Abstract
Purpose: To report the case of a 14-year-old male patient, with bilateral atopic keratoconjunctivitis with corneal ulcer.
Methods: The patient complained of bilateral red, itchy eyes, decreased vision, photophobia, difficulty opening the eyelids upon awakening, palpebral edema, excessive tearing, along with yellowish mucous discharge. He had a two-year history of chronic blepharitis and recurrent episodes of conjunctivitis that were treated with Tobramycin and corticosteroid eye drops over the years. The patient’s past medical history was significant for atopic dermatitis (AD) and he had a family history for atopy. At the eye exam: his best-corrected visual acuity at the initial presentation was 0.2 in the right eye and 1.0 in the left eye. The following elements were found upon the slit lamp biomicroscopy: Eyelids - +4 palpebral edema (pseudoptosis), Dennie-Morgan fold and Herthoge’s sign were both present, tylosis; Conjunctiva - hyperaemia, cobblestone appearance of the tarsal papillae in both eyes, +2 chemosis; Cornea - corneal edema with a 8 mm × 4 mm epithelial defect in the inferior part of the cornea, covered partially by the lied, that stained positive with fluorescein dyes. Using the Evaluation Signs Severity for Allergic Ocular Diseases, a diagnosis of bilateral atopic keratoconjunctivitis with a grade 3 status for the right eye and a grade 2 status, was made. It was decided that he should be administered Olopatadine hydrochloride and Sodium cromoglicate eye drops, along with Moxifloxacin and steroid eye drops. The microbiological exam tested positive for staphylococcus aureus, and, based on the sensitivity pattern, Chloramphenicol eye drops had to be added to the treatment. After 2 weeks, his symptoms diminished, pain was significantly relieved and inflammation was markedly reduced, but the corneal ulcer persisted. In order to prevent corneal perforations, amniotic membrane transplantation (AMT) was used to promote epithelialization.
Results: A month later, the epithelial defect healed smoothly in an underlying vascular stromal scar and the visual acuity improved to 0.4 RE.
Conclusions: This case demonstrated the role of patient history and close clinical obser-vation in the diagnosis of AKC. As this case showed, the use of topic medication along with amniotic membrane transplantation (AMT) was successful in the treatment of atopic keratoconjunctivitis and secondary staphylococcal aureus keratitis.
Keywords: atopic keratoconjunctivitis, staphylococcus aureus keratitis, amniotic membrane transplantation (AMT)
Introduction
Atopic keratoconjunctivitis (AC) is an inflammation of the cornea and conjunctiva secondary to an immune response to external antigens. This inflammation is Ig E mediated and atopy can play a significant role in the clinical evolution. AC is in fact a syndrome affecting the entire ocular surface, including conjunctiva, lids, cornea, and tear film. The signs and symptoms of atopic keratoconjunctivitis have a meaningful effect on the patient’s quality of life and health, and are influenced by genetics, environment, immune regulation mechanisms, and ocular microbiota, all of which work together in a complex immunological response.
Case presentation
A 14-year-old male patient, with a two-year history of chronic blepharitis and recurrent episodes of conjunctivitis presented to our hospital. The patient complained of bilateral red, itchy eyes, decreased vision, photophobia, difficulty opening the eyelids upon awakening, palpebral edema, excessive tearing, along with yellowish mucous discharge. Tobramycin and corticosteroid eye drops have been prescribed over the years. This particular episode was severe and unremitting with his usual medication, hence the referral, making it impossible for the patient to go to school for 2 months.
The patient’s past medical history was significant for atopic dermatitis (AD) and he had a family history for atopy. According to the parents, the AD onset was at the age of 9. Previously, the patient underwent a Prick test that came positive against a panel of commonly occurring antigens. At presentation, his dermatological medications included systemic second-generation antihistamine and topic I-Modulia based medication. No other systemic diseases have been reported.
The general physical examination disclosed eczema of the face and chest.
His best-corrected visual acuity at the initial presentation was 0.2 in the right eye and 1.0 in the left eye. Intraocular pressures (IOP) were 15 mmHg in the right eye and 14 mmHg in the left eye.
