M, Boghaert S, De Laey JJ. Posterior microphthalmos. Bull Soc Belge Ophtalmol. 1989;231:101–10615. [PubMed] [Google Scholar]
- 24.Kharillah M, Messaoud R, Zaouali S, Ben Yahia S, Ladjimi A, Jenzri S. Posterior segment changes associated with posterior microphthalmos. Ophthalmology. 2002;109:569–574. doi: 10.1016/s0161-6420(01)00996-4. [DOI] [PubMed] [Google Scholar]