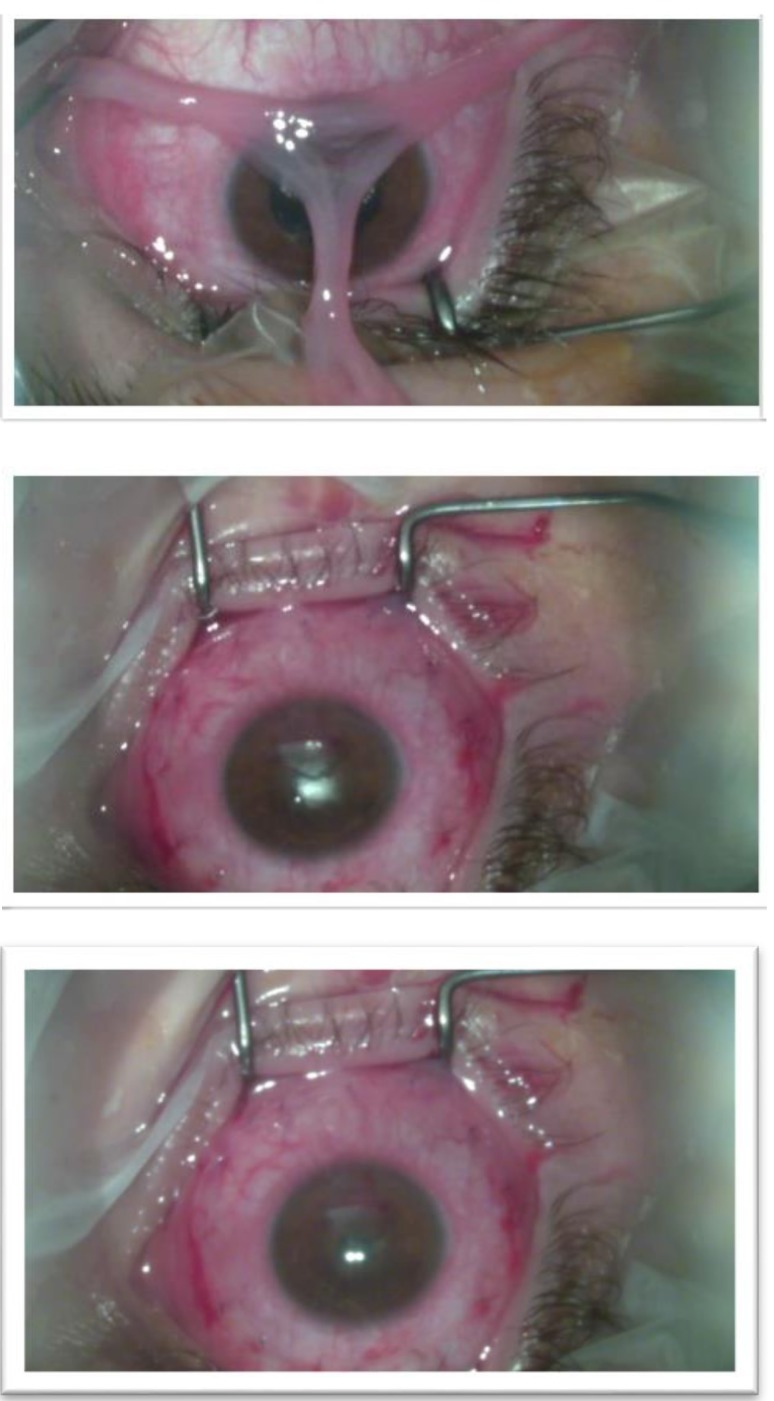
Regarding the slit lamp biomicroscopy, the following elements were found: Eyelids - +4 palpebral edema (pseudoptosis), Dennie-Morgan fold and Herthoge’s sign were both present, tylosis; Conjunctiva - hyperaemia, cobblestone appearance of the tarsal papillae in both eyes (Fig. 1) + 2 chemosis; Cornea - corneal edema with a 6 mm × 4 mm epithelial defect in the inferior part of the cornea, covered partially by the lied, that stained positive with fluorescein dyes (Fig. 2).
Fig. 1.
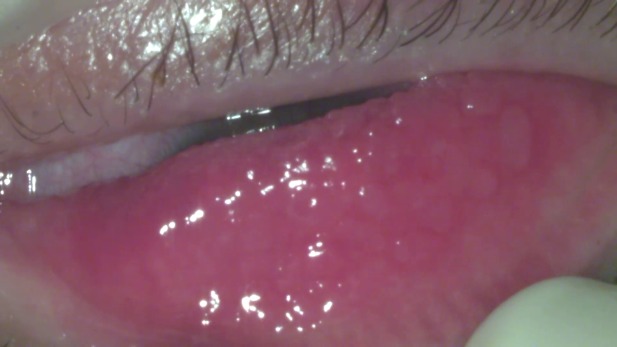
Cobblestone appearance of the tarsal papillae in both eyes
Fig. 2.
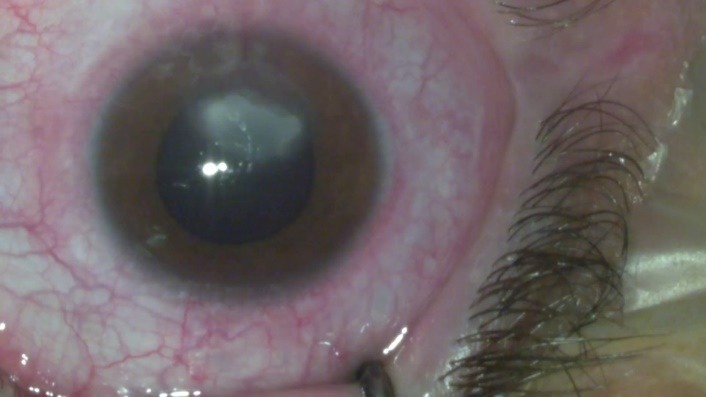
6mm x 4mm epithelial defect in the inferior part of the cornea
The ocular fundus examination could not be performed in the right eye due to the corneal edema and ulcer. The left fundus was normal.
Serological test (CBC) reveled hypereosinophilia, neutrophilia, monocytosis, increased levels of CRP, ECR and haemoglobin, lymphocytopenia.
Using the Evaluation Signs Severity for Allergic Ocular Diseases, a diagnosis of bilateral atopic keratoconjunctivitis with a grade 3 status for the right eye and a grade 2 status was made.
It was decided that he should be administered Olopatadine hydrochloride and Sodium cromoglicate eye drops, along with Moxifloxacin and steroid eye drops.
Conjunctival swab samples were collected. The microbiological exam tested positive for Staphylococcus Aureus, so an antibiogram was also demanded. Staphylococcus came susceptible to Chloramphenicol, Gentamicin, and Ofloxacin. Based on the sensitivity pattern of S. aureus to the following antibiotics Chloramphenicol (S=94.7%) (R=5.3%), Gentamicin (S=76.6%) (R=23.4%), Ofloxacin (S=69.1%) (R=30.9%) [1], it was decided to add Chloramphenicol eye drops to the treatment.
After 2 weeks of treatment, his symptoms diminished, pain was significantly relieved and inflammation was markedly reduced, but the corneal ulcer persisted.
It was also decided to use amniotic membrane transplantation (AMT) to promote epithelialization and to prevent corneal perforations (Fig. 4). Previously, a complete de-epithelization was performed so the corneal antigens loading decreased and a uniform re-epithelization could to be achieved (Fig. 3).
Fig. 4.
Amniotic membrane transplantation (AMT)
Fig. 3.

Complete de-epithelization
On the next follow-up, 1 month later, the epithelial defect healed smoothly in an underlying vascular stromal scar and the visual acuity improved to 0.4 RE, allowing him to continue his studies (Fig. 5). Further, the patient continued the Olopatadine hydrochloride and Sodium cromoglicate medication and the steroid eye drops for only for 2 weeks.
Fig. 5.

Postoperative follow-up, 1 month
Discussion
Background
Atopy affects 5-20% of the general population. Atopic keratoconjunctivitis occurs in 20-40% of the individuals with atopic dermatitis [2]. Although it can occur at any age, it is most common in patients between 3 to 25 years old, with 7 years as the average age of onset [3]. Regarding the role of genetic aspects in the pathogenesis of ACK, data showed that there is no simple correlation associated with a single gene defect or with the occurrence of its determined allelic form and the risk of contracting the disease.
It has been suggested that ACK represents a phenotypic model of overexpression of cytokine gene cluster on chromosome 5 q. This chromosomal area includes genes that regulate the expression of IL-3, IL-4, IL-5, and GM-CSF. The up-regulation of these factors is critical in modulating Th2 prevalence, Ig E production, as well as mast cell and eosinophil function. Moreover, their expression is influenced by environment, ocular microbiota, and immune regulation mechanisms [4] thus emphasizing the multifactorial pathogenesis. Only 35.3% of AKC patients have a family history of allergic diseases [5].
Pathogenesis
The first step of the process is sensitization: picogram quantities of environmental allergens reach the conjunctival mucosa. These particles are processed by Langerhans, dendritic or other antigen-presenting cells (APCs). Antigens are proteolytically cleaved and they subsequently bind to the antigen-recognition site of the major histocompatibility complex (MHC) class II molecules. Carried by APCs, the antigens are then presented to native Th0 lymphocytes that express antigen-specific receptors and recognize the antigenic peptides. This process probably occurs at the local draining lymph nodes. Multiple contacts and cytokine exchanges between APC and T cells are necessary to induce a Th2-type reaction. The cytokines released by the type-2 helper T-lymphocytes (interleukin-3, IL-4, IL-5, IL-6, IL-13 and granulocyte-macrophage colony stimulate factor – GM-CSF) stimulate the production of Ig E by the B cells.
The second step of the pathophysiology of keratoconjunctivitis allergy is the triggering of the mast cells residing in the conjunctival mucosa and the bearing of specific Ig E antibodies on the cell surface with the help of high affinity receptors. Exposure to environmental allergens in sensitized individuals causes the cross-linking of Ig E at the mast cell membrane level, with subsequent cell degranulation and release of histamine, tryptase, prostaglandins, and leukotrienes. These mediators trigger clinical manifestations of the acute phase of the disease (early phase). However, corneal mast cell degranulation also induces an activation of vascular endothelial cells, and thus expressions of chemokines and adhesion molecules, such as “Regulated-upon-Activation Normal T-cell Expressed and Secreted” (RANTES), monocytes chemotactic protein-1 (MCP-1), intracellular adhesion molecule (ICAM-1), vascular cell adhesion molecule (VCAM) and p-Selectin and chemotactic factors (IL-8, eotaxin). These factors initiate the recruitment phase of activated inflammatory cells in the conjunctiva. The late-phase reaction to allergen stimulation occurs hours after allergen exposure and is characterized by the recurrence or prolongation of symptoms due to the infiltration of eosinophils, neutrophils, and T lymphocytes into the mucosa.
It has been demonstrated that the stimulation of the corneal fibroblasts by the Th2 cytokines IL-4 and IL-13 results into a high release of eotaxin that induces a subsequent marked infiltration of eosinophils in the cornea. Activated eosinophils release cytotoxic proteins such as MBP-1, eosinophil peroxidase, eosinophil-derived neurotoxin and eosinophil cationic protein. Corneal fibroblasts also participate in collagen degradation, which leads to the subsequent corneal ulceration.
The infiltration and degranulation of eosinophils at the limbus are also responsible for the disruption of the corneal epithelium. Proteolytic enzymes, cytotoxic proteins, and oxygen radicals released by neutrophils contribute to the exacerbation of the corneal damage. Therefore, the corneal ulcer with plaque, which develops in patients with severe AKC, is composed of debris derived from eosinophils and epithelial cells. The giant papillae in AKC manifest a dense infiltration of eosinophils immediately beneath the denuded conjunctival epithelium [5].
Corneal Virulence of Staphylococcus aureus: Roles of Alpha-Toxin and Protein A in Pathogenesis
Eosinophils release an eosinophil cationic protein, a major basic protein, and eosinophil peroxidase, which have been implicated in corneal ulceration. Such eosinophilic activation perpetuates allergic inflammatory reactions in the cornea. Eosinophils and neutrophils can produce powerful oxidants such as superoxides and H2O2 in an attempt to kill S. aureus, which might have penetrated through the damaged tissue with decreased barrier functions [6].
Staphylococcus aureus can bind a variety of proteins present in the host extracellular matrix (ECM). The ability to bind ECM proteins is a function of ligand-specific adhesins collectively referred to as microbial surface components recognizing adhesive matrix molecules (MSCRAMMs). They are protein components of the microbial surface that are able to interact with and bind to a variety of relevant extracellular proteins. Among adhesins, two fibronectin-binding proteins, (FnbA and FnbB), three fibrinogen-binding proteins (ClfA, ClfB and Efb), a collagen-binding protein (Cna), the elastin-binding protein (EbpS) have been well characterized [7].
S. aureus produces and secretes many proteins, including coagulase, protein A, alpha-, beta-, gamma-, and delta-toxin, and leukocidin, all of which could contribute to the virulence of the organism. Alpha-toxin is a pore-forming hemolytic toxin that causes membrane damage to many types of cells. The cytolytic nature of alpha-toxin for several cell types could be an important mechanism for corneal epithelial and stromal tissue damage during S. aureus keratitis.
Protein A is a cell, wall-associated exoprotein that binds to the Fc region of immunoglobulin G. In addition, that can activate both the classical and the alternate complement pathways. The complement-activating function of protein A suggests that protein A could induce corneal inflammation and could be a critical factor in staphylococcal virulence. Protein A also inhibits opsonization and phagocytosis of staphylococci in vitro. As a result, protein A could help S. aureus avoid the host’s immune response and thus could contribute to the virulence of the organism [8].
Course
Clinical manifestations of the effects of eye include injection of conjunctival vascular bed due to vascular dilation evoked by vasoactive amines released during mast cell degranulation, accompanied by an influx of water from the intravascular space, to the extravascular space, resulting in tissue edema and eyelid swelling, progressing from a milky or pale conjunctiva aspect to conjunctival swelling or chemosis. Swelling appears 15-30 minutes after antigen exposure and diminishes slowly; a small quantity of white mucus secretion may form during the acute phase which can later becomes thick strands in the chronic form. These patients are prone to develop herpes simplex keratitis, corneal ectasia such as keratoconus, symblepharon formation, atopic (anterior or posterior polar) cataracts, retinal detachment.
Differential diagnosis
1. Vernal Keratoconjunctivitis (VKC),
2. Giant papillary conjunctivitis (GPC)
3. Superior limbic keratoconjunctivitis (SLK)
4. Bacterial Conjunctivitis and keratitis
5. Viral Conjunctivitis and keratitis
6. Protozoan and Helminth Keratitis
7. Fungal Keratitis
8. Ocular Rosacea
9. Central Sterile Corneal Ulceration
10. Dry Eye Syndrome
11. Neurotrophic Keratopathy
Prognosis
Complications result from persistent surface keratopathy, corneal scarring or thinning, keratoconus cataracts, and symblepharon formation. Significant keratopathy can be developed in 70%, corneal neovascularization in 60%, fornix foreshortening in 25%, and symblepharon in 20% during the course of the disease without a proper medication. In addition, medical treatment with corticosteroids can further promote the development of cataracts, glaucoma, and secondary corneal infections. Proper prophylactic measures, prompt effective treatment of exacerbations, and well-timed elective surgical intervention can reduce the incidence of poor vision and blindness. Patients should be observed at every few days or weeks until the ocular surface disease is stable. Moreover, when medically treating patients with steroids, a regular interval survey for drug-related adverse effects and complications is indicated [12].
Treatment
The treatment of AKC should include the involvement of an allergist for the identification of the provoking allergen(s) and education regarding the avoidance of triggers. The triggering antigen may be identified in a more sophisticated manner by RAST testing.
Regarding the medication, Dual-Action Anti-allergic Drugs are the first line of treatment in ocular allergy. At the same time, these drugs inhibit the histamine release from mast cells and histamine binding to H1 receptors along with a longer duration of action (4–6 h), high sedative effect, and anticholinergic activity. Corticosteroids are used for severe exacerbations of conjunctivitis and significant keratopathy, reducing the conjunctival activity that generally leads to corneal improvement. They are usually prescribed in short, but intensive (e.g. 2-hourly initially) courses, aiming for very prompt tapering. Although the risk of elevation of intraocular pressure is low, monitoring is advisable if long-term treatment is necessary. They may also have other serious side effects, such as causing cataracts, and potentiating infection.
Anti-leukotrienes demonstrated their efficacy in a pilot study by reducing signs and symptoms of ocular allergy after 15 days of treatment. The use of Omalizumab, an anti Ig E, may represent an interesting, still not tested, option for the most severe forms of ocular allergy. Adhesion molecule inhibitors may have a role in the treatment of chronic disease with a significant late-phase component. The reported potential side effects of these drugs seems to discourage their use, but, in very severe forms of ocular allergy. Chemokine Inhibitors are able to inhibit the activation of both the early and the late phases of inflammation in murine models of ocular allergy. Treatment with Immunomodulators may be at risk of folliculitis, acne, and herpes simplex [5].
Ma et al. found no difference between the amniotic membrane, mitomycin C or the autologous conjunctival grafts in the management of corneal healing process, but recommend the use of the membrane [9].
Several mechanisms of action are attributed to the membrane. These include the following: promotion of epithelialization, inhibiting scarring, inhibiting vascularization, reducing inflammation, providing a substrate for cell growth, antimicrobial effects and as a biological bandage. By virtue of its basement membrane, it provides a favorable substrate for new epithelial cells to migrate on, expand, and adhere. The use of the membrane as a bandage to cover inflamed or exposed areas, due to injury or surgery, not only favorably influences the healing process but also has a dramatic favorable effect on the symptom of pain and discomfort [10,11].
Conclusions
This case demonstrated the role of patient history and close clinical observation in the diagnosis of AKC. The early treatment was important to reduce the risk of further damage to the cornea. Signs and symptoms can change presentation, thus it is important to reevaluate the diagnosis and treatment. Clinicians must educate parents on the potential chronic nature of the condition, as well as the appropriate follow-up care. For the recurrent cases, parents can utilize preventative eye drops such as cromolyn sodium prior to the allergy season. Resolved cases of AKC should be followed annually in order to be monitored for recurrence. With the proper diagnosis, treatment, and patient education, AKC mostly has a good visual outcome. Future research, especially Gene therapy, will be geared towards finding safer and more effective drug alternatives.
References
- 1.Mir F, Rashid A, Farooq M, Irfan M, Ijaz A. Antibiotic sensitivity patterns of staphylococcal. Journal of Pakistan Association of Dermatologists. 2015;25(1):12–17. [Google Scholar]
- 2.Foster CS, Calonge M. Atopic Keratoconjunctivitis. Ophthalmology. 1990 Aug;97(8):992–1000. doi: 10.1016/s0161-6420(90)32477-6. emedicine.medscape.com/article overview. [DOI] [PubMed] [Google Scholar]
- 3.Kaiser P, Friedman N, Pineda R. The Massachusetts Eye and Ear Infirmary Illustrated Manual of Ophthalmology. 2nd Ed. Philadelphia: Saunders; 2004. pp. 124–127. [Google Scholar]
- 4.Leonardi A. Keratoconjunctivitis: pathogenesis and treatment. Prog Retin Eye Res. 2002;21:319–339. doi: 10.1016/s1350-9462(02)00006-x. [DOI] [PubMed] [Google Scholar]
- 5.Bonini S, Bonini S, Lambiase A, Magrini L, Rumi C, Del Prete G, Schiavone M, Rotiroti G, Onorati P, Rutella S. Vernal keratoconjunctivitis: a model of 5q cytokine gene cluster disease. Int. Arch. Allergy Immunol. 1995;107(1-3):95–98. doi: 10.1159/000236942. [DOI] [PubMed] [Google Scholar]
- 6.Fujishima H, Okada N, Dogru M, Baba F, Tomita M, Abe J, Matsumoto K, Saito H. The role of Staphylococcal enterotoxin in atopic keratoconjunctivitis and corneal ulceration. Allergy. 2012;67:799–803. doi: 10.1111/j.1398-9995.2012.02818.x. [DOI] [PubMed] [Google Scholar]
- 7.Arciola CR, Campoccia D, Gamberini S, et al. Prevalence of cna, fnbA and fnbB adhesin genes among Staphylococcus aureus isolates from orthopedic infections associated to different types of implant. FEMS Microbiol. Lett. 2005;246:81–86. doi: 10.1016/j.femsle.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 8.Callegan MC, Engel LS, Hill JM, O’Callaghan RJ. Corneal virulence of Staphylococcus aureus: roles of alpha-toxin and protein A in pathogenesis. Infect Immun. 1994;62:2478–2482. doi: 10.1128/iai.62.6.2478-2482.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma DH, See LC, Liau SB, Tsai RJ. Amniotic membrane graft for primary pterygium: comparison with conjunctival autograft and topical mitomycin C treatment. Br J Ophthalmol. 2000;84:973–978. doi: 10.1136/bjo.84.9.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azuara-Blanco A, Pillai CT, Dua HS. Amniotic membrane transplantation for ocular surface reconstruction. Br J Ophthalmol. 1999;83:399–402. doi: 10.1136/bjo.83.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dua HS, Gomes JAP, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49:51–77. doi: 10.1016/j.survophthal.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Power WJ, Tugal-Tutkun I, Foster CS. Long-term follow-up of patients with atopic keratoconjunctivitis. Ophthalmology. 1998 Apr;105(4):637–642. doi: 10.1016/S0161-6420(98)94017-9. [DOI] [PubMed] [Google Scholar]